Atlantic salmon (Salmo salar) are fed diets with up to 400 g marine lipids per kg, susceptible to lipid oxidation. Long-chain PUFA, abundant in marine lipids, may oxidise in the feed or in the fish body and in both cases expose the fish to oxidative stress. Small amounts of reactive oxygen species are constantly generated through normal metabolism, by electron transport, phagocytotic activity and by certain enzymes (oxygenases, cytochrome P450)(Reference Rice-Evans and Burdon1). Reactive oxygen species are very reactive towards all biomolecules (lipids, proteins and nucleotides) and the reaction of reactive oxygen species with PUFA generates an auto-oxidation cycle where the product of one cycle is a free radical, which may react with a new PUFA(Reference Frankel2). Animals have built up extensive defence systems against in vivo peroxidation, consisting of antioxidant enzymes, endogenous antioxidants such as glutathione (GSH) and ubiquinone (UQ), and nutritional antioxidants such as vitamins C and E and carotenoids. Normally, this defence keeps in vivo oxidation at an extremely low level and small amounts of reactive oxygen species are even essential for regulation of cell growth and development and intracellular signalling(Reference Rice-Evans and Burdon1, Reference Nordberg and Arner3). Oxidative challenge can be encountered when the formation of free radicals exceeds the capacity of the antioxidant defence. Such a situation may develop after tissue injury or inflammation, as a result of exposure to pollution or oxidative drugs and nutrients, or during deficiency of antioxidant nutrients. In Atlantic salmon juveniles and parr, oxidative challenge and increased lipid peroxidation have been demonstrated after hyperoxia caused by supersaturation of the water with O2(Reference Lygren, Hamre and Waagbø4), after feeding high levels of Cu and Cd(Reference Berntssen, Lundeby and Hamre5) and during vitamin E deficiency(Reference Hamre, Berge and Waagbø6). Oxidative stress leads to damage of biomolecules, tissues and organs and subsequently to disease. It is important that fish are fed diets with an optimal balance of the antioxidant nutrients and that they have a well-functioning endogenous antioxidant defence to cope with oxidative challenges present in the fish farming situation.
Vitamin E is a chain-breaking antioxidant that reacts with the lipid peroxyl radical formed in auto-oxidation of PUFA to prevent it from reacting with a new PUFA, and rendering the tocopheroxyl radical. It is now well accepted that vitamin C recycles the tocopheroxyl radical situated in the interface between water and lipid at the membrane surface to reduced vitamin E(Reference Tappel7, Reference Packer, Slater and Willson8). Vitamin C also works as a radical scavenger in the water phase of the organism. The results of Mårtensson & Meister(Reference Mårtensson and Meister9) demonstrate that the water-soluble endogenously synthesised antioxidant, GSH, reduces dehydroascorbic acid to ascorbate in newborn rats. In turn, oxidised GSH (GSSG) is reduced by NADPH produced in the pentose phosphate shunt(Reference Meister10). Therefore, the recycling, and thereby sparing, of dietary antioxidants may ultimately be coupled to energy metabolism.
UQ is the only endogenously synthesised lipid with redox function in vertebrates and exhibits a broad-tissue as well as intracellular distribution(Reference Dallner and Sindelar11). It was first identified as part of the mitochondrial respiratory chain, but is now recognised to occur in all membrane types and acts as an antioxidant in its reduced form, ubiquinol(Reference Ernster and Dallner12). In mitochondria, ubiquinol amplifies the antioxidant effect of vitamin E, apparently by regenerating the tocopheroxyl radical. UQ, in turn, can be reduced by succinate and NADH through the respiratory chain(Reference Maguire, Kagan and Ackrell13).
