The metabolic syndrome (MS) is characterised by the clustering of multiple metabolic disorders, including visceral obesity, hyperglycaemia, dyslipidaemia and hypertension(Reference Alberti1). Insulin resistance(Reference Reaven2, Reference Eckel, Grundy and Zimmet3) is generally accepted as its critical mechanism, which increases the risk of CVD and type 2 diabetes(Reference Isomaa, Almgren and Tuomi4, Reference Wilson, D'Agostino and Parise5). In the past two decades, with the changes in environment and lifestyles (including eating behaviours), the prevalence of the MS is rapidly increasing not only in developed countries(Reference Ford, Giles and Dietz6) but also in developing countries(Reference Tillin, Forouhi and Johnston7). Furthermore, the increasing prevalence of the MS among young people has also been reported recently(Reference Weiss, Dziura and Burgert8). The MS has become one of the most serious threats to public health worldwide. Although many strategies such as weight reduction, increased physical activity, balanced diet and drugs have been applied in MS management, more effective and feasible therapeutic measures are urgently required.
The human gut contains a large number of micro-organisms that are closely associated with nutrient absorption, vitamin production, metabolism and host immunity(Reference Bäckhed, Ley and Sonnenburg9). Newly emerging evidence has suggested that the increasing prevalence of the MS cannot be solely attributed to changes in the human genome or to too much energy intake or too little physical activity. Studies in rodents and human subjects have indicated that disorders of the host gut microbiota also play an important role in the development of these diseases(Reference Cani and Delzenne10–Reference Ley, Bäckhed and Turnbaugh13) and that the underlying pathways include improvement in the ability to extract energy from the diet(Reference Turnbaugh, Ley and Mahowald14, Reference Bäckhed, Ding and Wang15), synthesis of gut peptides involved in energy homeostasis, such as glucagon-like peptide-1 and peptide YY, and increase in circulating lipopolysaccharides related to the development of insulin resistance. Therefore, normalising the structure and function of the intestinal microbiota may be an ideal way to prevent and control the MS.
Probiotics have been defined by the FAO of the United Nations and the WHO (FAO/WHO) as live micro-organisms that confer health benefits on the host when administered in adequate amounts. Commonly used probiotics include the genera Lactobacillus, Bifidobacterium and Saccharomyces. The ability of live probiotics to reach and colonise host intestines after oral administration, even temporally, is generally considered to be one of the key criteria in conferring their health-promoting effects such as improving the intestinal microbiota and enhancing the natural defences of the host against various infections(Reference Salminen, Ouwehand and Benno16, Reference Goldin and Gorbach17). Recently, several safety issues regarding the use of probiotics came to light(Reference Ishibashi and Yamazaki18), e.g. translocation during gut barrier dysfunction, excessive immune stimulation and microbial resistance. Emerging evidence from various cell lines and animal studies has revealed that inactive probiotic cells (including cell components and metabolites) may also confer apparent health benefits on hosts(Reference Ouwehand and Salminen19, Reference He, Morita and Kubota20) and may be safer than their counterparts. In addition, inactive probiotic cells may be more advantageous than living cells for industrial production and consumer usage, e.g. in terms of easier and more convenient handling and distribution as well as use in different food product lines in addition to fermented milk and yoghurt, which are two of the most important probiotic foods. Therefore, more attention should be devoted to recognising the health-promoting effects of inactivated probiotics on hosts.
The present study was conducted to test the potential health-promoting effects of heat-inactivated Lactobacillus gasseri TMC0356 (TMC0356) on MS model rats and the possible underlying mechanisms.
Materials and methods
Animals and treatments
A total of sixty 7-week-old male Sprague–Dawley rats (160 (sem 20) g) were purchased from the Academy of Medical Science (certificate no: scxk (2008-19)). The animal facility was well ventilated and maintained at an ambient temperature of 23 ± 1°C, with 50–60 % relative humidity and 12 h light and dark cycles. The rats had free access to food and distilled water. After 7 d of adaptive feeding, the rats were randomly assigned to five groups according to their body weight (BW), with each group comprising twelve rats housed in groups of four per cage.
The five groups and their treatments were as follows: a control group fed a conventional diet and infused with distilled water; an MS model group fed a high-fat and high-salt (HFS) diet and infused with distilled water; low-, medium- and high-dose groups fed an HFS diet and infused with a water solution of TMC0356 at 41·8, 83·5 and 167·0 mg/kg BW per d, respectively. The heat-inactivated cell preparation of TMC0356 was provided by Takanashi Milk Products Company Limited. The components of the HFS diet provided by the Animal Centre of Sichuan University were 15 % lard oil, 15 % sucrose, 10 % yolk powder, 2 % cholesterol, 3 % salt and 55 % conventional diet. The macronutrients and percentages of energy in the conventional and HFS diets are presented in Table 1.
