Dysfunction of the vascular endothelium is considered to be an important early step in the development of atherosclerosis(Reference Vanhoutte, Shimokawa and Tang1), and improvements in endothelial function have been observed in studies of high-flavanol foods and beverages, including tea(Reference Vita2, Reference Ras, Zock and Draijer3), red wine(Reference Coimbra, Lage and Brandizzi4, Reference Andrade, Cesena and Consolim-Colombo5), cocoa and chocolate(Reference Fisher, Hughes and Gerhard-Herman6–Reference Njike, Faridi and Shuval13). A recent meta-analysis(Reference Shrime, Bauer and McDonald14) of eight randomised trials has concluded that the vascular effects of high-flavanol cocoa/chocolate were dose dependent and of sufficient magnitude to reduce cardiovascular risk. However, many of these studies(Reference Heiss, Dejam and Kleinbongard7, Reference Vlachopoulos, Aznaouridis and Alexopoulos15–Reference Faridi, Njike and Dutta17) involved acute exposure, lasting from a few hours to a few days. Sustained improvements in flow-mediated dilation (FMD) of the brachial artery with treatment periods lasting from 1 to 3 months have been reported by three previous studies(Reference Balzer, Rassaf and Heiss11, Reference Njike, Faridi and Shuval13, Reference Davison, Coates and Buckley18). However, sustained effects were not evident in a study of adults with CHD after 6 weeks of treatment(Reference Farouque, Leung and Hope9). Given that some vasodilatory drugs (e.g. nitroglycerin) exhibit tolerance with repeated dosing(Reference Knorr, Hausding and Kroller-Schuhmacher19), it is important to understand whether or not cocoa/chocolate may have the same effects on conduit artery vasodilation, even after sustained exposure.
Most vascular studies of cocoa/chocolate have focused on changes in endothelium-dependent vasodilation. There is preliminary evidence that cocoa products also reduce the augmentation index (AI), a measure of arterial stiffness(Reference Vlachopoulos, Aznaouridis and Alexopoulos15, Reference Vlachopoulos, Alexopoulos and Aznaouridis20). The AI is measured using pulse amplitude tonometry and pulse wave analysis(Reference Nichols and Epstein21). In individuals with stiff arteries, the pressure wave travels rapidly from the aorta to the peripheral arterioles, where it encounters resistance. This results in a reflected pressure wave that rapidly travels back to the heart, thus augmenting central systolic pressure(Reference Nichols and Epstein21, Reference Payne, Hurst and Stuart22). The AI increases with age(Reference Heffernan, Kuvin and Sarnak23) and is higher in individuals at elevated CVD risk(Reference Patvardhan, Heffernan and Ruan24). In a cross-sectional study of 198 adults, habitual cocoa consumption (>4·6 mg/d) has been reported to be associated with a lower AI, when compared with infrequent cocoa consumption(Reference Vlachopoulos, Alexopoulos and Aznaouridis20). However, a more recent study with a larger sample size has not confirmed this relationship, perhaps because retrospective recall is not the most effective way to assess habitual intake(Reference Recio-Rodriguez, Gomez-Marcos and Patino-Alonso25). Only one prospective, randomised study has examined the AI after the consumption of high-flavanol chocolate. Vlachopoulos et al. (Reference Vlachopoulos, Aznaouridis and Alexopoulos15) observed significant reductions in the AI 90–180 min after the consumption of a 100 g dose of dark chocolate than after a control procedure (sham eating). It is unknown whether acute effects on the AI are sustained with longer treatment or whether they are evident following the consumption of a more moderate dose of cocoa/chocolate. Thus, the purpose of the present study was to conduct a comprehensive assessment of large and small vessel dilatation, peripheral blood flow and arterial stiffness after treatment with cocoa and dark chocolate v. a matched control treatment over a 4-week period.