The requirements for dietary antioxidants such as vitamins C and E vary with the experimental conditions(Reference Hamre, Berge and Waagbø6, Reference Poston, Combs and Leibovitz14–Reference Watanabe, Takeuchi and Wada16). For vitamin E the reported requirements for salmonids vary between 5 and 60 mg/kg, dependent on dietary lipid content and quality and on vitamin C supplementation(Reference Hamre, Berge and Waagbø6, Reference Cowey, Adron and Walton17–Reference Hamre and Lie19). The vitamin E requirement may also be affected by astaxanthin and Se(Reference Bell, Cowey and Adron15, Reference Christiansen, Glette and Lie20) and possibly by other pro- and antioxidant nutrients. Further, vitamin C requirement in Atlantic salmon seems to be slightly modulated by vitamin E, where high dietary vitamin E increases the requirement(Reference Hamre, Berge and Waagbø6). This may be due to the regeneration of vitamin E by vitamin C(Reference Tappel7, Reference Packer, Slater and Willson8) which will consume reduced ascorbic acid.
Given the complex interactions of the antioxidant defence, it is difficult to compare results from different studies where one or two nutrients are investigated, since other nutrients may be supplemented at different levels and influence the results. In the present study, we used a multivariate approach to study effects and interactions between pro- and antioxidant nutrients and lipid on the nutrient-dependent and endogenous antioxidant defence. Seven different nutrients (vitamins C and E, astaxanthin, lipid, Fe, Cu and Mn) were given in high and low concentration according to a 27–3 reduced factorial design(Reference Thelin, Lundstedt, Seifert, Nortvedt, Brakstad and Kvalheim21). The low level of vitamins and minerals was chosen to be just above the minimum requirement, whereas the high level was below the anticipated toxic level, to cover the anticipated safe window of supplementation. For astaxanthin, the levels chosen were 10 and 50 mg/kg, covering the range of increase in fillet astaxanthin concentration, whereas dietary lipid level was 150 and 320 g/kg. Several responses were monitored: growth, feed conversion and fillet quality(Reference Hamre, Christiansen and Waagbø22), haematology, immune functions and antioxidant enzymes(Reference Lygren, Hamre and Waagbø23), cataract development(Reference Waagbø, Hamre and Bjerkås24) and lipid metabolism(Reference Torstensen, Lie and Hamre25). The present paper examines effects of the nutrient variation on the endogenous antioxidant defence.
Materials and methods
Fish and diets
The feeding experiment was carried out at Ewos Innovation AS in Dirdal, Norway, according to their fish-holding routines which are in accordance with the Animal Welfare Act of 12 December 1974 (no. 73, §§22 and 30). Post-smolt Atlantic salmon, 148 (sd 17) g, were distributed into sixteen indoor 2·8 m3 tanks and stocked at 180 fish per tank. Sixteen different diets (for compositions, see Table 1) with two levels of vitamin C, vitamin E, astaxanthin, Fe, Cu, Mn and lipid were fed in excess using automatic feeders. The diets were produced by Ewos Innovation AS (Dirdal, Norway). The low-fat-diets were formulated to contain (per kg): 450 g protein, 170 g fat, 65 g ash and 285 g N-free extracts. The formulation of the high-fat diets (per kg): was 450 g protein, 320 g fat, 65 g ash and 115 g N-free extracts. The micronutrients were added at just above the minimum requirement and at a level that was anticipated to be below the toxic level: vitamin E (as all-rac-α-tocopheryl acetate), 60 and 410 mg/kg; vitamin C (as Stay-C®), 30 and 2000 mg/kg; Fe, 70 and 1200 mg/kg; Cu, 7 and 100 mg/kg; Mn, 10 and 200 mg/kg. The minerals were added as water-saturated sulfates. Astaxanthin (as Carophyll Pink) was supplemented at 10 and 50 mg/kg. Other vitamins and minerals were added according to the National Research Council(26). Vitamins and astaxanthin were supplied by Hoffman-La Roche (Basle, Switzerland) and the minerals were from Merck (Darmstadt, Germany). Analysed levels of the dietary variables (Table 2) were close to the formulated ones.
