Overweight and obesity is a significant public health issue. Body weight gain, especially in the abdominal region, is associated with the development of insulin resistance, a complex metabolic disorder, which precedes the development of type 2 diabetes and also represents an independent risk factor for CVD(Reference Lebovitz1).
Tea (Camellia sinensis), which is one of the most commonly consumed beverages in the world, has long been considered to possess health protective properties, particularly in relation to the prevention of CVD and cancer(Reference Kahn and Mukhtar2). Recent studies suggest that regular consumption of tea polyphenols may also contribute to the prevention of type 2 diabetes(Reference Kao, Chang, Lee and Chen3). Indeed, extracts of green tea ameliorate insulin resistance in high-fat-fed and high-fructose-fed rodents(Reference Murase, Nagasawa, Suzuki, Hase and Tokimitsu4, Reference Wu, Juan, Hwang, Hsu, Ho and Ho5) and increase insulin sensitivity in male Sprague–Dawley rats(Reference Wu, Juan, Ho, Hsu and Hwang6). In man, regular consumption of oolong tea has been demonstrated to reduce plasma glucose levels in diabetic patients(Reference Hosoda, Wang, Liao, Chuang, Iha, Clevidence and Yamamoto7) and consumption of green tea has been demonstrated to acutely improve oral glucose tolerance in healthy subjects(Reference Tsuneki, Ishizuka, Terasawa, Wu, Sasaoka and Kimura8).
Epigallocatechin-3-gallate (EGCG), which is the major catechin found in green tea, has been implicated as an important bioactive molecule that may contribute to many of the health properties of tea, including effects on glucose regulation. For example, in cultured adipocytes extracts of green tea act as insulin-mimetics(Reference Broadhurst, Polansky and Anderson9), with EGCG being identified as the component responsible for the effect(Reference Anderson and Polansky10). In rodent models, dietary supplementation with EGCG has been demonstrated to improve glucose tolerance and insulin sensitivity(Reference Potenza, Marasciulo, Tarquinio, Tiravanti, Colantuono, Federici, Kim, Quon and Montagnani11, Reference Wolfram, Raederstorff, Preller, Wang, Teixeira, Riegger and Weber12). EGCG is also known to inhibit the activation of I-kappa kinase(Reference Chen, Wheeler, Malhotra, Odoms, Denenberg and Wong13–Reference Yang, Oz, Barve, De Villiers, McClain and Varilek15), a serine/threonine kinase implicated in the pathogenesis of insulin resistance(Reference Shoelson, Lee and Yuan16, Reference Tamura, Ogihara and Uchida17).
Accumulating cell and animal data suggest that regular consumption of EGCG may limit the development of insulin resistance and subsequent progression to type 2 diabetes during conditions of energy excess. However, these health benefits have yet to be substantiated in man. The purpose of the current study was to determine whether chronic dietary supplementation with EGCG would improve insulin resistance and associated metabolic risk factors (BMI, waist circumference, percentage body fat, blood pressure, total cholesterol, LDL-cholesterol, HDL-cholesterol, TAG) in non-diabetic, overweight and obese men. A secondary objective was to investigate the effect of EGCG consumption on self-reported mood, as tea is purported to have relaxation properties and EGCG per se has been shown to possess anxiolytic activity in mice(Reference Adachi, Tomonaga, Tachibana, Denbow and Furuse18, Reference Vignes, Maurice, Lante, Nedjar, Thethi, Guiramand and Recasens19).
Subjects and methods
Design and subjects
The study was conducted between July and December 2005 at Unilever's research facility located in Bedfordshire, UK. The protocol was approved by the Colworth Science Park ethical committee and the study was conducted in accordance with the Declaration of Helsinki. Male non-smokers, aged 40–65 years, with BMI>28 and < 38 kg/m2, fasting plasma glucose values below the diagnostic threshold for diabetes mellitus (fasting < 7·0 mmol/l) and with no significant history of disease, current disease or on medication were recruited on to the study (Fig. 1). Subjects were drawn from an existing database reflective of the local population or recruited through an agency. All subjects provided written informed consent.
