The high prevalence of elevated blood pressure (BP) is a major threat for human health, particularly its detrimental effects on the vasculature. This is a serious problem, as the number of subjects with high BP is rapidly increasing mainly due to the increasing prevalence of obesity, sedentary lifestyles and unhealthy dietary habits( Reference Singh, Suh and Singh 1 , Reference Yusuf, Reddy and Ounpuu 2 ). Altogether, high BP is estimated to cause 7·5 million deaths per year (12·8 % of all deaths) and 57 million disability-adjusted life years or 3·7 % of total disability-adjusted life years worldwide( 3 ). Unfortunately, most people are not aware of the fact that they have elevated BP, which is a major but modifiable risk factor for CVD, which means that lives and costs can be saved. Health authorities recommend adoption of a healthy diet and lifestyle as the primary treatment to lower BP( Reference Whitworth 4 , Reference Mancia, De Backer and Dominiczak 5 ). Any food component that lowers BP contributes to the prevention of CVD. A considerable number of nutrients such as K, PUFA and protein have been studied for their ability to reduce BP( Reference Savica, Bellinghieri and Kopple 6 ). Also, protein hydrolysates, which are composed of a mixture of peptides of different chain lengths and free amino acids, have received a lot of attention lately( Reference Meisel, Bernhard and Fairweather-Tait 7 ).
NWT-03 is a novel protein hydrolysate derived from the enzymatic digestion of the protein lysozyme (isolated from hen egg whites) using the endoprotease enzyme Alcalase®. Both lysozyme and Alcalase® are widely used in the food industry. NWT-03 has been identified during screening procedures for its angiotensin-converting enzyme (ACE) activity-inhibiting peptides (unpublished results) and has recently been found to attenuate renovascular inflammation and damage and to restore aortic endothelium-dependent relaxation in Zucker diabetic fatty rats( Reference Wang, Landheer and van Gilst 8 ). Generally, ACE inhibitors have emerged as important agents in managing high BP and reducing cardiovascular risk( Reference Basile and Toth 9 , Reference Enseleit, Hurlimann and Luscher 10 ). The in vitro and animal data so far generated with NWT-03 support its potential use as a functional food ingredient aiming at reducing BP. In other words, we hypothesised that a daily intake of NWT-03 lowers systolic and diastolic BP (SBP and DBP). Therefore, in the present study, we assessed the effects of 1, 2 and 5 g doses of NWT-03 on daytime, 36-h and night-time SBP and DBP as measured with an ambulatory BP monitor (ABPM) in generally healthy male and female subjects with normal BP (SBP<130 mmHg and DBP<85 mmHg), high-normal BP (SBP 130–139 mmHg and DBP 85–89 mmHg) or mild hypertension (SBP 140–159 mmHg and DBP 90–99 mmHg).
Methods
The Clinical Research Ethics Committee of the Cork Teaching Hospitals approved the study protocol. Written informed consent was available from all patients before enrollment. The study is registered at www.clinicaltrials.gov as NCT02148198. The study was carried out by the clinical research organisation Atlantia Food Clinical Trials Ltd at University College Cork and the obtained data were transferred to Maastricht for further analysis.
Study population
The study included ninety-two healthy male and female subjects. Before entering the study, subjects had to attend a screening visit to obtain informed consent and to check inclusion and exclusion criteria. During the screening visit, BP, body weight, height and BMI were determined. For women of childbearing age, a urine sample was collected to perform a pregnancy test. In addition, medical history and general health were recorded. Inclusion criteria were as follows: aged between 35 and 75 years old; stable body weight (≤5 % change over the past 3 months); BMI between 25 and 35 kg/m2; and normal BP (SBP<130 mmHg and DBP<85 mmHg), high-normal BP (SBP 130–139 mmHg and DBP 85–89 mmHg) or mild hypertension (SBP 140–159 mmHg and DBP 90–99 mmHg). Exclusion criteria were as follows: pregnancy; lactation; hypersensitivity to the test product; acute or chronic coexisting illness (e.g. CVD, chronic kidney disease, gastrointestinal disorder, endocrinological disorder, immunological disorder and metabolic disease); diabetes mellitus type 1 and 2; treatment with diuretics, BP medication and medication interfering with the renin–angiotensin–aldosterone system (RAAS) (e.g. ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors and aldosterone receptor inhibitors); treatment with non-steroidal anti-inflammatory drugs within 2 weeks before the study and during the study; treatment with nasal decongestants and other over-the-counter or herbal preparations within 2 weeks before the study and during the study; drug and alcohol abuse (males>twenty-one units/week and females>fourteen units/week); heavy coffee intake (i.e. >4 cups/d) within 2 weeks before the study and during the study; and participation in an experimental study within 60 d before the study and during the study. To reach the ninety-two eligible subjects for inclusion, 200 subjects were screened.