Table 1 Main composition of the experimental diets
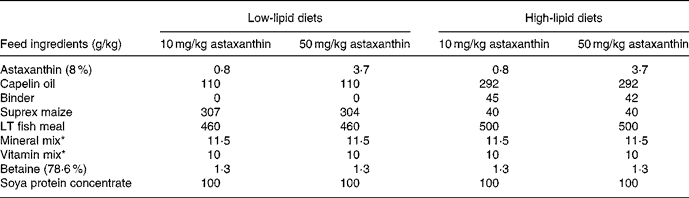
* Added non-variable vitamins and minerals (per kg dry diet): 25 μg vitamin D3, 10 mg vitamin K, 15 mg thiamin, 10 mg riboflavin, 10 mg pyridoxine, 50 mg niacin, 5·0 mg folic acid, 1·0 mg biotin, 0·01 mg cyanocobalamin, 20 mg pantothenic acid, 1625 g choline, 500 mg Mg as magnesium sulfate.7H2O, 100 mg Zn as zinc sulfate.7H2O. The vitamins were from Hoffman-La Roche (Basle, Switzerland) and the minerals from Merck (Darmstadt, Germany).
Table 2 Variable nutrient levels (1, high; −1, low) in the experimental diets and analysed nutrient concentrations (mg/kg or g/kg) of diets with high or low levels, respectively, of the nutrient in question
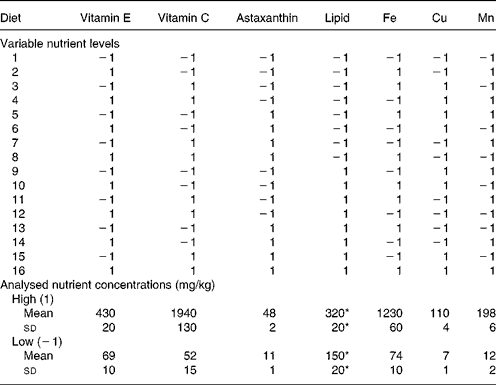
* g/kg.
During the experiment, mortalities were recorded and dead fish removed daily. Weight was determined by bulk weighing and subsequent counting of all the fish in each tank. The mean temperature, O2 level and salinity during the experimental period were 8·2 ± 0·4°C, 12·6 ± 0·7 mg/l and 29·2 ± 1·2 g/l, respectively. The fish were exposed to continuous light.
Experimental design
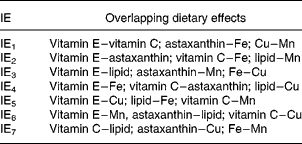
The experiment was carried out as a 27–3 reduced factorial design (Table 2). This implies that the seven independent dietary variables were added at two levels and that the number of dietary combinations (cases) was systematically reduced from 128 (27) to sixteen (24) according to Box et al. (Reference Box, Hunter and Hunter27). With a full factorial design it would be possible to calculate effects of the single nutrients (main effects) and all the interaction effects (IE), but with seven variables one would need 128 treatments. Reducing the design to 27–3 and sixteen treatments gives loss of resolution, since the main effects and interactions overlap with one another. In this particular design, it is possible to calculate the main effects separated from each other and from the effects of two-factor interactions. There are three possible interpretations (overlap) of each two-factor interaction (Table 3) and the three-factor interactions overlap with the main effects. Three-factor interactions are usually small compared with main effects and two-factor interactions, which were considered in the present study. The advantage of this design is the possibility to screen a greater number of nutrients than in traditional designs, and to identify interactions between them. The use of one replicate per dietary treatment may be considered a disadvantage, but the fact that every nutrient level is replicated eight times increases the confidence of the study. A reduced factorial experiment is described by Langsrud et al. (Reference Langsrud, Ellakjær and Næs28), who evaluated the effects of varying six different ingredient and processing factors on the sensory quality of cheese. The experiment was set up as a 26–2 reduced factorial design with sixteen treatments (cases). Sundberg's tutorial(Reference Sundberg29) describes factorial designs in general and includes an example with a 24–1 reduced factorial design.