Table 1 Comparison of the conventional and high-fat and high-salt (HFS) diets (100 g)

Measurements and analysis
During the experimental period, the daily food intake was recorded, and the BW was measured weekly. Body length and blood pressure (BP) were measured, and 1-ml fasting blood samples were collected at 0, 5, 10 and 15 weeks from the caudal vein for the analysis of fasting blood glucose (FBG) and serum lipid levels. After 15 weeks, the rats were anaesthetised with diethyl ether, 5-ml fasting blood samples were collected from the femoral vessels and the organs were excised aseptically for the analysis of various parameters. All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of Sichuan University.
Experimental parameters
Food intake
Food and water were provided once a day, diet surpluses were recorded every weekend and the average daily food and energy intake in each group was calculated weekly.
Body weight and length
After overnight fasting for 14 h, BW were recorded weekly using an electronic scale, measured three times per rat and the mean values were then computed. After anaesthesia with diethyl ether, body lengths were measured using a flexible rule (from nose to anus). Lee's index was used to reflect the degree of obesity in the rats. Lee's index = ((BW)− 3/body length) × 103.
Biochemical indices
Sera were isolated from the fasting blood of the rats and stored at − 20°C until analysis. FBG, TAG, total cholesterol (TC), LDL-cholesterol, HDL-cholesterol and VLDL-cholesterol levels were measured using an automatic biochemical analyser (OLYMPUS AU400; Olympus). Fasting insulin was measured using commercial ELISA kits (R&D) and the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated (HOMA-IR = FBG × fasting insulin/22·5). Immune cells (neutrophils and lymphocytes) were counted using an automatic blood cell analyser (CA620; Boule Medical AB), and the Hb level was determined at the 15th week. A section (0·5 g) of the liver was homogenised with 4·5 ml of 1:1 chloroform–methanol, cooled at 4°C for 48 h, and then centrifuged for 15 min at 12 000 rpm. The supernatant was extracted to determine TC and TAG levels in the liver using commercial kits (Jiancheng).
Blood pressure
Systolic BP and diastolic BP of the caudal artery were measured using a non-invasive automatic sphygmomanometer (BP-98A, Softron). All examinations were performed after the rats were stable for 15–20 min.
Visceral coefficients
The liver, visceral fat (perinephric and testicular fat), thymus and spleen were washed with normal saline, wiped with filter paper and weighed. The liver:BW ratio, visceral fat:BW ratio, thymus index and spleen index were calculated.
Cytokines and antibodies
Fasting serum IgG, secretory IgA, C-reactive protein, TNF-α, IL-6 and adiponectin levels were measured using commercial ELISA kits (R&D) at the 15th week.
Data and statistical analysis
All data analyses were performed using SPSS 17.0 (SPSS Corporation), and the graphs were prepared using Origin 8.0 (Origin Lab Corporation). The results were expressed as means and standard errors. The statistical differences of indices (including Lee's index, FGB, TAG, TC, LDL, VLDL and BP) among the five groups, which were determined three to four times (at 0, 5, 10 and 15 weeks) during the study, were analysed using repeated-measures ANOVA. Other indices that were measured only once were analysed by one-way ANOVA, and the least significant difference test was used to further compare the differences of these indices between the MS model and control groups and between the MS model and TMC0356 experimental groups. P< 0·05 was considered statistically significant.
Results
Food and energy intake
As indicated in Fig. 1, the average food and energy intake in all groups increased progressively before the 9th week, reduced significantly at the 11th week and thereafter continued to rise until the end of the study. The average daily food intake in the control group was significantly higher than that in the MS model group (23·3 (sem 0·5) v. 18·3 (sem 0·5) g, P< 0·001) and in the three TMC0356 groups. In contrast, the average daily energy intake in the control group was markedly lower than that in the MS model group (332·8 (sem 7·2) v. 356·4 (sem 9·5) kJ, P< 0·05). In addition, in the three TMC0356 groups (low-, medium- and high-dose groups), average daily food intakes (16·9 (sem 0·3), 16·9 (sem 0·3) and 16·7 (sem 0·4) g, respectively) and energy intakes (328·6 (sem 6·2), 327·9 (sem 5·9) and 323·9 (sem 7·3) kJ, respectively) significantly decreased compared with those in the MS model group. Thus, it appeared that TMC0356 could decrease the food and energy intake in MS rats.