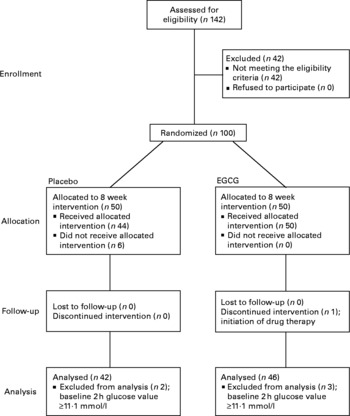
Fig. 1 Flow of participants through the phases of the study. EGCG, epigallocatechin-3-gallate.
This was a double-blind, randomized and parallel design study with groups matched for insulin resistance (homeostasis model assessment of insulin resistance, HOMAIR) and age. The study population was randomly assigned to receive active (800 mg EGCG/d) or placebo (800 mg lactose/d) dietary supplements which were consumed twice daily with food, one 400 mg capsule in the morning and another in the evening, for a period of 8 weeks. Randomization was performed by a study-independent statistician. Subjects with the highest levels of insulin resistance (HOMAIR scores) were selected from those recruited and divided into similar pairs using a statistical clustering algorithm based upon their HOMAIR score and age. Within each pair, subjects were randomly assigned to either placebo (one of five codes) or EGCG (one of five codes) treatment codes. Subjects received their supplement packs from the study staff. To conceal treatment allocation from the study staff, supplement packs were prepared and labelled by a third-party manufacturer (DHP, Wales, UK) in accordance with the randomization list.
Subjects visited the study site, where measurements were performed, at week 0 (visit 1) and after 8 weeks of dietary supplementation (visit 2), with the last EGCG capsule being consumed the evening before visit 2. Urine samples (first void of the day) and information on diet and physical activity levels were collected for 7 consecutive days before each study visit. Strenuous exercise and alcohol consumption were restricted for the 24 h before each study visit and subjects were prohibited from eating and drinking, except for water, from 21.00 hours on the day before the visit until the various measurements were completed. During the intervention phase consumption of green tea and the taking of dietary supplements or medications known to affect glucose and lipid metabolism were prohibited, but the intake of other flavonoid-containing food products was not limited. Subjects completed health and mood questionnaires on the evening before visit 1 and once a week during the intervention period.
Dietary supplements
A commercial green tea extract (TEAVIGO™; DSM), comprising >97 % pure EGCG, was used in the study. Size 0 vegetarian gelatin capsules containing 400 mg TEAVIGO™ or 400 mg lactose were prepared, by DHP, according to Good Manufacturing Practice. Capsules were weighed and sorted within ± 5 % weight range and placed within blister packs (fourteen capsules per pack). Four blister strips were placed into a labelled carton and each subject received two cartons in their intervention kit.
Anthropometric measurements
Height (cm), weight (kg), waist circumference (cm), percentage body fat and blood pressure (mmHg) were recorded. Waist circumference was measured at the site of the smallest circumference between the rib cage and the iliac crest, with the subjects in a standing position. Percentage body fat was measured by bioelectrical impedance using a handheld device (OMRON BF 302). Blood pressure was measured manually on the upper arm using a sphygmomanometer (UA-787, A and D). Three measurements were taken, at 5 min intervals, whilst participants rested in a semi-recumbent position and with participants rested for at least 5 min before the first measurement. The first measurement was discarded. The second and third measurements were used to derive mean blood pressure values. Non-smoking status and alcohol abstinence were verified using Micro CO meter (Micro Medical Ltd) and AlcoMate Pro (AK Solutions) monitors, respectively. All equipment was calibrated before use.
Blood sampling, oral glucose tolerance test and biochemical analysis
Prior to ingestion of a glucose load, blood samples were taken at − 10, − 5 and 0 min for analysis of fasted insulin levels and at − 10 and 0 min for analysis of fasted glucose levels. Blood samples for other measures were taken as convenient during the − 10 to 0 min period. All blood samples were stored at − 20°C before analysis.
Glucose (75 g), containing 125 mg U-13C-uniformly labelled glucose, was administered as Lucozade (Glaxo SmithKline) in a volume of 394 ml. Blood samples were collected at 30, 60, 90 and 120 min for glucose and insulin analysis.
Glucose, cholesterol, TAG, LDL-cholesterol, HDL-cholesterol and HbA1c analysis was performed using an ABX Pentra 400 and assay systems supplied by Horiba ABX. Insulin analysis was performed by time-resolved fluorescence immunoassay (product code B080-101; Perkin Elmer, MA, USA) on an AutoDelfia (Perkin Elmer) automated analyser. All assays were performed in accordance with the manufacturer's instructions.