Table 3 Overlap pattern for two-factor interaction effects (IE) whereby each IE has three possible interpretations
Sampling procedure
Samples were taken from each batch of the diets and stored at − 20°C. Fish were sampled at the start and after both 14 and 23 weeks. The fish were starved for 48 h before sampling. Sixteen randomly sampled fish from each tank were anaesthetised with metomidate (7 g/l). Blood was drawn from the caudal vein into heparinised tubes, and centrifuged at 1000 g for 10 min at 4°C to separate blood cells and plasma. Plasma from the sixteen fish was pooled together and subsamples were frozen on dry ice. The fish were killed with a blow to the head, and liver and the whole fillet were removed. The tissue samples from the sixteen fish per tank were pooled together, homogenised on ice and frozen as subsamples on dry ice. Samples were stored at − 80°C.
Chemical analyses
α-Tocopherol was analysed by HPLC by the method of Lie et al. (Reference Lie, Sandvin and Waagbø30). Total ascorbic acid was also analysed by HPLC according to Mæland & Waagbø(Reference Mæland and Waagbø31). The feed samples were hydrolysed with phosphatase before extraction. Astaxanthin was analysed according to Torrissen(Reference Torrissen32) and total lipid, gravimetrically, according to Lie et al. (Reference Lie, Waagbø and Sandnes33). The analyses of dietary Fe, Cu and Mn were carried out using flame atomic absorption as described by Liaset et al. (Reference Liaset, Julshamn and Espe34). Thiobarbituric acid-reactive substances (detection limit 1·17 nmol/g wet weight) were analysed according to Hamre et al. (Reference Hamre, Næss and Espe35) and GSH by the method of Svardal et al. (Reference Svardal, Mansoor and Ueland36).
A method modified from Lang et al. (Reference Lang, Gohil and Packer37) and Podda et al. (Reference Podda, Weber and Traber38) was applied for analyses of UQ and ubiquinol. A 0·05–0·1 g homogenised sample was dissolved in 1 ml distilled water, 1 ml 0·2 m-SDS and 2 ml ethanol in screw-capped 10 ml glass tubes and extracted three times with 3 ml hexane. The extracts were pooled, evaporated to dryness under N2 (room temperature) and dissolved in mobile phase, before injection into the HPLC. The HPLC system consisted of a pump (L-7100; Merck-Hitachi, Darmstadt, Germany) an auto-injector (AS3000; Spectra System, San Jose, CA, USA), a UV detector (UV1000; Spectra System) and a LC-18 analytical column (4·6 × 150 mm; 3 μm particle size; Supelco, Bellefonte, PA, USA). The mobile phase consisted of 85 % methanol and 15 % tetrahydrofuran, flow rate was 1·5 ml/min and detection was performed at 290 nm (ubiquinol) between 0 and 6·5 min and at 275 nm (UQ) from 6·5 min. Quantification was performed with external standards for UQ and a correction factor for ubiquinol of 0·28, based on the extinction coefficients (1 %; 1 cm) EUQ275nm = 165 and Eubiquinol290nm = 46(Reference Podda, Weber and Traber38). Recoveries of UQ standard from liver and plasma were 99 (sd 7) and 102 (sd 4) %, respectively, and linearity was found in the area 2 ng–20 μg UQ injected into the column. Muscle samples gave peaks in the chromatogram which overlapped with UQ. Therefore, muscle was not analysed for this compound.
Statistics
The software package Statistica for Windows version 4.5 (StatSoft Inc., Tulsa, OK, USA, 1993) was used for the statistical analyses. Significant effects of the dietary parameters or their two-factor interactions were calculated using multiple linear regression. Each response was calculated separately and only response models where all effects were significant were accepted as valid.