Fig. 1 Food intake was recorded daily, and the average daily food intake (weekly) was calculated. The average daily energy intake was computed weekly according to the energy density for each diet shown in Table 1. ![]() , Control;
, Control; ![]() , metabolic syndrome model;
, metabolic syndrome model; ![]() , low;
, low; ![]() , medium;
, medium; ![]() , high.
, high.
Effects of Lactobacillus gasseri TMC0356 on metabolic characteristics of metabolic syndrome rats
There was no significant difference in any of the parameters among the five groups at the beginning of the study.
Obesity
As shown in Fig. 2(a), BW in the MS model group was significantly higher (P< 0·01) since the 4th week compared with that in the control group. At the 15th week, the average BW reached 485·8 (sem 61·1) g in the MS model group and 404·6 (sem 25·9) g in the control group. The average BW of rats in the low- and medium-dose groups were significantly lower than that in the MS model group (P< 0·05) since the 11th week and the difference in their final BW was 50 and 51 g, respectively.
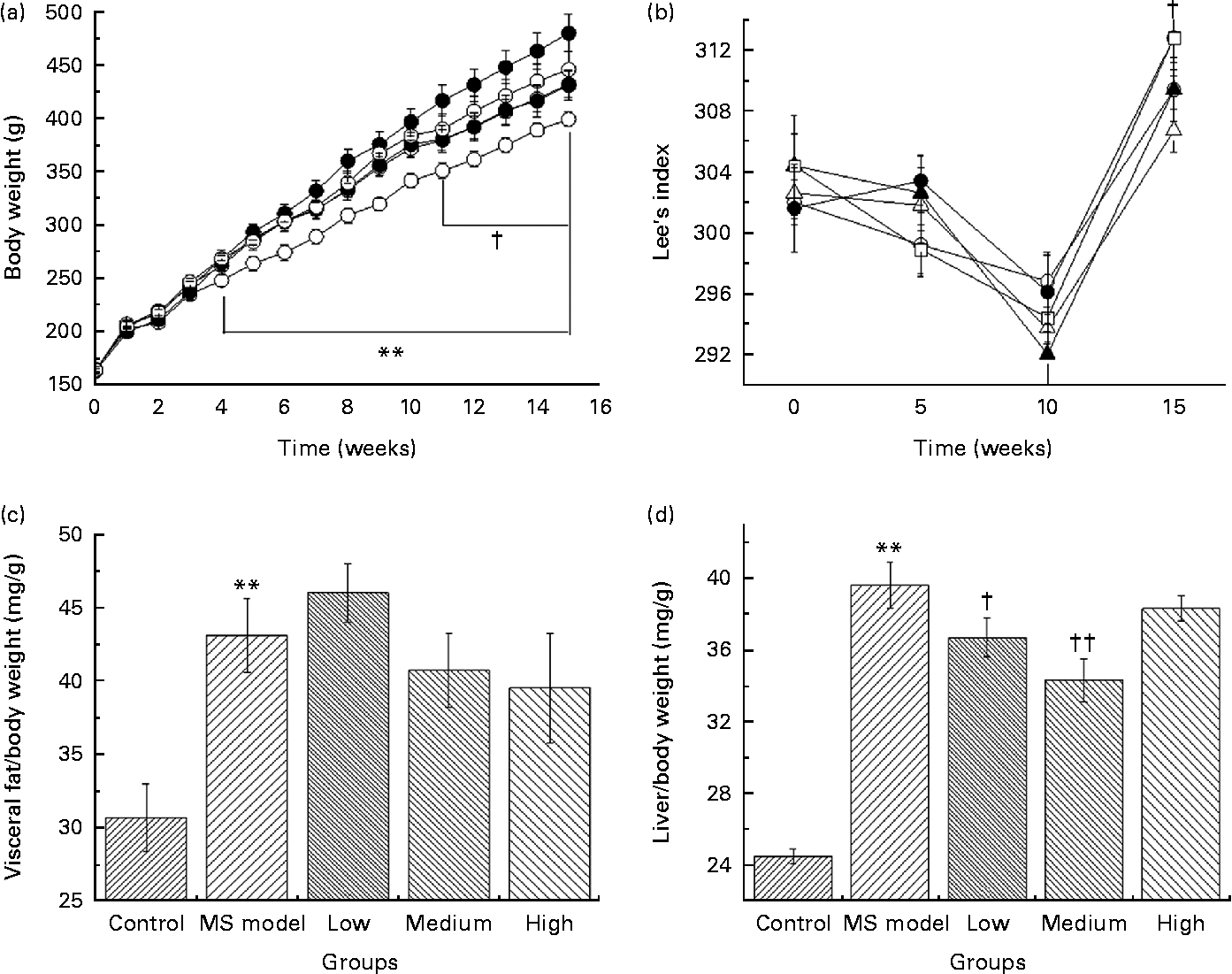
Fig. 2 (a) Body weight of rats in five groups over time. ** Mean values were significantly different for the control (![]() ) group compared with the metabolic syndrome (MS) model (
) group compared with the metabolic syndrome (MS) model (![]() ) group from the 4th week to the 15th week (P< 0·01). † Mean values were significantly different for the low- (
) group from the 4th week to the 15th week (P< 0·01). † Mean values were significantly different for the low- (![]() ) and medium (
) and medium (![]() )-dose groups compared with the MS model group from the 11th week to the 15th week (P< 0·05). (b) Effect of Lactobacillus gasseri TMC0356 (TMC0356) on Lee's index in MS rats; Lee's index is used to reflect the degree of obesity of the rats. Lee's index = ((body weight)− 3/body length) × 103. † Mean values were significantly different for the low-dose group compared with the MS model group (P< 0·05). Repeated-measures ANOVA was used to analyse the differences of Lee's index among groups.
