Trace amines, including tyramine and β-phenylethylamine (β-PEA), are biologically active amines occurring in the body in trace amounts. They are also found in the diet, derived from plants, bacteria and fungi(Reference Burchett and Hicks1). Foods rich in tyramine and β-PEA include cheeses, red wine, fermented foods, such as sausages, and cocoa-containing foods, such as chocolate(Reference Branchek and Blackburn2). The recently popularised probiotic foods owe their reputed beneficial effects on health to high levels of lactic acid-producing bacteria such as Lactobacillus, Lactococcus and Enterococcus species. These are said to be ‘friendly’ bacteria, displacing harmful bacteria of the gut flora(Reference Taylor and Mahenthralingham3) and combating infectious diarrhoea(Reference Fedorak and Madsen4). Their commercial promotion is also based on a perceived ability to alleviate bloating and abdominal distension(Reference Houghton and Whorwell5). Indeed, there is clinical evidence that bloating(Reference Kim, Camilleri and McKinzie6) and flatulence(Reference Nobaek, Johansson and Molin7, Reference Kim, Vazquez Roque and Camilleri8) associated with irritable bowel syndrome are reduced by probiotic bacterial supplements. It is not generally recognised, however, that the bacterial populations present in probiotic foods generate considerable levels of tyramine and β-PEA(Reference Marcobal, de la Rivas and Muñoz9).
Since trace amines are abundant in many foods and in drinks taken before or after meals (aperitifs, chocolates)(Reference Branchek and Blackburn2), in the present study we determined their effects on the gut and its vasculature. We examined the pharmacological effects of tyramine and β-PEA in isolated sections of gut from the guinea-pig or rat ileum which were electrically stimulated to simulate peristalsis. We also examined their effects on the mesenteric vasculature as an index of effects on blood flow to the gut, using rat isolated perfused mesenteric beds and compared these with the aorta and coronary vasculature.
Trace amines, such as tyramine and β-PEA, are classically regarded as having indirect sympathomimetic activity(Reference Trendelenburg, Blascho and Muscholl10). However, our recent studies have demonstrated a vasoconstriction by related amines that is independent of indirect sympathomimetic activity(Reference Fehler, Broadley and Kidd11–Reference Baker, Herbert and Broadley13). This raises the possibility that trace amines can also exert physiological effects through other receptors, such as the recently cloned trace amine-associated (TAA) receptors (Reference Borowsky, Adham and Jones14–Reference Lindemann, Ebeling and Kratochwil17). Thus, a secondary aim of the present study was to examine whether the effects of tyramine and β-PEA on the gut and its vasculature were mediated through sympathomimetic-dependent or -independent mechanisms.
Experimental methods
Isolated ileum
Male Sprague–Dawley rats (250–300 g; Harlan, Bicester, Oxon, UK) or Dunkin–Hartley guinea-pigs (250–400 g; Harlan) were killed by cervical dislocation and exsanguination. The guidelines for the care and use of laboratory animals were followed according to the Animals (Scientific Procedures) Act 1986.
Sections (2 cm) of ileum were isolated from rats or guinea-pigs and set up in tissue baths containing Krebs bicarbonate buffer (pH 7·4) at 37°C gassed with 5 % CO2 in O2. The Krebs bicarbonate buffer was made up in distilled water and had the following composition (mm): NaCl, 100; NaHCO3, 20; glucose, 10; MgSO4·7H2O, 1; KH2PO4, 1; KCl, 5; CaCl2·2H2O, 3. To simulate peristalsis, tissues were electrically stimulated with square-wave pulses (0·1 Hz, 5 ms pulse width, 10 V) via parallel platinum electrodes located inside and outside the lumen. Isometric tension was measured by force transducers (Dynamometer UF1, 57 g sensitivity range; Pioden Controls Ltd, Canterbury, Kent, UK) coupled to a PowerLab/4SP computerised data acquisition system (ADInstruments, Charlgrove, Oxon, UK). Data were analysed using Chart version 4.1.1 software (ADInstruments). β-PEA or tyramine was added cumulatively in half-logarithmic increments in molar concentration to the tissue baths until the maximum effect was achieved.