Study design
The study had a randomised, placebo-controlled cross-over design and included six treatment arms as follows: 1 g NWT-03 or placebo in period 1 and placebo or 1 g NWT-03 in period 2, 2 g NTW-03 or placebo in period 1 and placebo or 2 g NWT-03 in period 2, or 5 g NTW-03 or placebo in period 1 and placebo or 5 g NTW-03 in period 2 (Fig. 1). All treatment arms were stratified for BP (30 % normal BP, 40 % high-normal BP and 30 % mild hypertension). Both treatments in each arm had an intervention duration of 7 d and were separated by a 5-d washout period. Subjects visited the study centre 48 h before the start of each treatment (day 1). During this visit, an ABPM (Schiller BR-102; HCE) was attached to the non-dominant arm to measure BP at predetermined intervals over the following 36 h, when the subject did not yet consume NWT-03 or placebo. Subjects returned to the study site after the 36-h period (day 1) to have the monitor removed. At the same visit, subjects received their first treatment dose. Subjects were instructed to take one dose each morning for the next 6 d. On day 5 of each treatment, subjects returned to the study site to have an ABPM attached to measure 36-h BP for the second time. Finally, on day 7, the ABPM was removed. After a wash-out period of 5 d, subjects crossed over to the other treatment arm. In this period of 7 d, two 36-h BP recordings were performed again according a similar set-up as in the first period. Subjects were requested to return the empty packages or any unused study product at the end of both treatments to measure compliance. Subjects were instructed to follow their habitual diets and exercise routines and not to consume medications that could interfere with the assessment of the study product for the duration of the study.

Fig. 1 Overview of the study design (a) and the flow chart (b). ABPM, ambulatory blood pressure monitor. * First dose was administered at the study centre and the remaining doses were self-administered at home.
Measurements
Office BP was measured in the non-dominant arm using a validated semi-automatic device (Omron M10-IT), while the subjects were seated. Subjects had to rest for at least 5 min before BP was measured four times, 1 min apart. The average of the last three measurements was used. Subjects were asked not to exercise 24 h before, or consume caffeine or a tobacco product at least 30 min before an office BP measurement was taken. For the 36-h BP recordings, the ABPM monitor was used. For this, BP was measured at 30-min intervals from the time the monitor was attached: that is, from 08.00 to 22.00 hours. No measurements were collected during the first night. The monitor was programmed to start taking measurements again the following morning from 08.00 to 22.00 hours, at 30-min intervals throughout the day. From 22.00 to 08.00 hours in the morning, measurements were taken at hourly intervals. Subsequently, subjects came to the university, the monitor was removed, and the data were downloaded at the study site. Subjects were requested to abstain from excessive exercise at least 24 h before the ABPM was attached and while wearing the monitor.
Analysis of the BP measurements
Effects of NWT-03 and placebo on daytime, night time and 36-h BP were calculated by subtracting the BP value before treatment from the BP value after treatment. The percentage decline in nocturnal BP was calculated according to the formula: (daytime BP−night-time BP)×100/daytime BP( Reference Ohkubo, Hozawa and Yamaguchi 11 ). The 36-h BP variability was calculated as the sd of the 36-h BP. These calculations were performed for both SBP and DBP.