)-dose groups compared with the MS model group from the 11th week to the 15th week (P< 0·05). (b) Effect of Lactobacillus gasseri TMC0356 (TMC0356) on Lee's index in MS rats; Lee's index is used to reflect the degree of obesity of the rats. Lee's index = ((body weight)− 3/body length) × 103. † Mean values were significantly different for the low-dose group compared with the MS model group (P< 0·05). Repeated-measures ANOVA was used to analyse the differences of Lee's index among groups. ![]() , High. (c, d) Effect of TMC0356 on visceral fat:body weight ratio and liver:body weight ratio in MS rats. Values are means, with standard errors represented by vertical bars. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared to the MS model group: † P< 0·05, †† P< 0·01.
, High. (c, d) Effect of TMC0356 on visceral fat:body weight ratio and liver:body weight ratio in MS rats. Values are means, with standard errors represented by vertical bars. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared to the MS model group: † P< 0·05, †† P< 0·01.
Lee's index (Fig. 2(b)) in the low-dose group decreased dramatically compared with that in the MS model group (P< 0·05); however, no differences were detected between the MS model group and the medium- and high-dose groups.
Visceral fat:BW ratio (Fig. 2(c)) in the MS model group was significantly higher than those in the control group (P< 0·01). Fat:BW ratio in the TMC0356 groups was not significantly different from those in the MS model group; however, liver:BW ratio (Fig. 2(d)) in the low- and medium-dose groups decreased significantly (P< 0·05, P< 0·01).
Glycometabolism
FBG levels in the MS model group was significantly higher than that in the control group since the 5th week (P< 0·05, Fig. 3(a)); FBG levels in the medium-dose group reduced dramatically compared with that in the model group (P< 0·05).

Fig. 3 (a) Effects of Lactobacillus gasseri TMC0356 (TMC0356) on fasting blood glucose (FBG) in metabolic syndrome (MS) rats. ** Mean values were significantly different for the control (![]() ) group compared with the MS model (
) group compared with the MS model (![]() ) group (P< 0·01). †† Mean values were significantly different for the medium (
) group (P< 0·01). †† Mean values were significantly different for the medium (![]() )-dose group compared with the MS model group (P< 0·01). † Mean values were significantly different for the low- (
)-dose group compared with the MS model group (P< 0·01). † Mean values were significantly different for the low- (![]() ) and medium-dose groups compared with the MS model group (P< 0·05).
) and medium-dose groups compared with the MS model group (P< 0·05). ![]() , High. (b–d) Effects of TMC0356 on serum insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and serum adiponectin in MS rats. HOMA-IR = FBG × insulin/22·5. The difference of FBG among groups was analysed using repeated-measures ANOVA, and the insulin, HOMA-IR and adiponectin among groups were analysed by one-way ANOVA. Values are means, with standard errors represented by vertical bars. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared with the MS model group: † P< 0·05, †† P< 0·01.
, High. (b–d) Effects of TMC0356 on serum insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and serum adiponectin in MS rats. HOMA-IR = FBG × insulin/22·5. The difference of FBG among groups was analysed using repeated-measures ANOVA, and the insulin, HOMA-IR and adiponectin among groups were analysed by one-way ANOVA. Values are means, with standard errors represented by vertical bars. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared with the MS model group: † P< 0·05, †† P< 0·01.