Mesenteric bed
Male Sprague–Dawley rats (250–300 g) were killed by cervical dislocation and exsanguination. The superior mesenteric artery was cannulated and the mesenteric arterial bed excised and placed in a perfusion chamber. The bed was perfused at a constant flow rate (4 ml/min) with Krebs bicarbonate solution, warmed to 37°C and gassed with 95 % O2–5 % CO2. Perfusion pressure was monitored by means of a pressure transducer (Elcomatic EM 750; Elcomatic Ltd, Glasgow, UK) connected to a computer data acquisition system (ADInstruments PowerLab, Chart 5). Increasing bolus doses (100 μl) of β-PEA or tryramine were injected, each dose added after recovery of the response to the previous dose.
Aortic ring preparations
Rings (3 mm) were cut from the thoracic or abdominal aorta of male Sprague–Dawley rats (250–300 g) or Dunkin–Hartley guinea-pigs (250–400 g) and passed over fixed and mobile hooks. The latter was connected to an isometric transducer (Dynamometer UF1, 57 g sensitivity range; Pioden Controls Ltd, Canterbury, Kent, UK), a resting tension of 1·5 g applied and tension recorded on a computerised data acquisition system (ADInstruments PowerLab, Chart 5). Tissues were bathed in Krebs bicarbonate buffer (37°C) gassed with 95 % O2–5 % CO2. Endothelium was removed by rolling the ring around a wooden cocktail stick. Endothelium denuding was confirmed by failure of acetylcholine (100 μm) to relax aortae pre-contracted with the selective thromboxane A2 agonist, U46619 (9,11-dideoxy-9α, 11α-epoxymethanoprostaglandin F2α;1 μm). After 1 h equilibration, isotonic KCl (60 mm) was added to contract the tissue to test for tissue viability and as a standardisation response. After washout, non-cumulative concentration–response curves (CRC) for β-PEA or tyramine were constructed by adding successive concentrations in half-logarithmic increments with washout between each. CRC were obtained either in the absence or presence of the inhibitors, prazosin (1 μm), ICI-118,551 (1 μm), cocaine (10 μm) and pargyline (10 μm), which were incubated with the tissue for 15 min before commencing the CRC.
Pig coronary artery rings
Left anterior descending coronary arteries were dissected from porcine (either sex) hearts after transport at 4°C from the abattoir. Rings (5 mm long) were mounted in tissue baths and isometric tension recorded as described for aortic rings. Tissue viability was initially determined by contracting with 60 mm-KCl. After washout, intact endothelium was confirmed by at least 70 % relaxation to10 μm-bradykinin in rings precontracted with U46619 (5 nm). Acetylcholine contracts rather than relaxes pig coronary arteries(Reference Baker, Herbert and Broadley13, Reference Meyers, Banitt and Guerra18) and is therefore unsuitable for testing for the presence of endothelium and endothelium-derived relaxation. Baseline tensions were re-established by washing before obtaining a cumulative CRC to tyramine or β-PEA.
Measurement of responses and analysis of results
In isolated ileum preparations, increases in the baseline contractions to β-PEA or tyramine were plotted as a percentage of the maximum response to methacholine (acetyl-β-methylcholine) added at the start of the experiment or as a percentage of the maximum contractile response.
Responses of the mesenteric vascular bed were measured as the peak fall in perfusion pressure after each dose of tyramine or β-PEA and plotted as a percentage of the constriction by phenylephrine. Constrictions of aortic and coronary artery ring preparations above baseline were measured at the peak response to each concentration of β-PEA or tyramine and expressed as a percentage of the contraction to KCl (60 mm) added at the beginning of an experiment. CRC for the mean responses with their standard errors were plotted against log molar concentration.
Geometric mean EC50 values (molar concentration for 50 % of maximal response) and their 95 % CI were calculated. To enable comparisons with a standard contractile agent in the ileum, responses were also expressed as a percentage of the maximum response to methacholine; because the maximum did not always reach 50 % of the methacholine maximum, the EC30 values (molar concentration for 30 % of the maximum response to methacholine) were calculated. EC30 and EC50 values and maximum responses expressed as a percentage of methacholine (ileum) or KCl contractions (aorta and coronary artery) were compared by Student's t test for paired or unpaired two-set data or by ANOVA followed by post hoc Bonferroni's tests for multiple comparisons. P < 0·05 was deemed significant.