Study products
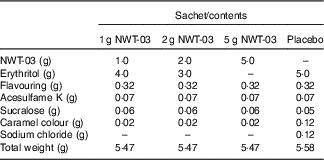
NWT-03 was provided by Newtricious R&D BV. It was produced through enzymatic hydrolysis of the lysozyme protein, using Alcalase® under alkaline conditions. After hydrolysis, the enzyme was deactivated via heat treatment, and insoluble material was removed by centrifugation and filtration. NWT-03 was dried using a spray-drying technique. Both NWT-03 and the placebo (erythritol as inert filling material) were packed in dry-powder sachets. The product formulation of the different sachets is presented in Table 1. Each sachet had to be dissolved in 200 ml of water before consumption. Sachets had to be stored at room temperature (15–25°C). Both NWT-03 and placebo had a tea-mint flavour and were identical in colour and taste. In comparison with the NWT-03 products, the placebo product contained a small amount of sodium chloride (0·12 g/d), which was too low to effect changes in BP during the study.
Table 1 Nutritional composition of the study products
Statistics
A Shapiro–Wilk test was used to evaluate whether the data were normally distributed. Independent samples t tests were used to check for carryover effects between the NWT-03 and placebo periods. Comparisons between the effects of 1, 2 and 5 g NWT-03 and placebo on daytime BP, night-time BP, 36-h BP, percentage decline in nocturnal BP and 36-h BP variability were made using paired samples t tests for all subjects combined and for the three BP categories separately. Data are expressed as mean values and standard deviations. All statistical tests were performed two-sided at 5 % level of significance using SPSS 20.0 for Mac OS X (SPSS Inc.).
It was calculated that a sample size of twenty-eight subjects per treatment group (1, 2 and 5 g NWT-03) was needed to show an effect size of 7 mmHg in daytime SBP with a power of 80 % with an sd of the response of 9 mmHg( Reference Nakamura, Mizutani and Ohki 12 , Reference Cicero, Rosticci and Veronesi 13 ) and an α of 0·05 (two-tailed).
Results
Baseline characteristics
In Table 2, characteristics of the subjects during the screening visit are presented. All subjects completed the study. All but one subject were 100 % compliant. One subject missed a single dose during their first ‘treatment’. Some minor adverse events not related to the study products were observed both during the NWT-03 and placebo period (e.g. headache, toothache, cough, bloated feeling, ear pain, dry lips, wrist pain, rash, sore throat and stomach pain). No protocol deviations were observed except for one subject taking all twenty units that were dispensed instead of the intended fourteen units of experimental products.
Table 2 Baseline characteristics of the study subjects (Mean values and standard deviations)
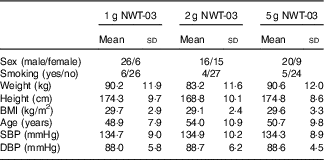
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Blood pressure
Table 3 and the online Supplementary Table S1 show the absolute values before and after treatment with NWT-03 and placebo, and the effects of NWT-03 and placebo on daytime, night-time and 36-h BP. Values are presented separately for the three different doses (1, 2 and 5 g/d) of NWT-03 and the three different BP categories (normotensive, high normotensive and mild hypertensive). There were no indications for carryover effects or non-normality of the data. A 1 g dose of NWT-03 had no effect on BP in any of the three BP groups. In mild-hypertensive subjects, 2 g NWT-03 significantly decreased daytime SBP (7·9 mmHg; P=0·006), daytime DBP (4·2 mmHg; P=0·009), 36-h SBP (6·9 mmHg; P=0·015) and 36-h DBP (3·5 mmHg; P=0·035) compared with the changes in the placebo arm. No significant effects of 2 g NWT-03 were observed in subjects with normal and high-normal BP. In mild-hypertensive subjects, 5 g NWT-03 significantly decreased night-time SBP (14·8 mmHg; P=0·008) and night-time DBP (8·4 mmHg; P=0·020) compared with the changes in the placebo arm. In subjects with normal and high-normal BP, no significant effects of 5 g NWT-03 were observed. Effects for men and women were comparable (data not shown).
Table 3 Comparisons between the changes in daytime, night-time and 36-h blood pressure after NWT-03 and placebo (Mean values and standard deviations)

SBP, systolic blood pressure; DBP, diastolic blood pressure.
* P values for the difference between the active and the placebo period are based on the paired samples t test.