Experimental methods
Participants
In the present study, thirty overweight or moderately obese individuals (BMI 25–37 kg/m2), aged 40–64 years, participated (mean age 52·6 (se 0·40) years). To avoid fluctuations in endothelial function across the normal menstrual cycle, only women who were postmenopausal (no menses >12 months) and were not taking hormone therapy were enrolled. Exclusion criteria included the use of medications with cardiovascular or metabolic effects, use of medications for sleep or mood disorders, current use of tobacco, intention to lose weight, history or current diagnosis of CVD, blood pressure (BP) ≥ 140/100 mmHg, diabetes or screening glucose levels ≥ 1260 mg/l and Raynaud's syndrome. The use of vitamins and other supplements was discontinued 2 weeks before participation and throughout the study period. The participants were required to abstain from eating chocolate and/or cocoa-containing products, except those provided during the study period. The study criteria were met by fifty-seven participants after a phone screening, and thirty-seven were eligible following a clinic screening that included a chemistry panel (including liver and kidney function), complete blood count, lipid profile, 12-lead electrocardiogram, brief physical examination and review of medical history. Of the initial thirty-seven eligible participants who were enrolled into the study, seven were excluded due to weight loss >10 % of body weight (n 1), dislike of test products (n 2), change in work schedule (n 1), change in medication (n 1), presence of an undisclosed medical device that would interfere with data collection (n 1), and not providing any reason for study withdrawal (n 1). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of The Pennsylvania State University. Written informed consent was obtained from all the subjects.
Study design and intervention
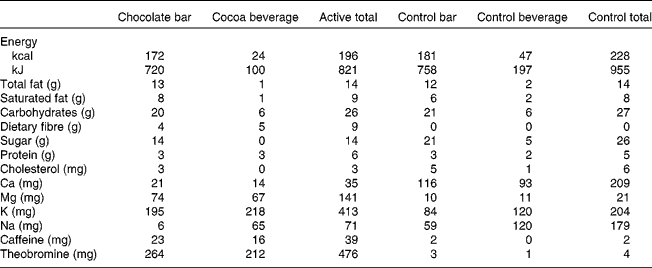
The present study was a randomised, double-blind, two-period, cross-over design. The 4-week treatment periods (cocoa/chocolate v. control) were separated by a minimum of 2 weeks. On each day of the cocoa/chocolate treatment, the participants consumed 37 g/d dark chocolate (Hershey's® EXTRA DARK Chocolate) and a sugar-free cocoa beverage (total dose of natural cocoa = 22 g/d, total flavanols = 814 mg/d). The control treatment included a low-flavanol chocolate bar and a cocoa-free beverage mix with no added sugar (total flavanols = 3 mg/d). The participants were instructed to consume the test products in place of a daily snack. The beverages and bars were similar in appearance and were matched for total fat, saturated fat, protein and carbohydrates (Table 1).
Table 1 Nutrient profiles of test materials
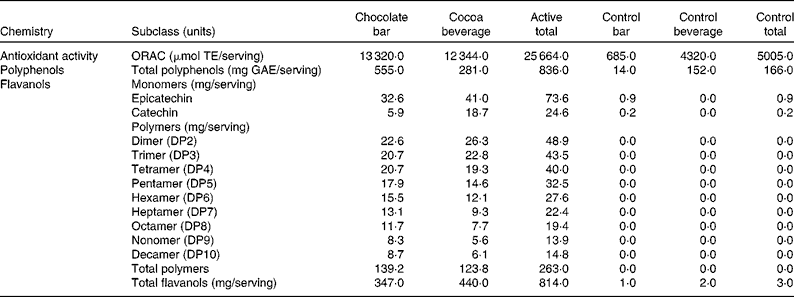
The total antioxidant activity of the active and control products was measured using the oxygen radical absorbance capacity assay(Reference Ou, Hampsch-Woodill and Prior26, Reference Huang, Ou and Hampsch-Woodill27) (Table 2). The concentrations of total polyphenols were measured using the colorimetric method of Singleton & Rossi(Reference Singleton and Rossi28).The concentrations of total flavanols were determined using three different methods (each analytical method was optimised to assess a specific subgroup of flavanols). The concentrations of flavanol monomers, epicatechin and catechin, were measured using liquid chromatography/APCI-MS(Reference Nelson and Sharpless29), and those of the flavanol dimer through decamer polymers (DP2–10) were measured using liquid chromatography/MS(Reference Hurst, Stanley and Glinski30, Reference Robbins, Leonczak and Johnson31). The concentrations of total flavanols were measured using the colorimetric DMAC (4-dimethylaminocinnamaldehyde) method. The DMAC assay is unique in that it measures the concentrations of all flavanols including those of monomers (epicatechin and catechin), gallated flavanols, and flavanol polymers (DP2 to DP10) and polymers greater than DP10(Reference Payne, Hurst and Stuart22).
