l-Citrulline – a non-essential amino acid abundantly found in watermelon – is the immediate precursor for the synthesis of L-arginine(Reference Davis, Webber and Fish1,Reference Papadia, Osowska and Cynober2) . Through increased l-arginine bioavailability, l-citrulline stimulates the synthesis of endogenous nitric oxide (NO)(Reference Boger3) that may exert several beneficial cardiometabolic and vascular health effects(Reference Levine, Punihaole and Levine4–Reference Hadi, Arab and Moradi6), thereby reducing the risk on developing CVD(Reference Bryan7). A recent review indeed found that l-citrulline supplementation consistently increased both plasma l-arginine and cyclic guanosine monophosphate concentrations, which is an indicator of NO production(Reference Allerton, Proctor and Stephens8). We already reported in our very recent meta-analysis of human trials that l-arginine improved longer-term (i.e. 3 d to 6 months) fasting and 2-h postprandial vascular endothelial function, which was explained by an increased NO bioavailability(Reference Smeets, Mensink and Joris9). Remarkably, increasing evidence now indicates that dietary l-citrulline supplementation may be an even more efficient intervention to increase l-arginine and NO bioavailability than supplementation with l-arginine itself(Reference Moinard, Nicolis and Neveux10–Reference Figueroa, Wong and Jaime12). In fact, dietary l-arginine is – in contrast to l-citrulline – partly metabolised to l-ornithine and urea by the enzyme arginase in the gastrointestinal tract and the liver resulting in lower plasma l-arginine concentrations as compared with dietary l-citrulline that bypasses intestinal and first hepatic metabolism(Reference Allerton, Proctor and Stephens8,Reference Castillo, deRojas and Chapman13,Reference van de Poll, Siroen and van Leeuwen14) .
Two meta-analyses of human intervention studies have already reported that l-citrulline supplementation decreases blood pressure(Reference Yang, Li and Zhang15,Reference Barkhidarian, Khorshidi and Shab-Bidar16) . Also, l-arginine intake has previously been shown to lower TAG concentrations(Reference Sepandi, Abbaszadeh and Qobady17) and to play an important role in glucose metabolism(Reference Hu, Han and Rezaei18). It is therefore of interest to study if l-citrulline supplementation may also beneficially affect cardiometabolic risk markers, such as total cholesterol, glucose and insulin concentrations. Studies on these cardiometabolic risk markers are, however, equivocal. In addition, several trials already investigated the effects of dietary l-citrulline on various vascular function markers addressing different aspects of the vasculature during the fasting and postprandial phase, but effects have not been systematically and quantitatively reviewed. Moreover, results of watermelon consumption on vascular function and cardiometabolic risk markers have not been quantitatively summarised before. Therefore, the aim was to investigate and compare the effects of l-citrulline supplementation and watermelon consumption on vascular function and cardiometabolic risk markers in randomised controlled trials (RCT) involving adults. Focus was on longer-term and postprandial effects, while different vascular function markers were considered that exist along the pathway between diet andCVD.
Methods
Eligibility criteria
Human RCT investigating postprandial or longer-term effects of l-citrulline supplementation or watermelon consumption with an appropriate control group on (i) non-invasive vascular function parameters and (ii) cardiometabolic risk markers were included. Vascular function parameters included brachial artery flow-mediated vasodilation (FMD), pulse wave analysis (PWA), pulse wave velocity (PWV), and related outcomes. Cardiometabolic risk markers included circulating glucose, serum insulin and total cholesterol concentrations. Only articles published in English involving an adult study population were included. Longer-term was defined as supplementing l-citrulline or consuming watermelon for at least one day.
Search method and data extraction
Two systematic searches using the electronic databases PubMed, EMBASE, MEDLINE and the Cochrane Library were performed to retrieve potentially relevant articles published before June 2021. A search was conducted for studies assessing effects on: (i) the vascular function parameters and, (ii) cardiometabolic risk markers. The following search terms were used: flow-mediated dilation (or vasodilatation or vasodilation or dilatation or FMD), endothelial (or endothelium) function (or dysfunction), vascular reactivity, PWA, PWV, l-citrulline (or citrulline malate), watermelon (or citrullus lanatus), glucose, insulin (or proinsulin) and insulin resistance (or clamp or glucose tolerance test or matsuda or HOMA-IR or QUICKI). Detailed information on the search terms used can be found in Supplemental Table 1.