Nocturnal decline in blood pressure
Table 4 shows the percentage decline in nocturnal BP before and after treatment with NWT-03 and the placebo. Again, values are presented separately for the different doses of NWT-03 and the different BP categories. In mild-hypertensive subjects, 5 g NWT-03 showed a trend to increase the percentage decline in nocturnal SBP compared with placebo (P=0·087). In normotensive and high-normotensive subjects, no significant effects of 5 g NWT-03 were observed. In normotensive subjects, 2 g NWT-03 decreased the percentage decline in nocturnal DBP compared with an increase in the placebo period (P=0·050). In high-normotensive and mild-hypertensive subjects, no significant effects of 2 g NWT-03 were observed. In addition, no significant effects of 1 g NWT-03 were observed.
Table 4 Comparisons between changes in the nocturnal decline in blood pressure after NWT-03 and placebo (Mean values and standard deviations)

SBP, systolic blood pressure; DBP, diastolic blood pressure.
* P values for the difference between the active and the placebo period are based on the paired samples t test.
Blood pressure variability
The 36-h BP variability before and after treatment with NWT-03 and the placebo are shown in Table 5. No significant effects of NWT-03 were observed, irrespective of the dose of NWT-03 or the BP category of the subjects.
Table 5 Comparisons between changes in 36-h blood pressure variability after NWT-03 and placebo (Mean values and standard deviations)

SBP, systolic blood pressure; DBP, diastolic blood pressure.
* P values for the difference between the active and the placebo period are based on the paired samples t test.
Discussion
Our results show that 2 g NWT-03 lowered daytime SBP and DBP in subjects with mild hypertension, but not in subjects with normal or high-normal BP. The identification of a sensitive subpopulation is in line with a recent meta-analysis of Fekete et al.( Reference Fekete, Givens and Lovergrove 14 ) in which subjects with elevated BP at baseline had a greater reduction in SBP and DBP after lactotripeptide supplementation. However, these effects of 2 g NWT-03 need to be confirmed as no effects were found for 1 or 5 g NWT-03 in any of the three subgroups. In addition to the effects on daytime BP, we also observed that 2 g NWT-03 lowered 36-h SBP and DBP, and 5 g NWT-03 lowered night-time SBP and DBP in subjects with mild hypertension.
The potential of NWT-03 to lower BP can be explained by its earlier observed ACE-inhibitory activity in vitro. ACE is part of the renin–angiotensin system in which it inactivates bradykinin and cleaves inactive angiotensin-I into active angiotensin-II, which in turn stimulates the production of aldosterone. Both angiotensin-II and aldosterone increase BP via vasoconstriction and an increase in blood volume, respectively( Reference Atlas 15 ). Here, we evaluated the effects of three increasing NWT-03 doses, namely 1, 2 and 5 g. The dose of 5 g was chosen because earlier studies had already reported that other hydrolysates have an optimal effect at or about this dose. For example, Ballard et al.( Reference Ballard, Bruno and Seip 16 ) used 5 g of a whey protein hydrolysate per d to observe a vascular endothelial response. In addition, Cadee et al.( Reference Cadee, Chang and Huang 17 ) used 3·8 g of a bovine casein hydrolysate per d and observed a decrease of respectively –10·7 and –6·9 mmHg in SBP and DBP compared with the baseline after a 4-week intervention period. In fact, the Food and Drug Administration has approved a claim for this protein hydrolysate, which has been called C12 Peption® and for which the targeted daily intake has been set at 4·2 g/d. The European Food Safety Authority, however, concluded that no causal relationship existed between the intake of this C12-peptide and the maintenance of normal BP. Furthermore, the dose of 5 g NWT-03 was used in a proof-of-concept study by our group to assess the short-term effects of the hydrolysate on arterial stiffness in overweight or moderately obese subjects with impaired glucose tolerance or type 2 diabetes mellitus (unpublished results). The additional doses of 1 and 2 g were chosen to assess the dose-ranging effect of NWT-03. Interestingly, but at the same time difficult to understand, is the finding that daytime and 36-h SBP and DBP significantly decreased in subjects with mild hypertension, but only in the 2 g NWT-03 group, and not in the 1 or 5 g NWT-03 groups. This is not only in contrast with studies that have suggested that protein hydrolysates exert optimal effects around the relatively high intake of 5 g, but it also implies that no dose–response relationship exists for the potential effect of NWT-03 on BP.