Table 2 Flavanol compounds and antioxidant activity

ORAC, oxygen radical absorbance capacity; TE, Trolox equivalents; GAE, gallic acid equivalents; DP, degree of polymerisation.
Before each blood draw and vascular test, the participants were asked to avoid the consumption of alcohol and high-flavanoid foods for 48 h; food lists were provided to guide appropriate food choices. Vigorous exercise was prohibited on test days. Waist and hip circumferences were measured in triplicate by nurses, according to a standardised protocol(32). All tests were conducted between 07.00 and 11.00 hours.
Before randomisation, blood sample collection and vascular assessments were carried out after a 12 h fast. At the end of each treatment period, vascular assessments were carried out 2 h after the consumption of the last dose of test products (including one bar and one beverage). In keeping with several previous vascular studies, the 2 h time point was selected to coincide with the peak plasma concentrations of flavanols(Reference Baba, Osakabe and Yasuda33) and peak vascular response in studies of acute exposure(Reference Heiss, Dejam and Kleinbongard7, Reference Balzer, Rassaf and Heiss11, Reference Schroeter, Heiss and Balzer16). Fasting blood samples were collected at the end of each 4-week treatment period from thirteen of the thirty participants.
Ultrasound measures of the diameter and flow-mediated dilation of the brachial artery
FMD and arterial diameter were measured using high-frequency ultrasound as described previously(Reference Celermajer, Sorensen and Gooch34–Reference West, Hecker and Mustad36) in a quiet, dimly lit room at 22–24°C. Our laboratory has previously reported robust test–retest reliability for this measurement(Reference West, Wagner and Schoemer35). The brachial artery above the elbow of the right arm was scanned in a longitudinal section after 15 min of rest, and continuous cross-sectional images were recorded at rest (1 min), during cuff inflation (5 min), and during increased blood flow after cuff release (2 min). An automated rapid cuff inflator set to 250 mmHg (Hokanson) was placed on the forearm, distal to the ultrasound probe. Changes in arterial diameter were measured using external B-mode ultrasound imaging (Acuson Aspen 128XP equipped with a 10 mHz linear array transducer; Acuson) by a single well-trained sonographer (P. W.). Images were gated using R-wave detection so that scans could be assessed at end diastole.
Quantification of arterial diameter and flow-mediated dilation
Automated edge detection software (Brachial Analyzer; MIA) was used to quantify arterial diameter continuously, throughout the test period. The peak arterial diameter was determined as the largest diameter recorded in the 2 min post-deflation segment. Resting diameters are reported as the average diameter in all the images collected over a 1 min period. FMD was calculated by two independent scorers as percent change in arterial diameter at peak dilation v. baseline and is reported as a percent. FMD is reported as the average of the two scores.
Measurement of reactive hyperaemia (change in flow volume following cuff release)
Using duplex pulsed Doppler, we measured the average flow velocity (m/s), maximum flow velocity, and velocity time integral during the resting baseline period and immediately after cuff release. Flow (ml/min) was calculated as described previously(Reference West, Wagner and Schoemer35).
Peripheral arterial tonometry
During the FMD test, the EndoPAT2000 (Itamar Medical Limited) was used to measure relative changes in peripheral pulse wave amplitude before v. after occlusion(Reference Bonetti, Pumper and Higano37). The EndoPAT technique has been validated as a procedure to measure endothelium-dependent vasodilation(Reference Bonetti, Pumper and Higano37). We have demonstrated a low variability for this measure over repeated visits(Reference McCrea, Skulas-Ray and Chow38). On the index fingers of the right (ischaemic) and left (control) hands of the participants, two flexible probes were placed. Measurements were taken at baseline (5 min), during occlusion (5 min) and during reactive hyperaemia (5 min). The reactive hyperaemia index (RHI) was calculated as the ratio of the average pulse wave amplitude during hyperaemia (60 to 120 s of the post-occlusion period) to the average pulse wave amplitude during baseline in the occluded hand over the same values in the control hand multiplied by a baseline correction factor. Low RHI scores have been reported to be prospectively associated with increases in CVD risk over a 6-year follow-up period(Reference Rubinshtein, Kuvin and Soffler39). The Framingham reactive hyperaemia index (fRHI) is an alternative calculation derived from the same raw data. The fRHI uses the period from 90 to 120 s after cuff release, does not incorporate a baseline correction factor, and has a natural log transformation applied to the resulting ratio(Reference Hamburg, Keyes and Larson40). The fRHI is inversely correlated with other CVD risk markers, such as BMI and the total:HDL-cholesterol (HDL-C) ratio(Reference Hamburg, Keyes and Larson40, Reference Hamburg and Benjamin41). The EndoPAT device calculates the AI from the shape of the pulse wave under resting conditions. The AI can be adjusted to a heart rate of 75 beats/min to correct for the independent effect of heart rate on this measure(Reference Wilkinson, MacCallum and Flint42). Both unadjusted and adjusted AI are reported here.