Results
Results on growth, survival and feed conversion have been reported by Hamre et al. (Reference Hamre, Christiansen and Waagbø22). The average specific growth rate was 0·92 %/d with a positive effect of high dietary lipid (P < 10− 6), increasing the final weight by 12 %, and a slight negative effect of high dietary Fe (P < 0·05). The average feed conversion rate was 1·07 and high dietary lipid reduced feed conversion by 0·14 (P < 10− 5). There were between zero and two mortalities per tank. The most important factor for determining astaxanthin concentration in muscle was dietary astaxanthin (P < 10− 4), but there was also a positive effect of lipid in week 14 (P = 0·0003). Astaxanthin in the liver was affected by dietary astaxanthin, lipid, vitamin E, Fe and Mn. Muscle, but not liver, lipid was increased by high dietary lipid (P < 10− 6). In the liver, the lipid level was affected by vitamin E and Mn but the magnitude of the effects was less than 10 %(Reference Hamre, Christiansen and Waagbø22).
Ascorbic acid concentration in liver, plasma and fillet (Table 4) was mainly influenced by dietary vitamin C, as none of the other dietary variables gave consistent responses or responses that were higher than 10 % of the mean. Vitamin C had a profound effect on plasma and tissue concentrations of ascorbic acid (P < 10− 6) and the models were very well correlated with the data (R 2 0·84–1·00).
Table 4 Biological effects of pro- and antioxidants and lipid on plasma and tissue ascorbic acid, α-tocopherol, vitamin A, iron, copper, manganese, glutathione and ubiquinone in Atlantic salmon (Salmo salar) fed the experimental diets for 14 or 23 weeks*

IE, interaction effect; NS, no significant effects of nutrients.
* The response models were obtained by multiple linear regression using the varied nutrients and their IE (see Table 3) as independent variables (value 1 or − 1). Models and effects were considered significant at P < 0·05, but only effects higher than 10 % of the mean or effects that were repeated at both samplings (weeks 14 and 23) are included in the Table.
† There were minor effects ( < 10 % of mean) which were not included in the Table.
‡ Ubiquinone in plasma was 100 % oxidised.
α-Tocopherol in liver, plasma and fillet (Table 4) was positively affected by high dietary vitamin E (P < 10− 5) and negatively affected by high dietary lipid (P < 0·006). In liver and fillet, there was a negative effect of IE3 (P ≤ 0·001; Table 3) on α-tocopherol concentration. In addition, high dietary Mn gave an increased concentration of α-tocopherol in fillet in week 14 (P < 10− 4). Vitamin A (Table 4) in liver in week 23 was positively and profoundly affected by high dietary lipid (P < 10− 6). The models for plasma and tissue α-tocopherol and vitamin A fitted the data well (R 2 0·83–1·00).
Plasma and liver Fe was increased by high dietary Fe (P < 0·04), while there was no effect of the other varied nutrients on tissue Fe levels (Table 4). No nutritional effects were seen on the concentration of Fe in bone. There were no effects of the dietary variables on tissue levels of Cu. Mn levels in liver, plasma and bone were increased by high dietary Mn (P = 0·04–0·002), and by high dietary lipid (P = 0·04–0·01), except in plasma in week 14. High dietary vitamin C decreased Mn concentration in plasma in week 23 (P = 0·02).
Tissue and plasma concentrations of GSH (Table 4) were only marginally affected by the dietary variation. Liver total concentration of GSH was positively influenced by high dietary lipid (P < 0·001) and negatively by IE3 (P < 0·004) and the fit between models and data was good (R 2 0·95–1·00). In plasma, IE3 decreased the fraction of reduced GSH at both samplings (P < 0·05), whereas the fraction was increased by high dietary vitamin E (P = 0·005) and reduced by high dietary Fe (P = 0·007) in week 23. There were no consistent effects, or effects exceeding 10 % of the mean, on liver percentage of reduced GSH, plasma total concentration of GSH or muscle GSH.