In the general population, SBP and DBP, on average, decrease by 10 % or more during night time compared with that during daytime, a phenomenon which is called dipping( Reference Grossman 18 ). It has been observed that subjects in whom the BP reduction during sleep is blunted have an increased risk for cardiovascular events and mortality( Reference Ohkubo, Hozawa and Yamaguchi 11 , Reference Fogari, Zoppi and Malamani 19 ). We found that 5 g NWT-03 significantly lowered night-time SBP and DBP by, respectively, 14·8 and 8·4 mmHg in subjects with mild hypertension compared with placebo (online Supplementary Table S1). Before NWT-03 consumption, SBP was approximately 5·4 % lower during night time in subjects with mild hypertension in the 5 g NWT-03 group. After NWT-03 consumption, the change towards 12·4 % lower values during the night time nearly reached statistical significance. For DBP, these values were respectively, 10·5 and 16·2 %, which were not statistically significant compared with the placebo period (Table 4). Ohkubo et al.( Reference Ohkubo, Hozawa and Yamaguchi 11 ) observed a significant inverse linear relation between nocturnal reduction in BP and cardiovascular mortality risk. The relative hazard for cardiovascular mortality was 1·64 and 2·13 in subjects with a 1–10 % nocturnal reduction in, respectively, SBP and DBP, compared with a relative hazard of 1·00 in subjects with an 11–20 % nocturnal reduction in BP. Because night-time BP is less variable than daytime BP, it is considered more closely related to cardiovascular risk( Reference Ogihara, Kikuchi and Matsuoka 20 ). However, the night-time BP values in the present study might be less reliable than daytime and 36-h values because fewer measurements of night-time BP were made compared with daytime and 36-h BP; night-time BP was measured for one night every hour (between 22.00 and 08·00 hours) and daytime BP was measured for 2 d every 30 min (from 08.00 to 22.00 hours). BP variability is linked to increased BP values and an increase in BP values is typically accompanied by an increase in BP variability( Reference Parati and Schumacher 21 ). In addition, BP variability contributes to CVD risk, independent of increased BP values( Reference Parati, Faini and Valentini 22 – Reference Johansson, Niiranen and Puukka 25 ). In contrast with what has been observed in previous studies( Reference Parati and Schumacher 21 ), we did not find a higher 36-h BP variability in subjects with a higher BP at baseline. In addition, we did not find a significant effect of NWT-03 on BP variability over the 36-h period.
A strength of our study is that we evaluated a potential dose–response relationship between NWT-03 and a variety of BP parameters. Also, differences in responsiveness between different tension groups were examined, and BP was measured for 36 h in a real-life setting. A limitation of the current study, however, is the lack of biochemical measurements to support the effects observed, particularly in the group that received 2 g/d of NWT-03. In addition, the power for the subgroup analysis may not have been adequate and the intervention duration of 7 d was relatively short. Finally, the male:female ratio was not equal between the three groups, although exploratory analyses did not suggest any sex-related effects. Conclusively, our findings need to be confirmed in future longer-term intervention trials with larger group sizes enriched with measurements of parameters reflecting the potential mechanism related to inhibiting the RAAS system.
To conclude, we here show that 2 g NWT-03 lowered daytime and 36-h BP in subjects with mild hypertension, and that 5 g NWT-03 lowered night-time BP in subjects with mild hypertension. However, as no dose–response relationship was evident, additional studies in human subjects are needed to confirm the observed effects.
Acknowledgements
The authors thank all volunteers for participating in the study, and the clinical research organisation Atlantia Food Clinical Trials Ltd at University College Cork for conducting the study.
The study was sponsored by the SME Newtricious, Oirlo, The Netherlands. ‘Newtricious’ had no role in the design, analysis or writing of this article. Atlantia Food Clinical Trial Ltd conducted the study and collected data.
J. P., N. S., S. M. and R. P. M conceived the research, designed the study, performed statistical analysis, interpreted data and wrote the manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000836