Blood pressure
On a separate day, fasting BP was measured using an automated, oscillometric device (Dinamap Pro 100; GE Medical Systems) after a 20 min rest period. The participants were seated, with their arms at the heart level, and appropriately sized cuffs were used. The time of day was held constant, and the average of three readings was recorded. This procedure was repeated 2 h after the consumption of the final dose of cocoa products. BP is reported as the mean of three readings collected during the 20 min rest period.
Metabolic parameters
Lipids and lipoproteins
Whole blood was drawn into serum separator tubes, allowed to clot and centrifuged. The concentrations of total cholesterol and TAG were determined using enzymatic procedures (Quest Diagnostics; CV < 2 % for both)(Reference Persson, Persson and Hagg43). The concentration of HDL-C was estimated according to the modified heparin–manganese procedure (CV < 2 %). The concentration of LDL-cholesterol (LDL-C) was calculated using the Friedewald equation:
The inter-assay CV was less than 3 %.
Inflammatory markers
The plasma concentrations of IL-1β, IL-6 and TNF-α were measured using high-sensitivity ELISA kits obtained from R&D Systems in duplicate (assay CV < 11 % for all). In samples collected 2 h after the consumption of the last dose of cocoa/chocolate or control, the serum concentration of high-sensitivity C-reactive protein was measured using latex-enhanced immunonephelometry (Quest Diagnostics; assay CV < 8 %).
Insulin and glucose
The concentration of insulin was measured by RIA using 125I-labelled human insulin and a human insulin antiserum (Linco Research; cross-reactivity with proinsulin < 0·2 %)(Reference Morgan and Lazarow44). The concentration of glucose was determined with an immobilised enzyme biosensor using the YSI 2300 STAT Plus Glucose & Lactate Analyzer (Yellow Springs Instruments). The homeostatic model of insulin resistance (HOMA-IR) was calculated as follows(Reference Bonora, Formentini and Calcaterra45):
Renin, angiotensin-converting enzyme and angiotensin
Changes in renin activity, angiotensin-converting enzyme activity and angiotensin II activity were measured based on previous research that showed reductions in angiotensin-converting enzyme activity after the consumption of chocolate(Reference Persson, Persson and Hagg43). RIA were used to measure plasma renin activity and angiotensin II activity (Diagnostic Systems Laboratories, Inc.). Angiotensin-converting enzyme activity was measured using an enzymatic assay at The Milton S. Hershey Medical Center core laboratory.
Statistical analyses
Variables were tested for normality and transformed where appropriate. Non-transformed values are reported as means with their standard errors. Treatment effects were examined using the mixed model procedure in the Statistical Analysis Systems statistical software package version 9.2 (SAS Institute). Initial models for the analysis of vascular parameters, BP and fasting blood variables included treatment (baseline, cocoa+chocolate, or control), period and treatment × period interaction as fixed effects, with participant as a random effect. There was no indication of carry-over: the treatment × period interaction was not significant. This interaction was removed from the final models. The treatment × sex interaction was significant only for the AI and the AI at 75 beats/min, and these results are presented separately by sex. Because the female participants were older, on average, data are presented after adjustment for age. Significant treatment effects (two-sided P≤ 0·05) were interrogated with Tukey's post hoc tests, where appropriate. The present study was powered (β = 0·90) to detect a relative increase in FMD of 25 %(Reference West, Wagner and Schoemer35) and a 0·25 unit change in RHI(Reference McCrea, Skulas-Ray and Chow38). Based on our previous work, the sample of thirty participants was sufficient to detect a 10 % change in LDL levels and a 7·2 unit change in the AI.