UQ (Table 4) in liver was influenced by dietary vitamin E, astaxanthin and lipid. High dietary lipid led to an increase, both in total concentration (P < 10− 6) and in fraction, of reduced ubiquinol (P ≤ 10− 6) in the liver. High dietary vitamin E gave slightly increased total concentration (NS in week 14 and P = 0·005 in week 23) and a slightly smaller fraction of reduced ubiquinol (P ≤ 0·002), while the opposite was true for high dietary astaxanthin (P ≤ 0·002). The net effect of vitamin E and astaxanthin on liver total concentration and fraction of reduced UQ was a similar concentration of reduced ubiquinol. There was a good fit between models and data concerning liver UQ (R 2 0·88–0·98). All UQ in plasma was oxidised. The total plasma concentration was increased by high dietary lipid (NS in week 14 and P = 0·04 in week 23) and decreased by high dietary astaxanthin (P ≤ 0·002). In week 23 there was a good fit between model and data for plasma UQ concentration (R 2 0·97).
The concentration of thiobarbituric acid-reactive substances in liver was 3·5 nmol/g wet weight and not affected by the dietary treatments while the fillet concentrations were below the detection limit of the method.
Discussion
The present study shows that the total concentration and redox state of GSH in Atlantic salmon tissues were only marginally affected by the high ranges of variations in diet composition. High compared with low dietary lipid level gave a difference in total GSH in the liver of maximally 10 %. In addition, IE3 had an effect of increasing total liver GSH from low to high by 4 %. IE3 is the interaction between lipid and vitamin E, between astaxanthin and Mn or between Fe and Cu. Since two-factor interactions are normally higher for high than for low main effects(Reference Sundberg29), IE3 in this case is probably the interaction of lipid with vitamin E. There were also some effects on percentage of reduced GSH in plasma, where the difference between diets high or low in vitamin E was 22 %, and astaxanthin and IE3 changed the percentage of reduced GSH by − 22 and − 15 %, respectively. There were no further effects of diet variation on tissue and plasma GSH, including GSH in muscle. The total concentration of GSH in the liver was approximately ten times that in muscle and 100 times that in plasma.
The dietary effects on UQ were both more diverse and of greater magnitude than those on GSH. Dietary lipid had the most profound effect, increasing the total concentration of UQ in the liver by 29–48 % and in the plasma by 20 %. The redox state of UQ in the liver responded 26–31 % on high compared with low lipid. Vitamin E had a slight positive effect on total concentration of UQ and a slight negative effect on percentage of reduced UQ in the liver, while the opposite was true for astaxanthin. A high level of astaxanthin also lowered plasma total concentration of UQ. UQ in muscle was not determined due to problems with overlapping peaks in the chromatogram. Thus, lipid and the lipid-soluble antioxidants vitamin E and astaxanthin affected GSH and UQ status in tissues and plasma of Atlantic salmon, while vitamin C, Fe, Cu and Mn had no effect on these variables. The dietary variation had no effect on liver thiobarbituric acid-reactive substances, and the fillet level did not increase to above the detection limit of the method in response to any diet. Therefore, the fish do not seem to have been subject to adverse oxidative stress.
According to the hypothesis of Hoffman et al. (Reference Hoffman, Spetner and Burke39), the GSSG:GSH ratio has a great impact on the redox potential of the cell, since GSH is present at very high concentrations, i.e. 1–10 mm. Further, the redox potential regulates phosphorylation and dephosphorylation of proteins involved in cell-cycle regulation; in the reduced state these proteins are phosphorylated and the cells proliferate, while in the oxidised state, the proteins are dephosphorylated and the cells are at rest. Kemp et al. (Reference Kemp, Go and Jones40) propose that the thioredoxin system and protein cysteine/cystine couples also participate in the regulation of the cell redox potential and that different cell compartments have different redox regulators, although the concentrations of these compounds are only in the range of 50 μm(Reference Rebrin, Rajindar and Sohal41). Mechanisms involved in post-transcriptional redox regulation of proteins are the reversible formation of disulfide bridges internally in protein, between subunits or between separate proteins and glutathionation of protein cysteine residues. This causes conformational changes in the proteins(Reference Dalle-Donne, Rossi and Giustarini42), which may lead to phosphorylation–dephosphorylation(Reference Hoffman, Spetner and Burke39) or other changes in activity. Cellular concentrations of GSH are regulated through two principal pathways, de novo synthesis of GSH, where the rate-limiting enzyme is glytamyl cysteine ligase (also known as γ-glutamylcysteine synthetase), and by reduction of GSSG by GSH reductase. Glytamyl cysteine ligase is stimulated by oxidative stress and GSH depletion and inhibited by high concentrations of GSH(Reference Krzywanski, Dickinson and Iles43). Both glytamyl cysteine ligase and GSH reductase were induced by ischaemia–reperfusion of rat heart, which is known to be accompanied by increased oxidative stress(Reference Renner, Sagstetter and Gotz44).