Materials
The following drugs were used: acetylcholine chloride, atropine sulfate, bradykinin triacetate, cocaine hydrochloride, 5-hydroxytryptamine (5-HT) hydrochloride (serotonin), methacholine (acetyl-β-methylcholine) chloride, pargyline hydrochloride, (R)-( − )-phenylephrine hydrochloride, 2-phenylethylamine hydrochloride (β-phenylethylamine), ( ± )-propranolol hydrochloride, prazosin hydrochloride, ritanserin, tyramine hydrochloride (p-tyramine), U46619 (9,11-dideoxy-9α, 11α-epoxymethanoprostaglandin F2αα) (Sigma-Aldrich, Poole, Dorset, UK), ICI-118,551 hydrochloride (( ± )-1-(2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy(-3-((1-methylethyl) amino(-2-butanol hydrochloride) (Tocris Bioscience, Bristol, UK) and phentolamine mesylate (Rogitine®; Alliance Pharmaceuticals, Chippenham, Wilts, UK).
All chemicals for the Krebs bicarbonate buffer were of analytical grade and were obtained from Fisher Scientific (Loughborough, Leics, UK) unless otherwise stated. All drugs were dissolved in distilled water, except for prazosin, ICI-118,551 and U46619, which were dissolved in dimethyl sulfoxide and ritanserin which was dissolved in alcohol. Neither dimethyl sulfoxide nor alcohol at the concentrations employed exerted significant effects on coronary artery or ileum responses, respectively.
Results
Ileum
Both guinea-pig and rat electrically stimulated ileum exhibited concentration-related contractions in response to tyramine and β-PEA (Fig. 1). Tissue sections also contracted to tyramine and β-PEA without electrical stimulation. In guinea-pig unstimulated ileum, the geometric mean EC30 values for tyramine and β-PEA were 1·89 (95 % CI 0·88, 4·06) × 10− 4 and 6·54 (95 % CI 3·68, 11·6) × 10− 4m, respectively, yielding a potency ratio of 0·3. The maximum responses as a percentage of methacholine maximum contraction were 73·0 (sem 2·0) and 53·9 (sem 5·9) %, respectively. In unstimulated guinea-pig ileum, the corresponding maxima were 78·0 (sem 2·0) and 58·6 (sem 1·7) %, respectively. In guinea-pig electrically stimulated ileum, the non-selective α- and β-adrenoceptor antagonists phentolamine (10− 6m) and propranolol (10− 6m) failed to block the contractions to tyramine (Fig. 2 (a)) or β-PEA (Fig. 2 (b)). The geometric mean EC50 values for tyramine in unstimulated preparations in the absence (2·56 (95 % CI 1·24, 5·20) × 10− 4m or presence of both antagonists (3·48 (95 % CI 2·88, 4·21) × 10− 4m) were not significantly different. Similarly, the EC50 values for β-PEA in the absence (2·51 (95 % CI 1·50, 4·20) × 10− 4m) or presence of phentolamine (2·51 (95 % CI 1·87, 3·37) × 10− 4m), propranolol (2·51 (95 % CI 2·02, 3·13) × 10− 4m) and both antagonists (2·51 (95 % CI 1·87, 3·37) × 10− 4m) did not differ significantly. The increase in baseline tension of guinea-pig electrically stimulated ileum by β-PEA was not blocked by the non-selective muscarinic acetylcholine receptor antagonist, atropine (10− 6m), whereas contractions to electrical stimulation were abolished. The EC50 values for β-PEA in the absence (5·32 (95 % CI 1·8, 15·3) × 10− 4m) or presence of atropine (3·16 (95 % CI 2·04, 4·91) × 10− 4m; n 4) did not differ significantly. The 5-HT2A/B/C receptor antagonist, ritanserin (10− 6m)(Reference Hoyer, Clarke and Fozard19), abolished 5-HT contractions (Fig. 3 (a)) but did not shift the CRC for the contraction of guinea-pig ileum to β-PEA (Fig. 3 (b)). The EC50 values in the absence (3·16 (95 % CI 1·52, 6·58) × 10− 4m) or presence of ritanserin (5·01 (95 % CI 1·16, 21·7) × 10− 4 m) were not significantly different.

Fig. 1 Typical traces of the contractile effects of β-phenylethylamine (β-PEA) on electrically stimulated guinea-pig (a) and rat (b) isolated intestine. Both show β-PEA increasing the baseline tension.