Titles and abstracts of all potentially eligible articles were first screened independently by two of the authors. The full text of selected articles was then read. Discrepancies were resolved by discussion until consensus was reached. Studies were excluded if relevant data were missing. Also, studies were not included if the intervention involved infusion of l-citrulline or multiple consecutive bolus ingestions for studies investigating postprandial effects. After inclusion, data were extracted relating to participant (i.e. age, BMI, sex and health status), treatment characteristics (i.e. watermelon or l-citrulline intervention, study duration, relevant outcome parameters with accompanying measures of variability), the total number of dropouts and funding received. If the amount of l-citrulline was not provided, it was calculated using the information that 1000 g of fresh watermelon flesh corresponds on average to 2 g of l-citrulline(Reference Rimando and Perkins-Veazie19). It should be noted, however, that fresh watermelon flesh may contain slightly more l-citrulline than watermelon rind(Reference Rimando and Perkins-Veazie19). If available, data on circulating markers of l-arginine metabolism (i.e. l-citrulline, l-arginine, l-ornithine – an intermediate amino acid involved in the conversion of l-arginine back into l-citrulline(Reference Luiking, Engelen and Deutz20) – and NOx (i.e. nitrate and nitrite) concentrations) were also extracted.
Risk of bias and GRADE assessment
Studies were assessed for their risk of bias (i.e. low, some concerns or high risk) using the Cochrane Risk of Bias 2 (RoB 2) assessment tool for randomised parallel and cross-over trials(Reference Sterne, Savovic and Page21). In addition, the quality of evidence (i.e. very low, low, moderate or high) was determined by the GRADE assessment. RCT were qualified as high-quality studies by using this assessment tool, whereas downgraded RCT provided moderate quality of evidence. Finally, (very) low quality of evidence was derived from double or triple-downgraded RCT(Reference Schuneman, Brozek and Guyatt22).
Statistical analyses
Quantitative meta-analyses were conducted when at least two independent studies investigated the impact of l-citrulline supplementation or watermelon consumption on one of the vascular function parameters or cardiometabolic risk markers. All statistical analyses were performed using Stata 12.1 (Stata Corporation, College Station). As described(Reference Ras, Hiemstra and Lin23), effect sizes for all outcomes in cross-over trials were calculated by subtracting the mean value at the end of the treatment period from the mean value at the end of the control period. Mean changes in the control group were subtracted from mean changes in the intervention group for outcomes in parallel studies. Mean changes were defined as the difference between the end and start of the study values. Multiple study arms were included if results were only provided for different study populations separately (i.e. men v. women, or young v. old participants). For postprandial studies, the time point with the most pronounced effect was taken if multiple time points were measured.
For all outcomes, summary estimates of weighted mean differences (WMD) and 95 % CI were determined using fixed-effect meta-analyses and visualised using forest plots. The inverse of the within-subject variance (i.e. 1/(standard error)2) was used as weighing factor. If only one study was available, no weighting was involved in the calculation of the statistical summary. Heterogeneity was quantified using I2, that is, the percentage of variability in effect estimate that is due to heterogeneity rather than sampling error. If relevant heterogeneity between studies was present, as indicated by an I2 above 50 %, random-effect meta-analyses were performed.
Results
Study selection
A total of 1983 potentially relevant articles were retrieved from both systematic searches and 1928 papers were excluded for different reasons, such as no inclusion of a watermelon or l-citrulline intervention, no adult study population or no RCT. The full text of the fifty-five remaining articles were reviewed and twenty-six were included in the present meta-analysis (Fig. 1).