Steady-state measures of insulin sensitivity (HOMAIR) and insulin secretion (HOMA%B) were derived from mean fasting plasma glucose and mean fasting plasma insulin values as described by Matthews et al. (Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner20). Non-steady-state measures of insulin sensitivity (OGTTISI) and insulin secretion (OGTTInsulinogenic index) were derived from the oral glucose tolerance test (OGTT) according to Matsuda & DeFronzo(Reference Matsuda and DeFronzo21) and Phillips et al. (Reference Phillips, Clark, Hales and Osmond22), respectively. The total area under the curve for plasma glucose was calculated using the trapezoid method where the interpolation between two adjacent observed points is a straight line. The area under the curve was only calculated if plasma glucose values were available at all time-points.
Measurement of physical activity and diet
Physical activity was measured by an accelerometer (ActiWatch™; Cambridge Neurotechnology Ltd) worn on the non-dominant arm at the wrist and was defined as the average activity counts per epoch for the wake period (average wake movement). Data were recorded at 1 min epochs and processed using the software (version 5.32) supplied by the manufacturer.
Dietary information was collected in 7 d diaries, coded using DIDO (Diet In Data Out)(Reference Price, Paul, Key, Harter, Cole, Day and Wadsworth23) and analysed using a program based on McCance and Widdowson's The Composition of Foods and supplements(24).
Health and mood questionnaires
Mood was assessed using the University of Wales Institute of Science and Technology (UWIST) mood adjective checklist which measures dimensions of energetic arousal, tense arousal, hedonic tone and general arousal(Reference Matthews, Jones and Chamberlain25). General health was evaluated using a modified version of the gastrointestinal symptom checklist developed by Svedlund et al. (Reference Svedlund, Sjodin and Dotevall26). The questionnaire was reworded by study staff using simple non-technical terms and additional questions were added to evaluate the frequency of occurrence of common physical symptoms and sensations.
Statistical analysis
The primary end-points (insulin sensitivity, insulin secretion and glucose tolerance measures derived from the OGTT) were analysed by mixed model analysis of covariance according to a pre-established analysis plan. The variables analysed were change from baseline, with baseline included in the model as a covariate. Subject was included as a random effect and the treatment group as a fixed effect. To control for potential confounders further analysis was performed, in which a number of additional covariates (alcohol consumption (g alcohol), diet (g carbohydrate, g fat, g protein, total estimated energy in kJ), physical activity (average wake movement, counts/min) and body weight change) were included and retained in the model if significant at the 10 % level. Additional end-points were analysed using a similar approach. For UWIST score data week number was included as an extra repeated measures term. Data are represented as mean and standard deviation unless stated otherwise. Statistical analysis was performed with SAS statistical software version 9.1.3 (SAS Institute Inc., Cary, NC, USA). Significance was set at P ≤ 0·05 (two-sided).
Results
Data were analysed on a per-protocol basis, with subject exclusion occurring before release of the double-blind procedure. There were 100 subjects entered on to the study. Six subjects did not receive their allocated intervention as they failed to attend study visit 1 because of work commitments or due to moving out of the area. Five subjects with suspected diabetes mellitus (2 h plasma glucose values ≥ 11·1 mmol/l) at baseline were excluded, as HOMAIR has been reported to provide a poor estimate of insulin resistance in diabetic patients(Reference Laakso27) and a further subject was discontinued from the intervention because of initiation of drug therapy. Consequently, the data reported here are for eighty-eight subjects (Fig. 1).
To monitor compliance subjects were required to return all packaging and unused capsules. Each subject received 112 capsules and on average 3·2 (sd 4·5) capsules per subject (placebo, 4·0 (sd 5·8); EGCG, 2·4 (sd 2·7)) were returned, indicating that >95 % of doses were taken. No significant differences between the EGCG or placebo groups were detected for any of the assessed gastrointestinal and general health parameters (data not shown).
Baseline characteristics for the placebo and EGCG groups are shown in Table 1. Except for HbA1c, which was higher in the EGCG group (P = 0·043) and fat intake, which was higher in the placebo group (P = 0·040), no significant differences between any of the group means were detected at baseline (Table 1). It was found that 57·1 % of the placebo group and 58·7 % of the EGCG group were classified as obese (BMI ≥ 30). A subset of subjects also manifested with glucose intolerance: 19·5 and 20·0 % with impaired fasting glucose; 7·3 and 6·7 % with impaired glucose tolerance; 4·9 and 8·9 % with combined impairments (impaired fasting glucose and impaired glucose tolerance), for placebo and EGCG groups, respectively, categories being defined according to recent American Diabetes Association guidelines(Reference Genuth, Alberti and Bennett28). The presence of obesity and glucose intolerance did not differ between the groups.