Fig. 2 Concentration–response curves for increases in baseline contractions of guinea-pig electrically stimulated ileum to (a) tyramine and (b) β-phenylethylamine (β-PEA). (a) Tyramine responses plotted as percentages of the maximum response to methacholine (acetyl-β-methylcholine) in the absence (▲; n 7) and presence of phentolamine (10− 6 m) and propranolol (10− 6 m; ■; n 4). (b) β-PEA responses plotted as percentages of the maximum response, in the absence (■) and presence of phentolamine (10− 6m; ▲), propranolol (10− 6 m; ![]() ) or phentolamine and propranolol (◇). Values are means, with standard errors represented by vertical bars.
) or phentolamine and propranolol (◇). Values are means, with standard errors represented by vertical bars.

Fig. 3 (a) Baseline contractions of guinea-pig electrically stimulated ileum to 5-hydroxytryptamine (5-HT; 3 × 10− 6m), plotted as percentages of the maximum response to methacholine (acetyl-β-methylcholine), before (□) and in the presence of ritanserin (■; 10− 6m). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that pre-ritanserin (P < 0·05). (b) Concentration–response curves for increases in baseline contraction of guinea-pig electrically stimulated ileum to β-phenylethylamine, plotted as percentages of the maximum response to methacholine, in the absence (■) and presence of ritanserin (10− 6m; ▲). Values are means (n 4), with standard errors represented by vertical bars.
Mesenteric vascular bed
The responses of the vascular bed of the gastrointestinal tract to increasing bolus doses of tyramine and β-PEA were measured in rat isolated perfused mesenteric beds. Resting perfusion pressure was little affected by tyramine and β-PEA. However, when perfusion pressure was raised by infusion of the α-adrenoceptor agonist, phenylephrine (100 μm), tyramine and β-PEA caused dose-related falls in perfusion pressure indicative of vasodilatation (Fig. 4). The maximum pressure falls were to 84·2 (sem 4·3) and 59·9 (sem 5·4) % of the phenylephrine-induced rise in pressure, respectively.
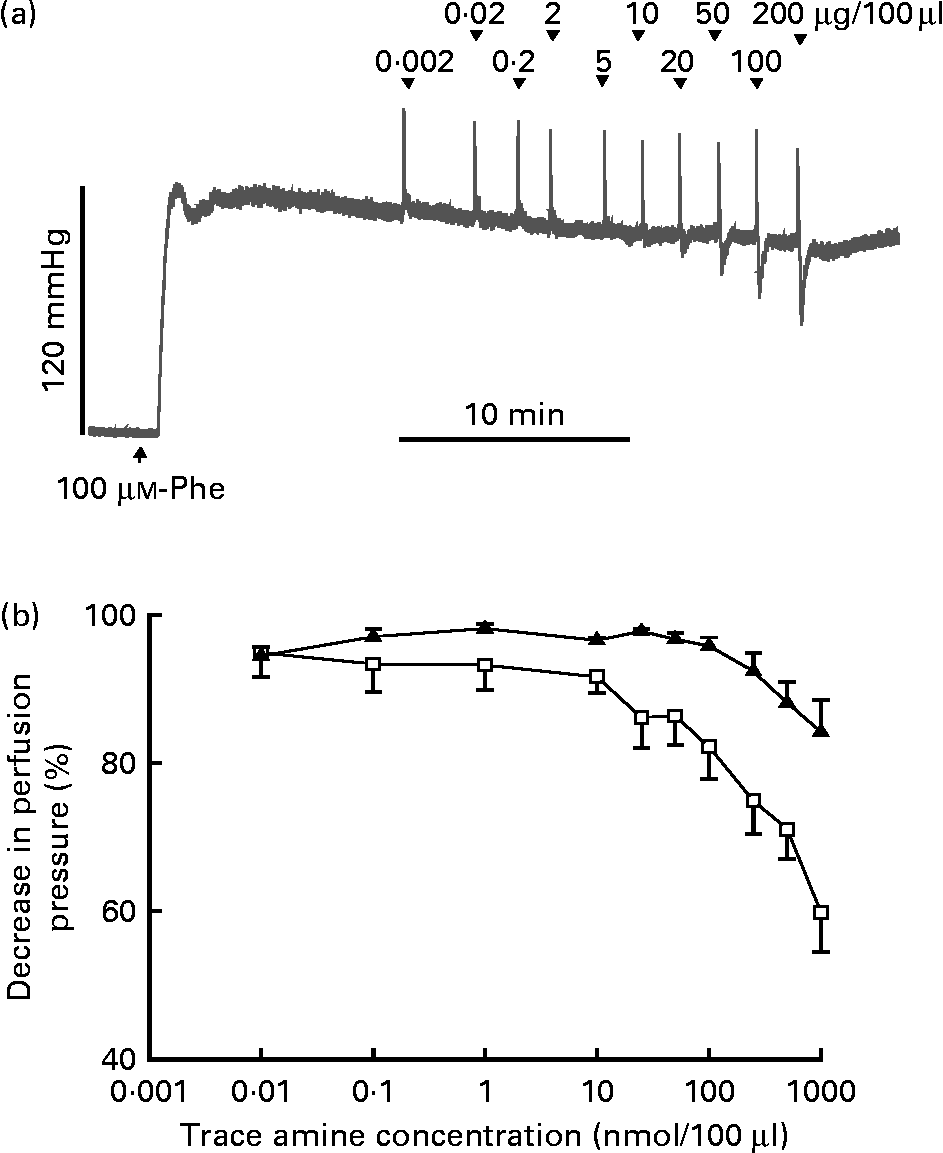
Fig. 4 Vasodilator effects of β-phenylethylamine (β-PEA) and tyramine in rat isolated perfused mesenteric vascular beds. (a) Typical trace for falls in perfusion pressure of the mesenteric bed with increasing doses of β-PEA with resting perfusion pressure raised by infusion of phenylephrine (Phe, 100 μm). The initial spike increases in pressure are injection artifacts. (b) Dose–response curves for falls in mesenteric vascular pressure to tyramine (▲; n 4) and β-PEA (□ n 4), plotted as percentages of the increase in pressure by Phe against concentration (μg/100 μl). Values are means, with standard errors represented by vertical bars.