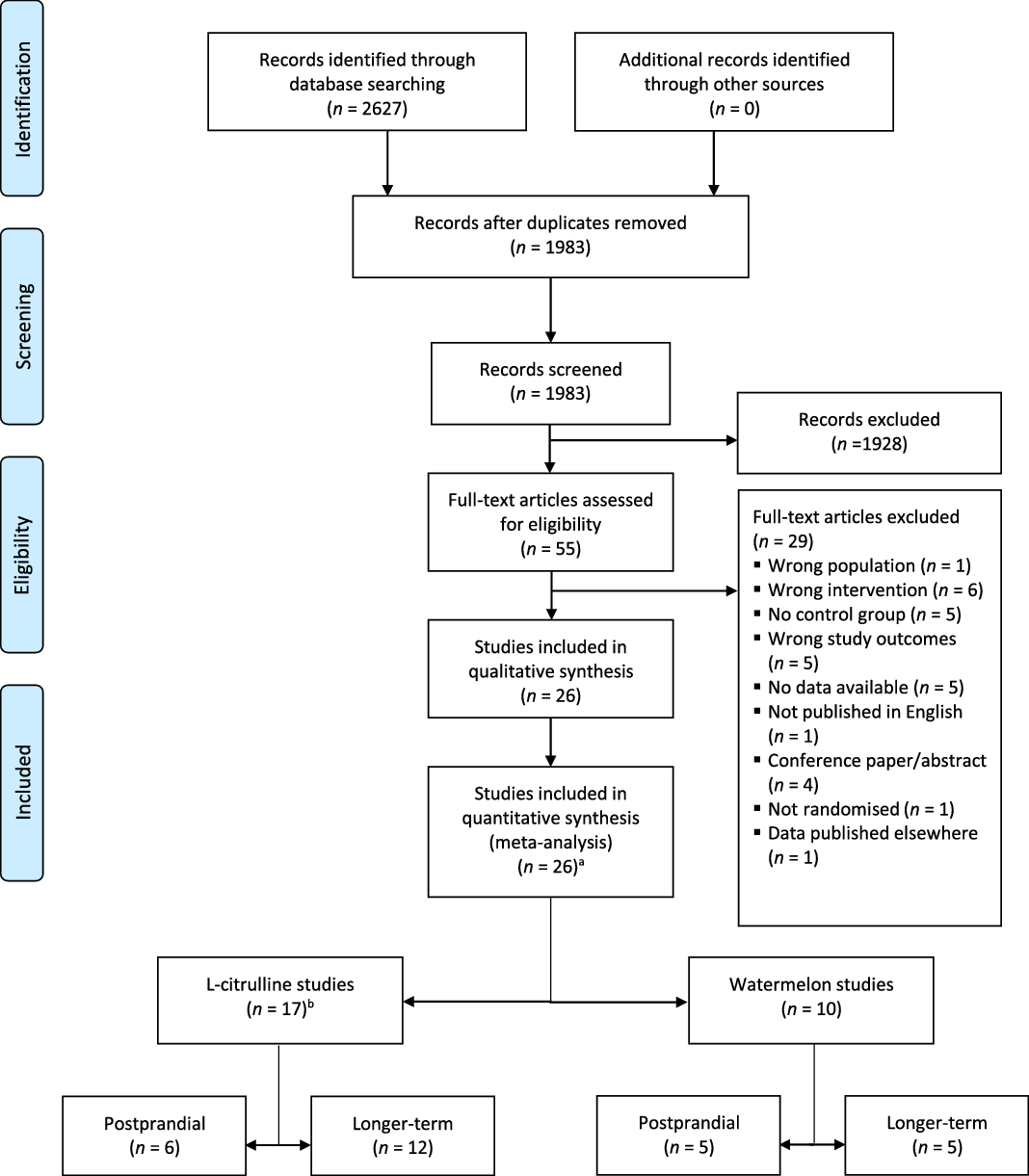
Fig. 1. PRISMA 2009 flow diagram of the study selection process of human randomised controlled trials that investigated postprandial and longer-term effects of l-citrulline supplementation or watermelon consumption on vascular function and cardiometabolic risk markers. a one study included a l-citrulline and watermelon intervention; b one study examined both postprandial and longer-term effects.