Results
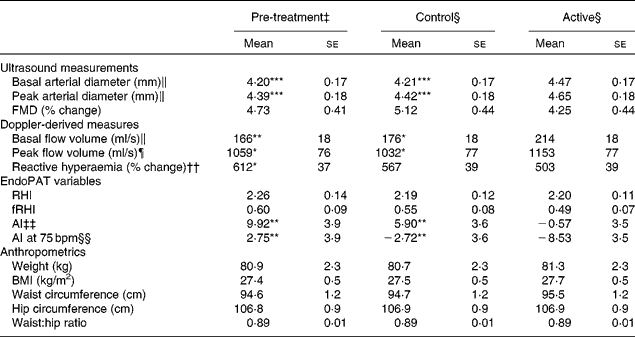
The baseline characteristics of the participants are given in Table 3. We observed marked peripheral vasodilation following the cocoa+chocolate treatment (Table 4). The increase in the diameter of the brachial artery was evident before reactive hyperaemia (P= 0·001) and at the peak dilation following cuff release (P= 0·0001). As a result, FMD (expressed as percent change from basal diameter) did not differ by treatment. In addition, resting (P= 0·04) and peak (P= 0·03) hyperaemic blood flow significantly increased following the chocolate v. control treatment (mean increase = 22 and 12 % for the basal and peak hyperaemic blood flow, respectively). There were no treatment differences for RHI and fRHI, the measures of endothelial function derived from the EndoPAT device (Table 4). Fig. 1 shows that the reductions in arterial stiffness were largest for the female participants, resulting in a significant treatment × sex interaction, for the AI (P= 0·05) and the AI at 75 beats/min (P= 0·02). The dark chocolate/cocoa treatment decreased the AI by 83 % and the AI at 75 beats/min by 129 % in women, relative to the pre-treatment value (P= 0·01). In contrast, in men, the AI was not significantly different after the chocolate treatment.
Table 3 Characteristics of the participants before treatment* (Least-squares mean values with their standard errors)
* Pre-treatment values for other CVD risk factors are presented in Tables 4–6.
Table 4 Effects of treatments on vascular outcomes and anthropometrics† (Adjusted mean values with their standard errors)

FMD, flow-mediated dilation; RHI, reactive hyperaemia index; fRHI, Framingham reactive hyperaemia index; AI, augmentation index; AI at 75 bpm, the augmentation index normalised for a heart rate of 75 beats/min.
Mean values were significantly different from those of the active group: * P≤ 0·05, ** P≤ 0·01, *** P≤ 0·001.
† Data were obtained from the mixed model procedure in SAS. Tukey's post hoc comparison test was used for multiple comparisons.
‡ Pre-treatment testing was carried out in the fasting state.
§ At the end of each 4-week treatment period, vascular tests were carried our 2 h after the consumption of the last treatment dose.
∥ There was a significant main effect of treatment (P= 0·0001).
¶ There was a significant main effect of treatment (P= 0·02).
†† There was a significant main effect of treatment (P= 0·05).
‡‡ There was a significant main effect of treatment (P= 0·0008).
§§ There was a significant main effect of treatment (P= 0·05).
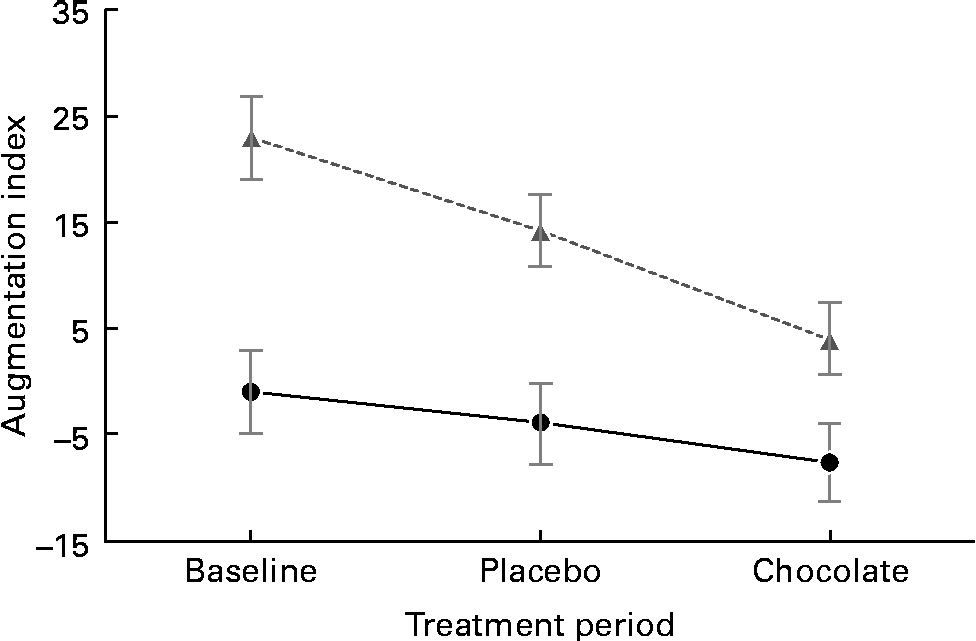
Fig. 1 Sex difference in vascular response to the cocoa+dark chocolate treatment. Women (![]() ) exhibited significant reductions in the augmentation index, whereas men (
) exhibited significant reductions in the augmentation index, whereas men (![]() ) did not (sex × treatment interaction, P= 0·01).