To evaluate the function of the pancreas, serum insulin levels and HOMA-IR were determined. The insulin level in the MS model group increased compared with that in the control group; however, the difference was not significant (P>0·05, Fig. 3(b)). Insulin levels in the three TMC0356 groups were found to be reduced compared with that in the MS model group; however, a statistical difference was observed only in the medium-dose group (P< 0·05). HOMA-IR in the MS model group increased markedly compared with that in the control group (P< 0·01), and it decreased significantly in all the three TMC0356 groups compared with that in the model group (P< 0·01, P< 0·05, Fig. 3(c)). Interestingly, adiponectin, a cytokine that can increase insulin sensitivity and improve inflammatory status, was augmented in the low- and medium-dose groups compared with those in the MS model group (P< 0·05, Fig. 3(d)).
Lipid metabolism
As indicated in Fig. 4, TC and LDL-cholesterol levels increased and HDL-cholesterol levels decreased markedly in the MS model group compared with those in the control group (P< 0·01). Unexpectedly, TAG and VLDL-cholesterol levels in the MS model group reduced progressively and were eventually significantly lower than those in the control group (P< 0·01). LDL-cholesterol levels decreased and HDL-cholesterol levels elevated dramatically in the medium-dose group compared with those in the MS model group (P< 0·01); however, serum TAG, TC and VLDL-cholesterol levels in the three TMC0356 groups did not improve during the study.

Fig. 4 (a–e) Effects of Lactobacillus gasseri TMC0356 (TMC0356) on serum TAG, total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C) and VLDL-cholesterol (VLDL-C) levels in metabolic syndrome (MS) rats. Serum lipids were determined at 0, 5, 10 and 15 weeks during the study. Repeated-measures ANOVA was used to analyse the differences of lipid profiles among groups. Values are means, with standard errors represented by vertical bars. ** Mean values were significantly different for the control (![]() ) group compared with the MS model (
) group compared with the MS model (![]() ) group (P< 0·01). Mean values were significantly different for the medium (
) group (P< 0·01). Mean values were significantly different for the medium (![]() )-dose group compared with the MS model group: † P< 0·05, †† P< 0·01.
)-dose group compared with the MS model group: † P< 0·05, †† P< 0·01. ![]() , Low;
, Low; ![]() , high. (f, g) Effects of TMC0356 on hepatic TC and TAG levels in MS rats. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared with the MS model group: † P< 0·05, †† P< 0·01.
, high. (f, g) Effects of TMC0356 on hepatic TC and TAG levels in MS rats. ** Mean values were significantly different compared with the control group (P< 0·01). Mean values were significantly different compared with the MS model group: † P< 0·05, †† P< 0·01.
As indicated in Fig. 4(f and g), hepatic TC and TAG levels in the MS model rats were significantly higher than those in the control group (P< 0·01). These reduced markedly in the three TMC0356 groups, particularly in the low- and medium-dose groups, when compared with those in the MS model group (P< 0·05, P< 0·01).
Blood pressure
BP was measured at 5, 10 and 15 weeks (Fig. 5). The differences in systolic BP and diastolic BP between the control and the MS model groups gradually increased and reached significance at the 15th week (P< 0·05). However, no improvement was detected in the three TMC0356 groups compared with the MS model group.

Fig. 5 (a, b) Effects of Lactobacillus gasseri TMC0356 on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in metabolic syndrome (MS) rats. Because the sphygmomanometer broke, initial blood pressure was not measured and blood pressure was only measured at 5, 10 and 15 weeks during the study. The differences in blood pressure among the groups were analysed by repeated-measures ANOVA. * Mean values were significantly different for the control (![]() ) group compared with the MS model (
) group compared with the MS model (![]() ) group (P< 0·05).
) group (P< 0·05). ![]() , Low;
, Low; ![]() , medium;
, medium; ![]() , high.
, high.
These results demonstrated that the HFS diet successfully induced the model rats with the MS and that heat-inactivated TMC0356 could improve metabolic characteristics such as obesity, hyperglycaemia and hyperlipidaemia of MS rats.
Effects of Lactobacillus gasseri TMC0356 on immune function of metabolic syndrome rats
Organ coefficients and immune cells
As shown in Table 2, there were no significant differences in the thymus index, spleen index, neutrophil and lymphocyte counts in the MS model group compared with those in the control group (P>0·05), except for Hb (P< 0·01). The thymus index and Hb in the medium-dose group and the lymphocytes in all the three TMC0356 groups increased markedly compared with those in the MS model group (P< 0·05).