Table 1 Baseline characteristics of the study population
(Mean values and standard deviations)
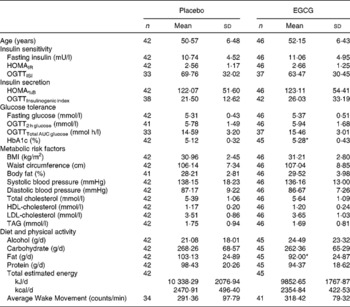
AUC, area under the curve; EGCG, epigallocatechin-3-gallate; HOMAIR, homeostasis model assessment of insulin resistance; HOMA%B, homeostasis model assessment of pancreatic β-cell function; OGTT, oral glucose tolerance test.
Mean values were significantly different from those of the placebo: *P ≤ 0·05.
To evaluate the effect of dietary intervention, change from baseline at week 8 was compared between the placebo and EGCG groups for each parameter. No significant differences were detected between the two groups for any of the insulin sensitivity, insulin secretion or glucose tolerance measures (Table 2). However, EGCG treatment was found to reduce diastolic blood pressure (P = 0·014) (Table 2; Fig. 2). Although not significant, a trend towards a reduction in systolic blood pressure (P = 0·096) was also noted. Importantly, the blood pressure values for the two groups did not differ significantly at baseline (Table 1). The EGCG intervention had no significant effect on any of the other metabolic risk factors measured (Table 2). Over the course of the intervention no significant change in dietary intake measures (g alcohol/carbohydrate/fat/protein or total estimated energy in kJ) was detected (data not shown).
Table 2 Effect of dietary supplementation on metabolic variables and risk factors, data shown as change from baseline at week 8†
(Mean values with their standard errors)
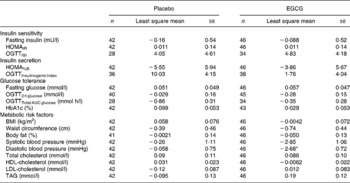
EGCG, epigallocatechin-3-gallate; HOMAIR, homeostasis model assessment of insulin resistance; HOMA%B, homeostasis model assessment of pancreatic β-cell function; OGTT, oral glucose tolerance test.
Value was significantly different from that of the placebo: *P ≤ 0·05 (mixed model analysis of covariance, baseline as a covariate, subject as a random effect and treatment group as a fixed effect).
† For details of subjects and procedures, see Table 1 and Subjects and methods section.
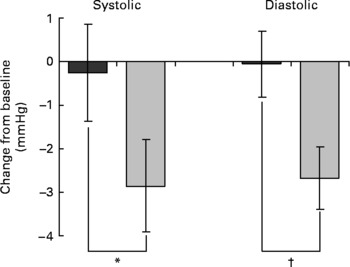
Fig. 2 Mean blood pressure change after 8 weeks of dietary supplementation (![]() , placebo, n 42;
, placebo, n 42; ![]() , EGCG, n 46). Mixed model analysis of covariance was used with baseline included as a covariate, subject as a random effect and treatment group as a fixed effect. Values are least square means with their standard errors depicted by vertical bars. Values were significantly different: *P = 0·096, †P = 0·014.
, EGCG, n 46). Mixed model analysis of covariance was used with baseline included as a covariate, subject as a random effect and treatment group as a fixed effect. Values are least square means with their standard errors depicted by vertical bars. Values were significantly different: *P = 0·096, †P = 0·014.
The EGCG intervention also had an effect on mood, as measured by the UWIST mood adjective checklist (Fig. 3). Across the 8-week period, the EGCG group scored higher on hedonic tone than the placebo group (29·11 (se 0·44) v. 27·84 (se 0·46), P = 0·048). Although not quite reaching significance the EGCG group also scored lower on tense arousal compared to the placebo group (11·06 (se 0·47) v. 12·33 (se 0·45), P = 0·056). Importantly, the baseline scores for both components (tense arousal, placebo 11·83 (sd 3·99), EGCG 11·29 (sd 3·06); hedonic tone, placebo 29·25 (sd 3·72), EGCG 29·10 (sd 3·68)) were virtually the same for the EGCG and placebo groups. Results for energetic arousal and general arousal did not differ significantly between the two groups.