Aortic rings
Tyramine and β-PEA were next examined in guinea-pig and rat isolated aortic ring preparations, from which the endothelium was removed. In contrast to the mesenteric vasculature, β-PEA and tyramine produced concentration-related vasoconstriction. These responses persisted in the presence of prazosin (1 μm), ICI-118,551 (1 μm), cocaine (10 μm) and pargyline (10 μm) to block α1- and β2-adrenoceptors, uptake of sympathomimetic amines and monoamine oxidases A and B, respectively (Fig. 5). The maximum vasoconstriction to tyramine (62·5 (sem 23·9) % of KCl contraction) was significantly less than to β-PEA (138·3 (sem 23·9) %) in rat aorta. Tyramine could therefore be regarded as a partial agonist.

Fig. 5 Vasoconstrictor effects of β-phenylethylamine (β-PEA) and tyramine in rat and guinea-pig isolated aortic rings. (a) Concentration–response curves (CRC) for constriction of guinea-pig aortic rings by β-PEA in the absence (□) and presence of the inhibitors prazosin (1 μm), ICI-118,551 (1 μm), cocaine (10 μm) and pargyline (10 μm) (◆). Values are means (n 6), with standard errors represented by vertical bars. (b) CRC for the constriction of guinea-pig aortic rings by tyramine in the absence (□) and presence of the inhibitors (◆). Values are means (n 5), with standard errors represented by vertical bars. (c) CRC for the constriction of rat aortic rings by tyramine (□ n 4) and β-PEA (◆; n 6) in the presence of the inhibitors. Values are means, with standard errors represented by vertical bars.
Pig coronary artery
To examine a smaller vessel rather than a major conductance vessel such as the aorta, we used isolated rings of pig left anterior descending coronary arteries with intact endothelium. Cumulative CRC to tyramine or β-PEA were obtained. Tyramine and β-PEA caused concentration-dependent vasoconstriction (Fig. 6) which reached maxima of 51·0 (sem 6·6) (n 4) and 74·3 (sem 9·1) %, respectively, of the KCl constriction. These constrictions were not prevented by the α1-adrenoceptor antagonist, prazosin (1 μm) (Fig. 6).

Fig. 6 Vasoconstrictor effects of β-phenylethylamine (β-PEA) and tyramine in isolated rings from pig left anterior descending coronary artery. (a) Typical trace for a concentration–response curve (CRC) for the constrictor response to β-PEA. (b) Typical trace for a CRC for the constrictor response to β-PEA in the presence of prazosin (1 μm). (c) CRC for tyramine in the absence (■) and presence of prazosin (□; 1 μm). Values are means (n 4), with standard errors represented by vertical bars. (d) CRC for β-PEA in the absence (▲) and presence of prazosin (△; 1 μm). Values are means (n 4), with standard errors represented by vertical bars.