Table 2 Comparison of organ coefficients and immune cells among groups (n 12) (Mean values with their standard errors)
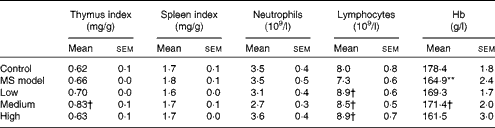
MS, metabolic syndrome.
Mean value was significantly different compared with the control group: ** P< 0·01.
Mean values were significantly different compared with the MS model group: † P< 0·05.
Cytokines and antibodies
Cytokines and antibodies were measured at the end of the study to evaluate the inflammatory immune response. As seen in Table 3, no statistical differences in these indices were observed between the MS model and control groups (P>0·05). Interestingly, serum C-reactive protein, TNF-α, IL-6 and IgG levels in the three TMC0356 groups, particularly in the medium- and high-dose groups, increased dramatically compared with those in the MS model group (P< 0·05, P< 0·01); however, the differences in serum secretory IgA among the groups did not reach significance (P>0·05).
Table 3 Comparison of cytokines and antibody levels among groups (n 12)† (Mean values with their standard errors)
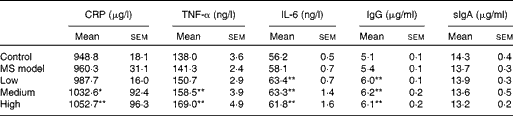
CRP, C-reactive protein; sIgA: secretory Ig A; MS, metabolic syndrome.
Mean values were significantly different compared with the MS model group: * P< 0·05, ** P< 0·01.
† All cytokines and antibodies were measured using ELISA kits at the end of the study.
These results demonstrated that the HFS diet did not lead to any change in the immunological function of the rats. Nevertheless, heat-inactivated TMC0356 could enhance immunity, particularly the inflammatory immune response in MS rats.
Discussion
The TMC0356 strain, which is tolerant to gastric acidity and bile toxicity and can adhere to gut epithelial cells and mucus in a strain-dependent manner, was originally isolated from a healthy Japanese adult. Several studies have clearly demonstrated that TMC0356 has a strain-dependent ability to regulate the host immune response, to improve IgE-mediated allergic disorders and to enhance the natural defences of the host against influenza virus infection and cancer(Reference Kawase, He and Kubota21, Reference Kawase, He and Kubota22). Recently, inflammatory factors such as TNF-α and IL-6 present in the supernatant of macrophage cells (J774.1) activated by lactobacilli, including TMC0356, were found to significantly inhibit adipocyte (3T3-L1) proliferation in vitro. These findings led to a hypothesis that functional food ingredients such as probiotics with immunoregulatory effects may be beneficial to MS management. In the present study, heat-inactivated TMC0356 was investigated to explore the possibility of improving the metabolic and immune characteristics of MS rats.
Gene-modified, drug-induced and diet-induced animal models are the three most widely used types in MS investigations. Despite the convenience, low cost, short induction period and high efficiency of gene-modified and drug-induced models, they are not better than diet-induced models in terms of replicating the pathological development of the MS in humans. Most published studies used high-fat diets to create an MS rat model. In the present study, considering the importance of hypertension in the definition of the MS and the fact that high salt intake is a typical dietary habit in the MS population, we used an HFS diet to induce the model rats with the MS. As expected, the metabolic parameters, including obesity, lipid profiles, FBG and BP of the MS model group fed the HFS diet were markedly different from those of the control group fed the conventional diet. These results demonstrated that the HFS diet could successfully duplicate the MS in rats.
Effects of Lactobacillus gasseri TMC0356 on the metabolic syndrome
Obesity is an independent risk factor for the MS, and BW control is recommended as the most important measure in MS management. The significant decrease in BW gain of MS rats implied that the TMC0356 might have a preventive effect against obesity. Recently, numerous experiments have demonstrated that the dysfunction of intestinal flora is closely associated with obesity, and the possible explanations include increasing energy harvest from diet, stimulation of fat storage and promotion of lipopolysaccharide transportation into the circulation, which is supposed to result in chronic low-grade inflammation and insulin resistance. To date, several probiotics possessing the ability to attenuate obesity have been discovered. Naito et al. (Reference Naito, Yoshida and Makino23) demonstrated that oral administration of the L. casei strain could reduce BW in diet-induced obese mice by improving insulin resistance. Lee et al. (Reference Lee, Park and Seok24) found that L. rhamnosus PL60 showed anti-obesity effects in diet-induced obese mice by producing conjugated linoleic acid. Tanida et al. (Reference Tanida, Shen and Maeda25) reported that L. paracasei ST11 could affect autonomic nerves, enhance lipolysis and then reduce BW and abdominal fat weight in high-fat diet-induced obese rats. The average food and energy intake of rats in the three TMC0356 groups were significantly lower than those of rats in the MS model group, and these findings indicated that TMC0356 may increase the satiety of MS rats, which could partly explain its alleviating effect on obesity. Previous studies have shown that some probiotics can both increase and prolong satiety in rodents or humans by gastrointestinal production of satiety hormones (e.g. cholecystokinin and glucagon-like peptide-1)(Reference Ohlson, Mahlapuu and Svensson26) and decreasing ghrelin secretion(Reference Parnell and Reimer27, Reference Delzenne, Cani and Daubioul28) to reduce the food intake, BW gain and fat accumulation. However, further studies are required to explore the effects of TMC0356 on host appetite.