As shown in Fig. 1, seventeen studies involved l-citrulline supplementation. Six studies with eight relevant study arms investigated postprandial effects(Reference Churchward-Venne, Cotie and MacDonald24–Reference Trexler, Keith and Lucero29), while twelve studies with twelve relevant study arms investigated longer-term effects(Reference Ochiai, Hayashi and Morita27,Reference Balderas-Munoz, Castillo-Martinez and Orea-Tejeda30–Reference Azizi, Mahdavi and Mobasseri40) . One trial investigated both postprandial and longer-term effects(Reference Ochiai, Hayashi and Morita27). Ten studies involved a watermelon intervention: five studies with five relevant study arms examined postprandial effects(Reference Cutrufello, Gadomski and Zavorsky25,Reference Blohm, Beidler and Rosen41–Reference Fan, Park and Zhang44) and the other five studies with five relevant study arms investigated longer-term effects of watermelon consumption(Reference Bailey, Blackwell and Williams45–Reference Figueroa, Wong and Kalfon49). One study included both a l-citrulline and watermelon intervention(Reference Cutrufello, Gadomski and Zavorsky25).
Study characteristics
Three studies did not receive any funding(Reference Balderas-Munoz, Castillo-Martinez and Orea-Tejeda30,Reference Safi, Mahjoob and Nateghi36,Reference Azizi, Mahdavi and Mobasseri40) , while one study did not report any information on funding or grants(Reference Ochiai, Hayashi and Morita27). Ten studies mentioned that their supplements were provided by Pronat Laboratories, NOW Foods, Milne Fruits or Cobell Ltd, without receiving any direct funding(Reference Figueroa, Alvarez-Alvarado and Jaime31,Reference Figueroa, Alvarez-Alvarado and Ormsbee32,Reference Orea-Tejeda, Orozco-Gutierrez and Castillo-Martinez35,Reference Sanchez-Gonzalez, Koutnik and Ramirez37,Reference Wong, Alvarez-Alvarado and Jaime39,Reference Bailey, Blackwell and Williams45–Reference Figueroa, Wong and Kalfon49) , while two studies reported that they received funding from the National Watermelon Promotion Board(Reference Blohm, Beidler and Rosen41,Reference Fan, Park and Zhang44) .
Studies investigating longer-term effects of l-citrulline supplementation had a study duration between 1 week and 4 months(Reference Ochiai, Hayashi and Morita27,Reference Balderas-Munoz, Castillo-Martinez and Orea-Tejeda30–Reference Azizi, Mahdavi and Mobasseri40) . In total, 311 participants were included ranging from fifteen to thirty-eight participants per study arm. Their mean age varied between 22 to 71 years, and their BMI from 23·7 to 35·0 kg/m2. The dose of l-citrulline ranged from 3 to 6 g per d. One study provided a dose of 100 mg/kg per d(Reference Sanchez-Gonzalez, Koutnik and Ramirez37). Five studies included a healthy study population(Reference Ochiai, Hayashi and Morita27,Reference Figueroa, Trivino and Sanchez-Gonzalez33,Reference Gonzales, Raymond and Ashley34,Reference Sanchez-Gonzalez, Koutnik and Ramirez37,Reference Schwedhelm, Maas and Freese38) . The other studies included participants with overweight and/or obesity(Reference Figueroa, Alvarez-Alvarado and Jaime31,Reference Figueroa, Alvarez-Alvarado and Ormsbee32,Reference Wong, Alvarez-Alvarado and Jaime39) , heart failure(Reference Balderas-Munoz, Castillo-Martinez and Orea-Tejeda30,Reference Orea-Tejeda, Orozco-Gutierrez and Castillo-Martinez35) , coronary artery disease(Reference Safi, Mahjoob and Nateghi36) or type 2 diabetes(Reference Azizi, Mahdavi and Mobasseri40). Postprandial effects were studied after 1 to 5 h(Reference Churchward-Venne, Cotie and MacDonald24–Reference Trexler, Keith and Lucero29). These studies included in total of 123 participants ranging from 7 to 27 per study arm. Their mean age was between 21 to 81 years, and their BMI from 23·5 to 29·0 kg/m2. Studies provided 5·6 to 10 g of l-citrulline. Six studies involved a healthy study population(Reference Churchward-Venne, Cotie and MacDonald24–Reference Trexler, Keith and Lucero29) and one study included participants with heart failure(Reference Kim, Schutzler and Schrader26) (Tables 1 and 2).
Table 1. Overview of studies involving longer-term l-citrulline supplementation on vascular function and cardiometabolic risk markers included in this meta-analysis

* PWA, pulse wave analysis; PWV, pulse wave velocity; FMD, flow-mediated vasodilation.
† PWV provided as cm/s instead of m/s. Values are converted into m/s before analyses.