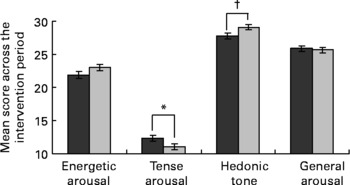
Fig. 3 Effect of dietary supplementation on mood, as measured by the University of Wales Institute of Science and Technology (UWIST) mood adjective checklist (![]() , placebo, n 42;
, placebo, n 42; ![]() , EGCG, n 46). UWIST data were analysed by mixed model repeated measures analysis of covariance, with baseline included as a covariate, subject as a random effect, treatment group as a fixed effect and week number as a repeated measures term. Values are least square means with their standard errors depicted by vertical bars. No baseline differences (P ≤ 0·05) were detected between the two groups. Values were significantly different: *P = 0·056, †P = 0·048.
, EGCG, n 46). UWIST data were analysed by mixed model repeated measures analysis of covariance, with baseline included as a covariate, subject as a random effect, treatment group as a fixed effect and week number as a repeated measures term. Values are least square means with their standard errors depicted by vertical bars. No baseline differences (P ≤ 0·05) were detected between the two groups. Values were significantly different: *P = 0·056, †P = 0·048.
To account for potential confounders and to test the robustness of the results we conducted multivariate analysis. Except for the OGTTInsulinogenic index measure of insulin secretion, inclusion in the model of additional covariates [alcohol consumption (g alcohol), diet (g carbohydrate, g fat, g protein, total estimated energy in kJ), physical activity (average wake movement, counts/min) and body weight change] did not substantially change the findings (data not shown). A significant difference (P = 0·048) between the placebo and EGCG groups was detected for OGTTInsulinogenic index (mean change: placebo 11·22 (se 4·02), EGCG − 0·25 (se 3·97)). However, after removal from the analysis of one subject in the placebo group, displaying an extreme OGTTInsulinogenic index value post-intervention but not at baseline, the difference was no longer significant.
Discussion
The effect of EGCG, on insulin resistance and associated metabolic risk factors, was assessed in a population of middle-aged, overweight and obese men. Subjects consumed 400 mg EGCG twice daily, which is comparable to drinking ten cups per day of moderate-strength green tea prepared from leaves with a relatively high EGCG content(Reference Khokhar and Magnusdottir29). At the end of the 8-week treatment period no change in insulin sensitivity, insulin secretion, glucose tolerance, BMI, waist circumference, percentage body fat, total cholesterol, LDL-cholesterol, HDL-cholesterol, TAG or systolic blood pressure was detected. However, diastolic blood pressure was found to be reduced and mood improved.
Dietary supplementation with green tea or EGCG is known to improve insulin sensitivity and oral glucose tolerance in rodents(Reference Murase, Nagasawa, Suzuki, Hase and Tokimitsu4–Reference Wu, Juan, Ho, Hsu and Hwang6, Reference Potenza, Marasciulo, Tarquinio, Tiravanti, Colantuono, Federici, Kim, Quon and Montagnani11, Reference Wolfram, Raederstorff, Preller, Wang, Teixeira, Riegger and Weber12). However, a similar effect was not observed in this human study. A number of other human intervention studies examining the effect of green tea on insulin resistance have recently been reported. In these studies a daily intake of 500 mg green tea catechins for 2 months(Reference Fukino, Shimbo, Aoki, Okubo and Iso30, Reference Fukino, Ikeda, Maruyama, Aoki, Okubo and Iso31), 540 mg EGCG for 3 months(Reference Chan, Koo, Ng, Tang, Yeung and Ho32) and 9 g green tea for 4 weeks(Reference Ryu, Lee, Lee, Kim, Seo, Kim, Kim, Baik, Choi and Choi33) was investigated. Similar to the present findings, consumption of green tea had no effect on insulin resistance, as determined by measures of HOMAIR or fasted serum insulin(Reference Fukino, Shimbo, Aoki, Okubo and Iso30–Reference Ryu, Lee, Lee, Kim, Seo, Kim, Kim, Baik, Choi and Choi33); although, Fukino et al. (Reference Fukino, Ikeda, Maruyama, Aoki, Okubo and Iso31)did observe a small but significant reduction in HbA1c. The discrepancy in findings between the human and animal studies may reflect species-specific differences. Alternatively, it may result from the lower dose of EGCG/green tea employed here and in the other human studies compared to the dose tested in rodents. Indeed, the oral bioavailability of EGCG is relatively low in man. Based on the results of pharmacokinetic studies conducted by ourselves (unpublished data) and reported by others(Reference Feng34), we would anticipate that a peak plasma concentration of EGCG in the region of 1 μm would result from the dose of EGCG we administered. In contrast, in vitro studies suggest that much higher (25–100 μm) concentrations of EGCG may be required to mimic the metabolic actions of insulin(Reference Waltner-Law, Wang, Law, Hall, Nawano and Granner35).