The remarkable decrease in liver:BW ratio, hepatic TAG and TC in the three TMC0356 groups than those in the MS model group suggested that TMC0356 can inhibit fat accumulation in the liver of MS rats. The liver plays a crucial role in glucose and lipid metabolism, and excessive lipid deposition in the liver inevitably impairs its function. Interestingly, lipid reduction in the liver by TMC0356 may normalise metabolic function, including lipolysis and glucose utilisation. Moreover, a decrease in fat relatively increases the lean body mass, which could elevate the BMR. In general, improvement in liver function and augmentation of BMR facilitate prevention of obesity.
TMC0356 significantly improved FBG, insulin, LDL-cholesterol and HDL-cholesterol levels, and these results indicated that it could ameliorate glycolipid metabolism. It is well known that insulin resistance is positively associated with the degree of obesity. In the present study, amelioration of obesity may have alleviated insulin resistance, which could be demonstrated by the decreased HOMA-IR and elevated adiponectin in the three TMC0356 groups. Improvement in insulin resistance led to improved glucose utilisation by the liver, muscle and adipose tissues. Therefore, TMC0356 may regulate glycolipid metabolism by improving insulin resistance caused by amelioration of obesity.
In addition, TMC0356 may directly regulate glucose and lipid metabolism. To date, several probiotics have been demonstrated to have anti-diabetic effects. Yun et al. (Reference Yun, Park and Kang29) reported that the L. gasseri BNR17 isolated from human breast milk could lower blood glucose levels and improve diabetic symptoms in db/db mice. Tabuchi et al. (Reference Tabuchi, Ozaki and Tamura30) administered L. rhamnosus GG to streptozotocin-induced diabetic rats and found significant improvement in glucose intolerance. Possible pathways such as reduction in glucagon secretion by the alteration of autonomic nerve activities(Reference Yamano, Tanida and Niijima31) and improvement in the disordered host immune response(Reference Matsuzaki, Yamazaki and Hashimoto32) were put forward.
Unexpectedly, serum TAG and VLDL-cholesterol levels reduced progressively in the MS model group and were eventually significantly lower than those in the control group in the present study. Recently, similar findings have been reported by several studies. Some experts attributed this to different animal species, components of the high-fat diet and the experimental period. In addition, a liver compensatory mechanism is now widely accepted(Reference Savransky, Nanayakkara and Li33). Liver is the major tissue for TAG synthesis. High-fat diets may severely damage liver function, which eventually causes decrease in TAG synthesis; however, an activated liver compensatory mechanism can increase TAG metabolism. The serum TAG and VLDL levels would increase again only when the liver damage exceeds the compensation function of the liver. This is also usually observed in patients at the end-stage of liver disease. Nevertheless, in the present study, liver TAG and TC did not decrease in the MS model group compared with the control group, which could not be explained by the liver compensatory mechanism. We propose that the HFS diet may obstruct the transportation of TAG and VLDL from the liver to the periphery. Moreover, the liver compensatory mechanism may have failed towards the end of our study and TAG catabolism in the liver was also reduced. To date, few studies recorded the variation trends of lipid levels in serum and liver tissue for a long time (15 weeks in the present study). More evidence is required to clarify this phenomenon.
Emerging evidence has demonstrated that milk fermented with certain probiotics possesses an anti-hypertensive effect, which is mainly attributed to the production of two tripeptides, Ile-Pro-Pro and Val-Pro-Pro; these are released from casein and may attenuate the development of hypertension as angiotensin-converting enzyme inhibitors(Reference Jäkälä and Vapaatalo34). However, TMC0356 exhibited no impact on BP in the present study. No study has reported that heat-killed probiotics could prevent hypertension yet. Therefore, we assume that the viability of probiotics may be essential for their anti-hypertensive activity and that this function is strain-dependent.
In general, heat-inactivated TMC0356 could ameliorate the MS in rats. However, we did not observe dose dependency, and the present results suggested that the medium dose may be the best concentration for preventing the MS in rats.