Table 2. Overview of studies involving postprandial l-citrulline supplementation on vascular function and cardiometabolic risk markers included in this meta-analysis

* BMI calculated by provided height and weight.
† FMD: flow-mediated vasodilation; PWV: pulse wave velocity.
‡ PWV provided as cm/s instead of m/s. Values are converted into m/s before analyses.
Five studies investigated longer-term consumption of a watermelon intervention(Reference Bailey, Blackwell and Williams45–Reference Figueroa, Wong and Kalfon49). Study duration varied from 16 d to 6 weeks. These studies included fifty-six participants with a mean age from 22 to 58 years and a mean BMI from 23·6 to 38·1 kg/m2. One study supplemented with watermelon juice providing 3·4 g of l-citrulline per d(Reference Bailey, Blackwell and Williams45). The remaining studies used watermelon powders or extracts providing 2·7 to 6 g of l-citrulline per d. Two studies included healthy participants(Reference Bailey, Blackwell and Williams45,Reference Figueroa, Wong and Hooshmand48) . The other studies included patients with pre-hypertension(Reference Figueroa, Sanchez-Gonzalez and Perkins-Veazie46) or obese patients with (pre)hypertension(Reference Figueroa, Sanchez-Gonzalez and Wong47,Reference Figueroa, Wong and Kalfon49) . Five studies investigated postprandial effects of watermelon consumption(Reference Cutrufello, Gadomski and Zavorsky25,Reference Blohm, Beidler and Rosen41–Reference Fan, Park and Zhang44) . In total, eighty-six participants ranging from six to twenty-seven per study arm were included. Mean age ranged from 21 to 35 years and mean BMI from 21·2 to 28·7 kg/m2. Varying amounts of (l-citrulline-enriched) watermelon juice(Reference Cutrufello, Gadomski and Zavorsky25,Reference Blohm, Beidler and Rosen41,Reference Martinez-Sanchez, Ramos-Campo and Fernandez-Lobato42) or watermelon(Reference Robert, Ismail and Winn43,Reference Fan, Park and Zhang44) were provided in the studies corresponding to 0·8 to 6·9 g of l-citrulline. Four studies involved a healthy study population(Reference Cutrufello, Gadomski and Zavorsky25,Reference Blohm, Beidler and Rosen41–Reference Robert, Ismail and Winn43) , whereas one study included overweight and obese participants(Reference Fan, Park and Zhang44) (see Tables 3 and 4).
Table 3. Overview of the studies investigating postprandial watermelon consumption on vascular function and cardiometabolic risk markers included in this meta-analysis

* BMI calculated by provided height and weight.
† FMD, flow-mediated vasodilation.
‡ Amount of watermelon calculated based on the Dutch nutritional centre indicating that 278 g of watermelon contain 100 kcal.
§ Amount of l-Citrulline not provided but calculated with the information that 1000 g of watermelon contains approximately 2 g of l-Citrulline.
Table 4. Overview of the studies investigating longer-term watermelon consumption on vascular function and cardiometabolic risk markers included in this meta-analysis

* BMI calculated by provided height and weight.
† PWA, pulse wave analysis; PWV, pulse wave velocity.
Longer-term and postprandial effects on vascular function markers
Longer-term (i.e. 1 week to 4 months) l-citrulline supplementation significantly improved FMD by 0·9 %-point (95 % CI 0·7, 1·1, P < 0·001, Table 5 and Fig. 2), while no heterogeneity was observed between studies (P = 0·85). No studies have investigated longer-term effects of watermelon consumption on FMD. Longer-term (i.e. 16 d–6 weeks) watermelon consumption significantly improved PWV by 0·9 m/s (95 % CI –1·5, –0·1, P < 0·001), while l-citrulline supplementation did just not reach statistical significance (WMD –0·2 m/s, 95 % CI –0·4, 0·0, P = 0·10, Table 5 and Fig. 3). Several PWA-related outcomes (i.e. augmentation index (corrected for heart rate), mean arterial pressure, transit time to reflected wave and augmentation index after a cold pressure test) were assessed in the longer-term trials. No effects of l-citrulline and watermelon were observed on the augmentation index (WMD 0·8 %-point, 95 % CI –2·4, 3·9, P = 0·63 and WMD –0·1 %-point, 95 % CI –6·9, 6·7, P = 0·99, respectively). After a cold pressure test, however, l-citrulline supplementation decreased the augmentation index by 7·8 % point (95 % CI –13·0, –2·6, P = 0·003, Table 5).