The mechanism by which high dietary lipid increased total GSH and UQ concentration in the liver of Atlantic salmon in the present study is not known, but one could speculate on at least two mechanisms. The lipid in salmon feeds has a high level of n-3 PUFA; in the present study n-3 fatty acids contributed to 20 % of total dietary fatty acids(Reference Torstensen, Lie and Hamre25). Increasing the dietary level of this lipid source may have led to increased oxidative stress and a response in the fish to increase the oxidant defence, for example, by stimulated synthesis of GSH and UQ. Another possibility is that increased dietary fat would contribute with more reducing equivalents into the cell, rendering it in a more reduced state. However, the GSSG:GSH ratio was not altered by the dietary treatments, i.e. dietary lipid appears not to have altered the redox status of the cytosol of the liver cells. UQ is present in both cellular and organelle membranes, and most abundant in Golgi vesicles, lysosomes and mitochondria(Reference Dallner and Sindelar11). Dietary lipid altered the ratio between oxidised and reduced UQ, indicating that the redox potential of the membranes was altered. Another possible explanation for the higher UQ in the livers of the high lipid groups is that increasing dietary lipid and hence increased levels of EPA may lead to increasing amounts of hepatic mitochondria as has been shown by Vegusdal et al. (Reference Vegusdal, Gjøen and Berge45) to occur in Atlantic salmon hepatocytes stimulated with EPA.
The effects of vitamin E and astaxanthin on UQ concentration and redox state in the liver and plasma of Atlantic salmon were minor, and the gain of total UQ by high vitamin E in the liver was compensated by a loss of reduced UQ, and vice versa for astaxanthin. Vitamin E also increased total superoxide dismutase in the muscle and liver of Atlantic salmon, while astaxanthin reduced catalase activity in both tissues(Reference Lygren, Hamre and Waagbø23). The mechanisms of these impacts are not known at present.
The low dietary levels of vitamins and minerals were chosen to be just above the minimal requirement of Atlantic salmon, while the high levels were below anticipated toxic levels. The supplementation was therefore within what may be considered a safe window and this may be the reason that the minerals and vitamin C did not affect GSH and UQ homeostasis, which is probably tightly regulated. In the present study, the dietary variation had no effect on tissue concentrations of Cu. High compared with low Fe increased plasma and liver concentrations of Fe by approximately 40 %, with no further effects of the other nutrients. Mn concentrations were increased in the liver by 9–13 %, in plasma week 23 by 80 %, and in bone by 58–65 %, in groups with high compared with low dietary Mn. High dietary lipid also caused considerably increased Mn concentrations, while vitamin C had a negative effect on plasma Mn in week 23.