Even though no effect on insulin resistance was observed, diet supplementation with EGCG was found to reduce diastolic blood pressure by approximately 2·5 mmHg. Although a modest reduction, this is clinically significant at the population level. Indeed, a reduction in diastolic blood pressure of 2 mmHg has been calculated to reduce hypertension by 17 % and reduce the risk of stroke and CHD by 15 and 6 %, respectively(Reference Cook, Cohen, Hebert, Taylor and Hennekens36). Consistent with the effect of EGCG on diastolic blood pressure observed here, habitual consumption of green tea has been associated with a reduced risk of hypertension in a Chinese population(Reference Yang, Lu, Wu, Wu and Chang37). Also, dietary supplementation with green tea, and more recently EGCG, has been shown to prevent blood pressure increases in rodents(Reference Potenza, Marasciulo, Tarquinio, Tiravanti, Colantuono, Federici, Kim, Quon and Montagnani11, Reference Henry and Stephenslarson38, Reference Negishi, Xu, Ikeda, Njelekela, Nara and Yamori39).
Although not investigated in the present study, the mechanism by which EGCG influences blood pressure may be linked to an impact on the endothelial cell and production of vasoconstrictors and vasodilators which subsequently impact on smooth muscle cell function and vascular tone. Indeed, EGCG induces dose-dependent vasodilation in pre-contracted rat aortic rings(Reference Lorenz, Wessler, Follmann, Michaelis, Dusterhoft, Baumann, Stangl and Stangl40) and in mesenteric vascular beds isolated from spontaneously hypertensive rats(Reference Potenza, Marasciulo, Tarquinio, Tiravanti, Colantuono, Federici, Kim, Quon and Montagnani11), effects being elicited at concentrations as low as 1 μm, albeit weakly. In cultured endothelial cells EGCG has been demonstrated to activate endothelial nitric oxide synthase and stimulate production of NO, which is a potent vasodilator(Reference Lorenz, Wessler, Follmann, Michaelis, Dusterhoft, Baumann, Stangl and Stangl40, Reference Persson, Josefsson, Persson and Andersson41). The acute actions of EGCG to stimulate endothelial production of NO are indicated to be via the phosphotidylinositol-3-kinase/Akt pathway(Reference Lorenz, Wessler, Follmann, Michaelis, Dusterhoft, Baumann, Stangl and Stangl40, Reference Kim, Formoso, Li, Potenza, Marasciulo, Montagnani and Quon42) and may, in part, be the result of the inhibitory effect of EGCG on I-kappa kinase activity(Reference Chen, Wheeler, Malhotra, Odoms, Denenberg and Wong13, Reference Kim, Tysseling, Rice, Gallis, Haji, Giachelli, Raines, Corson and Schwartz43–Reference Pan, Lin-Shiau, Ho, Lin and Lin45). EGCG may also influence NO production by modulating other signalling pathways(Reference Tang, Hu, Chen, Deng and Li46). In man, improvements in brachial artery flow-mediated dilation have been observed in coronary artery disease patients 2 h after EGCG administration(Reference Widlansky, Hamburg, Anter, Holbrook, Kahn, Elliott, Keaney and Vita47). Additionally, in male smokers consumption of green tea has been found to promote endothelial-dependent vasorelaxation(Reference Kim, Jeong, Hong, Ahn, Kim, Cho and Kang48, Reference Nagaya, Yamamoto, Uematsu, Itoh, Nakagawa, Miyazawa, Kangawa and Miyatake49). Other flavonoids have similarly been shown to influence vascular function in man(Reference Heiss, Schroeter, Balzer, Kleinbongard, Matern, Sies and Kelm50).