Table 5. Effects of longer-term l-Citrulline supplementation and watermelon consumption on vascular function markers

* No data available for forearm and femoral artery blood flow.
† n = 1; no weighting is involved in calculation of statistical summary of a single study.
‡ MAT/TT, maximum amplitude time/total time.

Fig. 2. Forest plot of human randomised controlled trials that investigated postprandial and longer-term effects of l-citrulline supplementation or watermelon consumption on brachial artery flow-mediated vasodilation (FMD). Solid squares represent individual studies, whereas the diamond square represents the weighed mean difference (WMD) in FMD as calculated by random-effect meta-analyses.

Fig. 3. Forest plot of human randomised controlled trials that investigated postprandial and longer-term effects of l-citrulline supplementation watermelon consumption on pulse wave velocity (PWV). Solid squares represent individual studies, whereas the diamond square represents the weighed mean difference (WMD) in PWV as calculated by fixed-effect meta-analyses.
Postprandial FMD was not affected by l-citrulline supplementation (WMD 0·1 %-point, 95 % CI −1·9, 2·2, P = 0·89) or watermelon consumption (WMD 0·4 %-point, 95 % CI −1·5, 2·3, P = 0·67). Only one study investigated postprandial effects of l-citrulline supplementation on PWV and found no significant effect (WMD −0·3 m/s, 95 % CI −1·1, 0·4, P = 0·41, Table 6). No postprandial studies investigated the effects of l-citrulline or watermelon on augmentation index and other PWA-related outcomes parameters.
Table 6. Postprandial effects of l-Citrulline supplementation and watermelon consumption on vascular function markers.

* No data available for all pulse wave analysis-related outcomes and photoplethysmography.
† n = 1; no weighting is involved in calculation of statistical summary of a single study.
Longer-term and postprandial effects on cardiometabolic risk markers
In only one study, longer-term effects on circulating glucose concentrations after watermelon consumption were reported, but no effects were observed (WMD 0·0 mmol/l, 95 % CI −0·0, 0·0, P = 1·00). However, a trend towards reduced glucose concentrations was observed after l-citrulline supplementation (WMD −0·9 mmol/l, 95 % CI −1·9, 0·8, P = 0·06). Total cholesterol concentrations were not changed after longer-term l-citrulline supplementation (WMD 0·0 mmol/l, 95 % CI −0·4, 0·4, P = 0·98, Table 7).
Table 7. Effects of longer-term l-Citrulline supplementation and watermelon consumption on cardiometabolic risk markers and circulating markers of l-arginine metabolism

WMD, weighted mean differences.
* No data available for insulin and l-ornithine concentrations.
† n = 1; no weighting is involved in calculation of statistical summary of a single study.
Postprandial glucose concentrations significantly decreased by 0·6 mmol/l after watermelon consumption (95 % CI −0·7, −0·4, P < 0·001), with significant heterogeneity between studies (P < 0·001). Effects of l-citrulline supplementation on postprandial glucose, insulin and total cholesterol concentrations were only studied in one trial and no effects were found. Moreover, only one trial included postprandial total cholesterol concentrations after watermelon consumption, which were not affected (see Table 8).
Table 8. Postprandial effects of l-citrulline supplementation and watermelon consumption on cardiometabolic risk markers and circulating markers of l-arginine metabolism

WMD, weighted mean differences.
* n 1; no weighting is involved in calculation of statistical summary of a single study.