Discussion
Tyramine and β-PEA contracted both guinea-pig and rat isolated gut preparations. In the guinea-pig ileum, tyramine consistently produced a greater maximum response (relative to the methacholine maximum contraction) than β-PEA, whether in electrically stimulated or unstimulated tissues. These responses were not inhibited by antagonists of the major neurotransmitters of the gut, namely noradrenaline, acetylcholine and 5-HT via adrenoceptors, muscarinic receptors and 5-HT2 receptors, respectively. A contractile response of the gut is opposite to that expected of sympathomimetic amines, such as noradrenaline and adrenaline, which relax the gastrointestinal tract(Reference Broadley20, Reference Van Rossum and Mujić21). The relaxation is due to direct activation of β1- and α1-adrenoceptors on the longitudinal smooth muscle(Reference Broadley and Grassby22). Indirect sympathomimetic amines such as tyramine have also been shown to reduce myogenic activity of rabbit ileum, an effect attributed to the release of noradrenaline from sympathetic neurones in the gastrointestinal wall(Reference Schmidt and Fleming23). However, the more usual response reported in the literature has been a contraction to tyramine and β-PEA of guinea-pig ileum(Reference Jamieson and Taylor24) and rabbit jejunum(Reference Van Rossum and Mujić21, Reference Innes and Kohli25), an effect that unlike the present findings was attributed to stimulation of 5-HT receptors(Reference Jamieson and Taylor24, Reference Innes and Kohli25). However, in these relatively old studies, the selectivity of available antagonists was poor. Furthermore, it was found that desensitisation of 5-HT receptors by prolonged exposure to 5-HT abolished the contractions to 5-HT but not β-PEA(Reference Innes and Kohli25), suggesting that β-PEA was not operating through 5-HT receptors.
These results have therefore shown that tyramine and β-PEA contract rather than relax the gut, a response that is inconsistent with a sympathomimetic action. Furthermore, the response is not abolished by antagonists of muscarinic or 5-HT receptors that are known to mediate contraction of the gut. Similarly, the vasoconstrictor actions of tyramine and β-PEA in rat and guinea-pig aortic and pig coronary blood vessels were resistant to adrenoceptor blockade. Therefore the vasoconstriction remaining in the presence of these antagonists was not due to noradrenaline release from sympathetic neurones in the aortic wall. Therefore β-PEA and tyramine were not behaving as indirect sympathomimetic amines by acting. In contrast to the aorta and coronary arteries, tyramine and β-PEA failed to constrict the mesenteric blood vessels supplying the gastrointestinal tract. Instead, when the pressure was raised by infusing phenylephrine, there was vasodilatation.
The present results overturn the dogma that has persisted for over 30 years that these amines owe their pharmacological actions to indirect sympathomimetic properties. They can clearly exert contractile effects on the gut and blood vessels via an alternative and hitherto unidentified pathway. Trace amines have been shown to bind to receptors now called TAA receptors(Reference Lindemann and Hoener16, Reference Lindemann, Ebeling and Kratochwil17, Reference Zucchi, Chiellini and Scanlan26). These receptors were identified by the selection of oligonucleotide sequences based on G-protein-coupled receptors for serotonin(Reference Borowsky, Adham and Jones14) or dopamine(Reference Bunzow, Sonders and Arttamangkul15) and amplification by PCR of novel DNA sequences. Cell lines were then transfected with the cloned sequences resulting in novel orphan receptors coupled to cAMP production. Several TAA receptors have now been cloned but only TAA receptors 1 and 4 are contenders for a functional role since only these recognise tyramine or β-PEA(Reference Lindemann and Hoener16, Reference Zucchi, Chiellini and Scanlan26). We therefore propose that tyramine and β-PEA exert their non-adrenergic responses via trace amine receptors. Human embryonic kidney (HEK293) cells stably expressing rat TAA 1 receptors show tyramine to be more potent than β-PEA for cAMP accumulation by potency ratios of 0·23(Reference Lindemann, Ebeling and Kratochwil17) or 0·29(Reference Bunzow, Sonders and Arttamangkul15). The TAA receptor 4 receptor expressed in COS-7 cells shows stimulation of cAMP for which β-PEA is 8·9-fold(Reference Zucchi, Chiellini and Scanlan26) more potent than tyramine. In the present studies, the potency ratios were 0·62 (EC50 ratio) in pig coronary artery, 0·13 (EC50 ratio in the presence of antagonists) in guinea-pig aorta and 0·30 (EC30 ratio in the presence of propranolol and phentolamine) in the unstimulated guinea-pig ileum. Tyramine was therefore marginally more potent than β-PEA, suggesting a role for TAA receptor 1. However, in the rat aorta tyramine was a weak partial agonist, which, assuming that it interacts with the same receptor as β-PEA, does not support the contention of TAA receptor 1 involvement. Furthermore, the contractile responses observed here are clearly not due to cAMP generation which would relax smooth muscle(Reference Broadley20). Further work is necessary to characterise the receptors involved in these native tissues and a major breakthrough would be the identification of a selective antagonist which is currently unavailable.