Tissue levels of the three trace elements studied are to a certain extent regulated at the intestinal level, at least when added in the inorganic form. This is especially true for Cu. The findings of the present study are in line with Berntssen et al. (Reference Berntssen, Lundebye and Maage46), where only minor tissue elevations of Cu were shown with high dietary levels of the element. This is because the element is strongly bound to intestinal tissue and excreted through apoptosis(Reference Berntssen, Hylland and Wendelar Bonga47). One would expect a minor increase in hepatic Fe with high dietary levels of inorganic Fe in accordance with Andersen et al. (Reference Andersen, Lorentzen and Waagbø48) and as was shown in the present study. A certain amount of Mn is absorbed and transported to the vertebra in line with findings from requirement studies(Reference Maage, El-Mowafi and Lygren49). Vitamin C concentrations in Atlantic salmon tissues increase in response to increasing dietary vitamin C(Reference Hamre, Berge and Waagbø6), as was the case in the present study. None of the other nutrients affected vitamin C status in plasma, liver and muscle of salmon. In view of the uptake kinetics and retention of vitamin C and the trace elements, one would expect that Cu would have no effect on GSH and UQ, while Fe, Mn and vitamin C may have been expected to have an effect due to their varying tissue concentrations and participation in the defence against oxidative stress. However, it is possible that the antioxidant enzymes superoxide dismutase and catalase were already saturated with Mn and Fe at the minimum dietary requirements. This is supported by the fact that the activities of superoxide dismutase in muscle and liver were not affected by Mn, while catalase activity was either not affected or reduced, as shown by Lygren et al. (Reference Lygren, Hamre and Waagbø4). Vitamin C is directly coupled to GSH in reactions regenerating oxidised antioxidants, such as vitamin E(Reference Mårtensson and Meister9), and it is therefore surprising that ascorbate had no effect on GSH status. Plasma and tissue concentrations of vitamin E(Reference Hamre, Berge and Waagbø6), astaxanthin(Reference Hamre, Christiansen and Waagbø22, Reference Bjerkeng, Hamre and Hatlen50) and lipid(Reference Hamre, Christiansen and Waagbø22, Reference Torstensen, Lie and Hamre25) increase with increasing dietary levels of these nutrients. In the present study, high dietary lipid also caused a decrease in α-tocopherol concentrations in plasma, muscle and liver. Therefore, the basis for the effects of lipid and lipid-soluble nutrients on GSH and UQ status was probably changes in plasma and tissue concentrations.
An interesting feature of the present experiment is that increases in the total concentrations of GSH and UQ and in the ratio of reduced to oxidised UQ in Atlantic salmon in response to high dietary lipid correlated with an increase in growth and condition factor and a decrease in the rate of feed consumption to body weight increase. A decrease in redox potential of tissues is known to occur in proliferating cells(Reference Hoffman, Spetner and Burke39, Reference Kemp, Go and Jones40), and cell proliferation may be correlated with increased energy supply and growth. Atlantic salmon has the ability to increase growth with increasing levels of dietary lipid to above 40 %(Reference Hillestad and Johnsen51), as long as the protein requirement is covered. This is in contrast to many other fish species such as Atlantic cod and Atlantic halibut which do not increase growth in response to high lipid levels(Reference Åsnes52, Reference Hamre, Bæverfjord and Harboe53).
In summary, vitamin C, vitamin E, astaxanthin, lipid, Fe, Cu and Mn were supplemented at high or low levels in the diet for Atlantic salmon in the present experiment, covering the safe window of supplementation for vitamins and minerals and the widow of supplementation in commercial aquaculture for astaxanthin and lipid. These variations in the diet had little impact on the total concentrations and redox status of GSH, where the only reproducible effect was that of high dietary lipid having a minor effect of liver total GSH concentration. The magnitude and diversification of dietary effects were greater on UQ, which responded to dietary lipid, astaxanthin and vitamin E, both with regard to total concentration and redox status. This shows that the endogenous redox regulation is quite stable and only to a limited extent responds to dietary manipulation of pro- and antioxidant nutrients. Dietary lipid and the lipid-soluble nutrients had the most prominent impacts and the lipid-soluble UQ was more sensitive to dietary changes than the water-soluble GSH.
Acknowledgements
The present study was funded by the Research Council of Norway (project no. 112317/120) and by NorAqua Innovation (now EWOS Innovation), Dirdal, Norway.
All authors except R. K. B. participated in the planning of the experiment; S. A. had the main responsibility for running the fish experiment; K. H., B. E. T., R. W. and S. A. participated in sampling. K. H., R. W. and A. M. were responsible for different parts of the analytical work. The preparation of the paper was headed by K. H. with inputs from the other authors.
There are no conflicts of interests connected to the present publication.