Longer-term and postprandial effects on circulating markers of l-arginine metabolism
Longer-term l-citrulline supplementation and watermelon consumption increased l-arginine concentrations (WMD 40·7 µmol/l, 95 % CI 19·1, 62·3, P < 0·001 and WMD 49·0 µmol/l, 95 % CI 43·5, 54·5, P < 0·001, respectively). Longer-term watermelon consumption (WMD 79·0 µmol/l, 95 % CI 66·1, 91·7, P < 0·001), but not l-citrulline supplementation (28·0 µmol/l, 95 % CI –15·7, 71·7, P = 0·21), also showed significantly increased l-citrulline concentrations in one study. l-Citrulline and watermelon did not affect NOx concentrations (WMD –1·6 µmol/l, 95 % CI –5·9, 2·7, P = 0·46 and WMD 8·5 µmol/l, 95 % CI –78·9, 95·9, P = 0·85, respectively, Table 6). Effects on l-ornithine concentrations were not studied.
l-citrulline supplementation increased significantly postprandial l-citrulline, l-arginine and l-ornithine concentrations (WMD 1647·5 µmol/l, 95 % CI 1362·7, 1932·1, P < 0·001, WMD 101·7 µmol/l, 95 % CI 87·7 to 115·8, P < 0·001 and WMD 55·8 µmol/l, 95 % CI 35·2, 76·3, P < 0·001, respectively). Postprandial l-arginine and l-citrulline concentrations were also significantly increased by watermelon consumption (WMD 27·9 µmol/l, 95 % CI 17·7 to 38·1, P < 0·001 and WMD 49·5 µmol/l, 95 % CI 21·9, 77·2, P < 0·001, see Table 8), while effects on l-ornithine concentrations were not investigated. Only one study examined the effects of l-citrulline supplementation on postprandial NOx concentrations and found no effects (WMD 0·1 µmol/l, 95 % CI –76·8, 77·1, P = 1·00). These effects were not investigated after watermelon consumption.
Risk of bias and GRADE assessment
Longer-term and postprandial l-citrulline studies were all classified as having a low risk of bias. Moreover, the included watermelon studies also had a low risk of bias (online Supplemental Tables 2a and b). GRADE assessment revealed that the included studies provided moderate or high quality of evidence.
Discussion
In this meta-analysis of human RCT, we found that longer-term l-citrulline supplementation significantly improved FMD, while watermelon consumption beneficially affected PWV. No postprandial effects of l-citrulline or watermelon on vascular function markers were observed. Watermelon consumption beneficially affected glucose concentrations during the postprandial phase, but other postprandial or longer-term effects on cardiometabolic risk markers were not observed.
Longer-term l-citrulline supplementation significantly improved vascular endothelial function as FMD increased by 0·9 %-point. Moreover, we here also provide evidence for an improved arterial stiffness as PWV decreased by 0·9 m/s after longer-term watermelon consumption. In line with this, beneficial effects on PWA outcomes were also found after both l-citrulline supplementation and watermelon consumption. These improvements in vascular function are of clinical relevance as they are associated with a reduced risk to develop cardiovascular events(Reference Thijssen, Bruno and van Mil50–Reference Zhong, Hu and Cui53). Longer-term l-citrulline supplementation nearly improved PWV, but effects were less pronounced as compared with those of watermelon. The amount of l-citrulline was comparable between the l-citrulline (2·7–6 g/d) and watermelon (3–6 g/d) studies, but l-citrulline studies were shorter (i.e. 1 to 4 weeks v. 6 weeks), which could be a possible explanation for the observed smaller effects. Moreover, the effects of l-citrulline could have been more pronounced following supplementation with higher dosages were provided, as dose-dependent effects were observed with a maximum effect reached at 10 g/d(Reference Moinard, Nicolis and Neveux10). Besides, differential effects between l-citrulline and watermelon on vascular function may also be explained by beneficial effects of other components next to l-citrulline provided watermelon, such as l-arginine, lycopene and vitamin C(54). In fact, meta-analyses have shown that these components beneficially impact vascular endothelial function as well(Reference Cheng, Koutsidis and Lodge55,Reference Ashor, Siervo and Lara56) and may also reduce arterial stiffness(Reference Ashor, Siervo and Lara57,Reference Mozos, Stoian and Caraba58) .
Since we spend most of the time in the postprandial phase, it is of interest to investigate if watermelon consumption and l-citrulline supplementation also affect vascular function during the postprandial phase. No effects on vascular function markers were found during the postprandial phase. However, most studies assessed vascular function 1 h postprandially. It cannot be excluded that effects on FMD have not been reached within 1 h as Borucki and colleagues(Reference Borucki, Aronica and Starke59) have indeed shown that 2-h and 3-h postprandial FMD, but not 1-h FMD, were improved after supplementation with l-arginine.