) did not (sex × treatment interaction, P= 0·01).
There were no significant treatment differences for body weight, BMI, waist circumference, hip circumference or the waist:hip ratio (Table 4). After 4 weeks of treatment, there were no changes in fasting BP or heart rate. There were modest, but statistically significant increases in systolic BP (P= 0·02), diastolic BP (P= 0·005) and heart rate (P= 0·02) 2 h after the consumption of the final dose of cocoa+chocolate relative to the control treatment (Table 5). Among the fasting blood variables, only insulin concentration and HOMA-IR showed significant increases after the control v. cocoa+chocolate treatment (Table 6).
Table 5 Blood pressure and heart rate after 4 weeks of treatment† (Adjusted mean values with their standard errors)
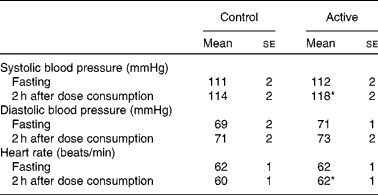
* Mean values were significantly different from those of the control treatment (P= 0·02).
† Data were obtained from the mixed model procedure in SAS. Tukey's post hoc comparison test was used for multiple comparisons.
Table 6 Effects of treatments on the fasting markers of metabolic variables, inflammation and renin/angiotensin-converting enzyme (ACE) activity‡ (Adjusted mean values with their standard errors)
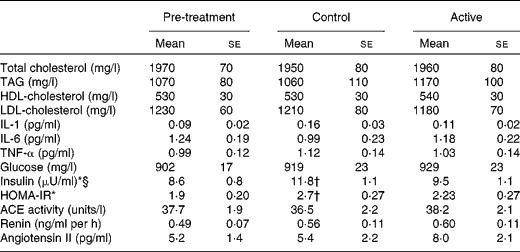
HOMA-IR, homeostatic model assessment of insulin resistance.
* There was a significant main effect of treatment (P= 0·01).
† Mean values were significantly different from those of the pre-treatment (P= 0·009).
‡ Data were obtained from the mixed model procedure in SAS. Tukey's post hoc comparison test was used for multiple comparisons.
§ Insulin: 1 μU/ml is equivalent to 6.45 pmol/l.
Discussion
In the sample of healthy, overweight adults in the present study, 4 weeks of daily consumption of cocoa and dark chocolate (equivalent to 22 g/d of natural cocoa) was associated with significant increases in basal and peak arterial diameter and increased arterial blood flow through the brachial artery. In only women, there were significant reductions in peripheral arterial stiffness. Taken together, these changes reflect peripheral vasodilation, both in the smaller resistance arteries and in the major conduit artery serving the forearm. This pattern (increased arterial diameter, together with greater flow volume and reduction in the AI) was also observed by Vlachopoulos et al. (Reference Vlachopoulos, Aznaouridis and Alexopoulos15) a few hours after the consumption of a single dose of high-flavanol cocoa. Reductions in the AI have been observed in habitual cocoa consumers(Reference Vlachopoulos, Alexopoulos and Aznaouridis20), although this finding is not universal(Reference Recio-Rodriguez, Gomez-Marcos and Patino-Alonso25), and previous studies have not presented data separately by sex. Given that women had a higher AI at baseline, it is unclear whether the sex difference in response to treatment is dependent on ceiling/floor effects, a true sex difference in physiology, or age. Future studies should enrol age-matched men and women to confirm the source of this effect.