A further observation from the present study was that dietary supplementation with EGCG had a beneficial effect on mood. Over the intervention period, subjects in the EGCG group reported feeling in a more positive mood compared to the placebo group and a tendency to feel less tense. This was in the absence of any baseline differences between the two groups. The present results suggest that EGCG treatment may have impacted central nervous system function. In mice, EGCG has been shown to be distributed in the brain after oral administration(Reference Suganuma, Okabe, Oniyama, Tada, Ito and Fujiki51). In addition, flavonoids have affinities for the benzodiazepine binding sites of γ-aminobutyric acid receptors (GABAA)(Reference Paladini, Marder, Viola, Wolfman, Wasowski and Medina52). γ-Aminobutyric acid is the principal inhibitory system in the brain mediating neurotransmitter receptor systems that regulate anxiety. In mice, both intracerebroventricular and oral administration of EGCG produce behavioural effects reflecting anxiolytic activity(Reference Adachi, Tomonaga, Tachibana, Denbow and Furuse18, Reference Vignes, Maurice, Lante, Nedjar, Thethi, Guiramand and Recasens19). The present study suggests that EGCG may have similar anxiolytic and sedative effects in man. Indeed, in man regular tea consumption results in lower post-stress cortisol levels and greater subjective relaxation, an effect that could be partially mediated through the effect of flavonoids on GABAA receptors(Reference Steptoe, Gibson, Vounonvirta, Williams, Hamer, Rycroft, Erusalimsky and Wardle53).
The present study has a number of limitations that do need to be acknowledged. The sample size was relatively small and a change in metabolic variables in response to the EGCG intervention may not have been detected if the effect size were small. Although overweight or obese and displaying a marked degree of insulin resistance, our study population was largely normoglycaemic. It is possible that significant glucose dysregulation may be required before the metabolic effects of regular EGCG consumption manifest in man and further studies are needed to explore this possibility. However, it is worth noting that in a study conducted in type 2 diabetic patients daily consumption of 900 ml water containing 9 g green tea did not influence insulin resistance, inflammation or adiponectin levels(Reference Chan, Koo, Ng, Tang, Yeung and Ho32). Furthermore, increases in insulin sensitivity have been demonstrated in both healthy persons and hypertensive subjects without diabetes or impaired glucose tolerance after short-term administration of dark chocolate(Reference Grassi, Lippi, Necozione, Desideri and Ferri54, Reference Grassi, Necozione, Lippi, Croce, Valeri, Pasqualetti, Desideri, Blumberg and Ferri55). With the exception of green tea, which is the primary dietary source of EGCG, the consumption of flavonoid-containing foods was not restricted during the study. Also, because of the nature of the study population the extrapolation of the present findings may be somewhat limited. Finally, it should be recognized that a relatively large number of comparisons have been performed in the present study which does increase the probability that the significant effects we report may have occurred by chance. Additional studies are therefore required to substantiate our findings.
In conclusion, diet supplementation with EGCG had no significant effect on insulin resistance or other associated metabolic risk factors, in a sample of overweight and obese men, but did reduce diastolic blood pressure. This antihypertensive effect may contribute to some of the cardiovascular benefits associated with habitual green tea consumption. Additionally, subjects in the EGCG group reported an improvement in mood compared to the placebo group, indicating that EGCG (or its metabolites) may also have anxiolytic effects in man. Further studies are now needed to confirm the present findings and to explore their mechanistic basis.
Acknowledgements
We acknowledge Ms Anna Gent and Ms Louise McKenna for technical support and Dr Oscar Franco for helpful suggestions during preparation of the manuscript. A. L. B. was responsible for the concept, data interpretation and preparation of the manuscript. J. L. and J. C. were responsible for conducting the study and for data collection and management. J. S. was responsible for data analysis. H. H. was involved in data interpretation. All authors contributed to the study design. The manuscript was critically reviewed by A. S., H. H., J. C., J. L., J. S., L. B. and S. J. The authors A. L. B., H. H., J. C., J. L. and J. S. are all employed by Unilever Corporate Research, which is a division of Unilever Plc, a company which has a significant commercial interest in tea. The authors S. J., L. B., A. S. and A. C. have no conflict of interest in relation to this study.