Effects of Lactobacillus gasseri TMC0356 on immunity of metabolic syndrome rats
The chronic inflammatory response is a common phenomenon that plays a key role in insulin resistance observed in the MS. Preliminary evidence(Reference Cani, Bibiloni and Knauf35–Reference Erridge, Attina and Spickett37) has demonstrated that a high-fat diet could increase intestinal permeability and alter gut microflora. These effects would then be followed by the elevation of lipopolysaccharides in circulation, i.e. metabolic endotoxaemia, which triggers excessive release of inflammatory markers and consequently results in metabolic disorders. Unexpectedly, we did not observe any increase in inflammatory factors in the MS model group compared with the control group at the end of the study. This may imply that the inflammatory response was confined to partial tissues (e.g. adipose tissue) at the present stage and that the systemic chronic inflammatory response may occur at a later stage of MS development. However, further studies are required to support this assumption. In addition, it may also imply that various mechanisms, and not solely chronic inflammation, are involved in the development of the MS. To our knowledge, the high-fat diets used to model metabolic disorders are not unified among studies. The percentage of energy provided by fat ranged from 40 to 70 %(Reference Buettner, Sch lmerich and Bollheimer38) in published literature, the basic fat component varied (animal-derived fat, e.g. lard or beef tallow, and plant oils, e.g. maize or safflower oil), and the type or strain of animals differed among laboratories. These factors could also inevitably lead to considerable variability among results.
In contrast, serum inflammatory cytokine and antibody levels in the rats orally fed TMC0356 were significantly increased compared with those in the MS model group. These results were in good agreement with those of a previous study(Reference Morita, He and Fuse39), indicating that TMC0356, a potent immunoregulatory agent, could enhance the host immune function. The present study was the first to demonstrate that a probiotic strain can alter the immunity of a host with MS.
Obesity-associated chronic inflammation has been found to be mainly induced by infiltrating macrophages that aggregate to constitute crown-like structures surrounding dead adipocytes in advanced obesity(Reference Suganami and Ogawa40). Endogenous ligands from damaged/dead adipose, e.g. SFA and deoxyadenosine monophosphate, are considered to be the main stimulators of this harmful chronic inflammation. However, lactobacilli are similar to other exogenous ligands, which can enhance host innate immunity. This inflammation should be different from obesity-associated chronic inflammation, which involves an acute phenotype and is considered as homeostatic. Enhancement of inflammatory immune responses with Lactobacillus, particularly with some selected probiotic strains, is considered to be a critical mechanism underlying the well-documented health-promoting effects of these probiotic strains on hosts, including anti-allergic and anti-pathogenic effects(Reference Miettinen, Vuopio-Varkila and Varkila41, Reference Erickson and Hubbard42). In a previous study, lactobacilli, including TMC0356, significantly suppressed adipogenic differentiation via stimulation of macrophage-derived inflammatory responses. Therefore, the underlying mechanism behind the potent health-promoting effect of TMC0356 on the MS may be, at least in part, through the inflammation caused by activating macrophages that inhibits adipocytes proliferation and reduces lipid accumulation.
In conclusion, TMC0356 can improve the metabolic characteristics of MS rats by suppressing appetite, and an elevated inflammatory immune response may also be involved in the possible mechanisms of health-promoting effects by TMC0356. Although it appears that these findings challenged the common view that inflammation is a risk factor for the MS, it is still reasonable to consider that many types of inflammation, particularly serious inflammatory responses, are harmful to the MS. However, based on the results from the present study, some types of low-grade inflammatory immune responses caused by functional ingredients, including probiotics such as TMC0356, under normal physiological conditions in a host or in the early stage of the MS, may be beneficial for controlling the MS. However, further studies are required to confirm its clinical effectiveness and related mechanism in humans.
Acknowledgements
The present study was supported by grants from the Ministry of Agriculture and Takanashi Milk Products Company Limited in Japan. The authors thank all the workers in the Animal Center of West China School of Public Health and Laboratory 126 of Sichuan University for their technical assistance during the experiment. The authors declare that there are no conflicts of interest. L. S., C. H., M. L., F. H. and Y. L. designed the study. L. S., C. G., X. J., M. L. and C. H. conducted the animal study. F. H., K. M. and M. H. provided technical support and prepared the TMC0356 sample. L. S., C. H. and F. H. analysed the data, and wrote and revised the final manuscript. This research was performed in the Department of Nutrition and Food Safety, West China School of Public Health, Sichuan University, Chengdu, People's Republic of China.