Concentrations of tyramine and β-PEA in the circulation comparable with those revealing effects on the gut and vasculature could be achieved by a number of means. Pressor responses are typically associated with a high dietary intake of tyramine, especially in individuals taking monoamine oxidase inhibitors to prevent its degradation in the intestine before reaching the circulation(Reference Zucchi, Chiellini and Scanlan26), in smokers who have lower monoamine oxidase A and B levels(Reference Berlin and Anthenelli27) and in women taking isoflavones as an alternative to hormone replacement therapy(Reference Hutchins, McIver and Johnston28). Plasma levels of tyramine are also elevated in hypertensive patients(Reference Andrew, Best and Watson29) and in patients with migraine headaches(Reference D'Andrea, Terrazzino and Leon30). Pressor responses are induced by the oral administration of doses of tyramine in the range 100–200 mg and the oral dose of tyramine to produce a 30 mmHg rise in systolic blood pressure is approximately 7 mg/kg (500 mg in a 70 kg subject)(Reference Peatfield, Littlewood and Glover31). Tyramine concentrations able to induce pressor responses can reach these levels in many fermented foods such as sausages (> 200 mg/kg)(Reference Suzzi and Gardini32) and goat (2000 mg/kg)(Reference Bonetta, Bonetta and Carraro33) and Dutch cheese (300 mg/kg)(Reference Komprda, Burdychova and Dohnal34). The concentrations of tyramine in the gut will be higher than in the circulation and in the range capable of stimulating the gut found in the present study.
From the present results we can hypothesise that trace amines, such as tyramine and β-PEA, found in the diet may have a physiological role in mammalian digestion. After ingestion, they stimulate the gut thereby facilitating digestion. The concomitant vasodilatation of the mesenteric vascular bed and increased blood flow to the gastrointestinal tract would provide energy to support the enhanced gut motility and release of digestive enzymes, and assist transport of absorbed nutrients from the gut(Reference Chou35). Increases in visceral blood supply are met by a compensatory restriction of blood supply to some other organs(Reference Chou35). The vasoconstrictor effects of tyramine and β-PEA on blood vessels other than those of the mesentery are demonstrated by the vasoconstriction observed here in the aorta and coronary arteries. Thus, the relief of bloating(Reference Kim, Camilleri and McKinzie6) and increased transit through the gut(Reference Niedzielin, Kordecki and Birkenfield36) that has been reported for probiotics could be due to increased gut activity by the generation of trace amines by their content of Lactobacillus, Lactococcus and Enterococcus species. Our hypothesis could also apply to other foods such as cocoa products, cheeses, sausages and red wine, which are often eaten before or after meals in the belief that they aid digestion. Our hypothesis will require verification in vivo to show that the effects of trace amines seen here in vitro can be extended to their oral administration, and reproduced after ingestion of trace amine-rich foods.
Acknowledgements
There are no conflicts of interest for any of the authors of the study.
The study was supported by grants from the British Heart Foundation and a Wellcome Trust Fellowship (to M. A. A.).
K. J. B. wrote the manuscript and interpreted the data; M. A. A. performed the mesenteric vasculature studies; A. A. H. performed the pig coronary artery studies; M. F. performed the rat aorta studies; E. M. J. and W. E. D. performed the ileum studies; E. J. K. designed and interpreted the rat aorta studies; W. R. F. designed and interpreted the ileum studies.