In contrast to several previous studies, more targeted measures of vascular endothelial function (FMD, RHI and fRHI) remained unchanged. However, we observed that significant vasodilation was evident both before and after the hyperaemic stimulus. We cannot rule out sustained improvement in tonic endothelial release of NO as a mechanism for the basal vasodilation observed in the present study. We encourage investigators to report arterial diameters and flow volume in addition to FMD in future publications.
There is convergent evidence from clinical trials(Reference Shrime, Bauer and McDonald14, Reference Hooper, Kroon and Rimm46), animal models(Reference Karim, McCormick and Kappagoda47–Reference Ottaviani, Momma and Heiss49) and in vitro studies(Reference Schroeter, Heiss and Balzer16, Reference Schnorr, Brossette and Momma48) that high-flavanol cocoa and chocolate enhance endothelium-dependent vasodilation, primarily by increasing NO production. In studies of human endothelial cells, cocoa flavanols have been reported to lower vascular arginase activity(Reference Schnorr, Brossette and Momma48), suggesting a possible mechanism for increases in NO levels. Several mechanistic studies have identified epicatechin(Reference Schroeter, Heiss and Balzer16) as a critical bioactive compound in cocoa. Recent studies have indicated that cocoa flavanols stimulate NO production in endothelial cells(Reference Heiss, Dejam and Kleinbongard7, Reference Balzer, Rassaf and Heiss11). The oral administration of ( − )-epicatechin, a flavanol found in very high concentrations in cocoa, has been reported to result in acute increases in NO production and vasodilation in healthy men(Reference Loke, Hodgson and Proudfoot50). It has also been demonstrated that the vasodilation observed following the consumption of flavanol-rich cocoa is associated with increases in circulating bioactive NO levels and that the inhibition of NO production prevents cocoa-induced vasodilation(Reference Fisher, Hughes and Gerhard-Herman6, Reference Schroeter, Heiss and Balzer16, Reference Heiss, Kleinbongard and Dejam51).
Previous studies(Reference Fisher, Hughes and Gerhard-Herman6, Reference Balzer, Rassaf and Heiss11, Reference Fisher and Hollenberg52–Reference Ried, Sullivan and Fakler54) on cocoa/chocolate have reported reductions in BP in adults with elevated BP(Reference Grassi, Necozione and Lippi55, Reference Grassi, Desideri and Necozione56) or no change in BP in normotensive individuals (for a review, see Hooper et al. (Reference Hooper, Kroon and Rimm46) and Ried et al. (Reference Ried, Sullivan and Fakler54)). In our normotensive sample, 4 weeks of cocoa+chocolate treatment had no effect on fasting BP. Systolic BP increased by 6 mmHg (v. 2 mmHg, after the control treatment, P= 0·03) 2 h after the consumption of a full day's dose of cocoa+chocolate by the participants. Monahan et al. (Reference Monahan, Feehan and Kunselman12) have recently examined the acute effects of increasing doses of cocoa on endothelial function and BP. They found that the highest dose (26 g) increased systolic BP by 6 mmHg (v. 3 mmHg in the control). As in the present study, small increases in systolic BP were accompanied by substantial decreases in peripheral vasoconstriction. Other studies involving the acute administration of cocoa or chocolate have reported no change(Reference Heiss, Dejam and Kleinbongard7, Reference Heiss, Finis and Kleinbongard10, Reference Vlachopoulos, Aznaouridis and Alexopoulos15, Reference Persson, Persson and Hagg43, Reference Berry, Davison and Coates57) or a decrease in BP(Reference Faridi, Njike and Dutta17). Although dietary fat has been shown to increase BP acutely(Reference Gosmanov, Smiley and Robalino58), our treatments were matched for fat content. It is more likely that the stimulant effects of caffeine or theobromine, inherent to cocoa and chocolate, may be responsible. The present results should be viewed with caution because a recent meta-analysis of thirteen longer-term studies has concluded that chocolate and cocoa have no effect on BP in healthy adults and that lower BP is observed in individuals with elevated BP(Reference Ried, Sullivan and Fakler54). We observed that the acute changes in the present study were well within the normotensive range.