Overall, cardiometabolic risk markers were not affected. A possible explanation could be that muscle glucose uptake and insulin sensitivity are improved, while fasting circulating concentrations remain unaffected or that the study duration (i.e. 7–16 d) was not long enough to exert long-term effects on fasting circulating glucose concentrations. However, we did observe a postprandial decrease in plasma glucose of 0·6 mmol/l after watermelon consumption. One mechanism by which watermelon may affect glucose concentrations is by increasing plasma l-arginine concentrations, which may increase NO bioavailability(Reference Allerton, Proctor and Stephens8) thereby affecting muscle glucose uptake(Reference Stamler and Meissner60). We indeed observed that the consumption of watermelon increased both plasma l-citrulline and l-arginine concentrations during the postprandial phase, which may in turn affect glucose and insulin metabolism via an increased NO bioavailability. However, no changes in longer-term glucose and insulin concentrations were observed.
l-Citrulline, either via supplementation or watermelon intake(Reference Assefa, Hur and Ro61), is not metabolised by intestinal and hepatic arginases, and released as l-arginine into the systemic circulation after conversion by the kidneys(Reference van de Poll, Siroen and van Leeuwen14). This may account for the observation that dietary l-citrulline increases l-arginine and NO bioavailability more efficient than intake of l-arginine itself(Reference Moinard, Nicolis and Neveux10–Reference Figueroa, Wong and Jaime12). We did observe in this meta-analysis that intake of l-citrulline and watermelon increased postprandial as well as longer-term l-arginine concentrations. However, no effects on plasma nitrate and nitrite concentrations were observed, which may be used as a marker for NO bioavailability. A better approach to assess NO bioavailability is to assess substrates and endogenous inhibitors of endothelial NO synthase, the enzyme responsible for NO production, such as l-arginine, homo-arginine and asymmetric dimethylarginine concentrations(Reference Forstermann and Sessa62,Reference Winkler, Kluge and Holzmann63) . Therefore, the observed absence of effects on plasma nitrate and nitrite concentrations should be interpreted with caution as they do not necessarily represent an absence of effect on NO bioavailability.
This is the first meta-analysis to compare the effects of watermelon consumption and l-citrulline supplementation on longer-term and postprandial vascular function and cardiometabolic risk markers. Our results are derived from studies with low risk of bias providing moderate-to-high quality of evidence. The protocol was however not pre-registered to PROSPERO and our systematic literature search did not include a search for grey literature. However, our meta-analysis did follow the AMSTAR 2.0 tool and Cochrane handbook(Reference Sterne, Savovic and Page21,Reference Shea, Reeves and Wells64) . Another potential limitation is the small number of longer-term studies, while only a limited number of postprandial studies was included. Subgroup analyses could therefore not be performed to investigate if observed results were depending on subject or treatment characteristics. More well-designed trials are thus still needed to further explore health effects and also the safety of watermelon consumption and l-citrulline supplementation. Unfortunately, the studies included did not report plasma l-citrulline concentrations after supplementation. Moreover, it was not mentioned which part of the watermelon was used, which can be important as differences exist in l-citrulline concentrations between rind and flesh. However, most studies did analyse the amount of l-citrulline in the study product. Finally, certain medications may affect l-citrulline metabolism. Furhter investigation is thus still needed to study whether the use of medication affects potential benefits of l-citrulline on CVD risk(Reference Irving, Carter and Soop65).
Conclusion
In conclusion, this meta-analysis provided evidence that longer-term l-citrulline and watermelon consumption may improve vascular function in adults, suggesting a potential mechanism by which an increased l-citrulline intake may beneficially affect cardiovascular health outcomes. No effects on postprandial vascular function markers were found. Longer-term effects on cardiometabolic risk markers were not observed, but more research is still needed to investigate the effects of l-citrulline and watermelon on cardiometabolic health.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
E. S. designed the study, performed the literature search, data extraction and statistical analyses, interpreted the data, and wrote the manuscript; R. P. M. designed the study, interpreted the data and wrote the manuscript; P. J. J. designed the study, performed the literature search, interpreted the data, wrote the manuscript and had overall responsibility.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521004803