Previous studies(Reference Grassi, Desideri and Necozione56, Reference Hooper, Kay and Abdelhamid59) have observed improvements in insulin levels or insulin resistance with the consumption of high-flavanol cocoa/chocolate. In a recent meta-analysis of seven clinical studies, a pooled analysis has revealed significant reductions in HOMA-IR ( − 0·67; 95 % CI − 0·98, 0·36), driven by reductions in serum insulin levels ( − 2·65 μU/ml; 95 % CI − 4·65, 0·65 μU/ml) following the consumption of cocoa/chocolate. The same interventions had no effect on the levels of fasting glucose, HbA1c or QUICKI (quantitative insulin sensitivity check index)(Reference Vanhoutte, Shimokawa and Tang1). In the present study, the control treatment was associated with increased fasting insulin levels and greater insulin resistance v. pre-treatment. Insulin resistance remained unchanged following the cocoa+chocolate treatment, and the two treatments did not differ. As expected, body weight and fasting lipid levels did not differ significantly from those observed during the control treatment or pre-treatment.
The present study has several limitations. The most important one is that we carried out all the vascular assessments 2 h after the consumption of the final dose, thus confounding the acute and chronic effects of cocoa+chocolate. However, time course studies have shown that the improvement in endothelial function is greatest 2 h after the consumption of the dose(Reference Balzer, Rassaf and Heiss11, Reference Schroeter, Heiss and Balzer16, Reference Heiss, Kleinbongard and Dejam51), and several previous studies have assessed post-treatment FMD at 2 h(Reference Njike, Faridi and Shuval13, Reference Faridi, Njike and Dutta17, Reference Berry, Davison and Coates57). Nested designs, in which acute responses are recorded at the end of a lengthy treatment period, are an effective strategy to tease out acute and chronic effects. Furthermore, we used a relatively high dose of cocoa products, including 37 g of dark chocolate and a beverage containing 11 g of natural cocoa. Several recent studies have suggested that smaller amounts of cocoa or dark chocolate can also enhance vasodilation(Reference Monahan, Feehan and Kunselman12). Monahan et al. (Reference Monahan, Feehan and Kunselman12) measured the acute change in FMD after varying doses of cocoa, ranging from 2 to 26 g. Significant increases in FMD (v. the fasting baseline) were observed 2 h after the ingestion of 5, 13 and 26 g of cocoa, with larger increases being observed after the consumption of higher doses. Although vascular effects of chocolate/cocoa are often attributed to their antioxidant compounds, we did not assess the measures of oxidative stress in the present study. This limits our ability to draw conclusions about the mechanism responsible for vasodilation.
In conclusion, in the present placebo-controlled, randomised trial, we observed substantial reductions in vascular constriction in the brachial artery in men and women after 4 weeks of daily consumption of cocoa and dark chocolate. The magnitude of blood flow during reactive hyperaemia, as measured by Doppler, also increased significantly with the cocoa+dark chocolate treatment. These changes may have been caused by the enhanced release of NO, although FMD of the brachial artery and RHI remained unchanged. We observed a substantial reduction in the AI in women, indicating greater compliance of conduit arteries or decreases in the constriction of peripheral arterioles. The cocoa+dark chocolate treatment produced no significant changes in fasting lipid, glucose, insulin or TAG levels. Although these data were obtained from only a subset of the study population, our findings are in agreement with those of studies showing little effect of chocolate on the lipid profile in individuals with normal cholesterol levels. Overall, the present study provides additional evidence that regular ingestion of natural cocoa and dark chocolate is beneficial for maintaining cardiovascular health, without adverse effects on body weight or body composition.
Acknowledgements
The General Clinical Research Center of The Pennsylvania State University is appreciated for providing its services. The present study was supported by National Institutes of Health Grant M01 RR 10732 and funded by The Hershey Company. D. L. M. and A. G. P. are employees of The Hershey Company. D. L. M. and A. G. P. contributed to the study design and manuscript development.
S. G. W., D. L. M., A. G. P. and A. C. S.-R. designed the research; M. D. M., M. J. P., N. P., P. W., L. F. G. and A. C. S.-R. conducted the research; A. C. S.-R. carried out the biochemical analyses; S. G. W. carried out the statistical analyses; S. G. W. prepared the manuscript; S. G. W. was responsible for its final content. All authors read and approved the final manuscript.
S. G. W. has received research funding and travel support from The Hershey Company. D. L. M. and A. G. P. are employees of The Hershey Company. None of the other authors has any personal or financial conflicts of interest.