Emerging evidence suggests that variation in blood pressure (BP) contributes to CVD( Reference Rothwell 1 – Reference Zis, Vemmos and Spengos 12 ). The method that we used in the present study to measure the rate of BP variation involves determining the slope for the change in BP between each reading over time. It provides an estimate of the rate or speed at which BP changes from reading to reading during daytime and night-time. It then provides a continuous hourly measurement of the rate of BP variation( Reference Hodgson, Croft and Woodman 13 ). A major advantage of this method is that it increases the power to detect smaller differences compared with the use of a single summary measurement such as the standard deviation. In a recently published trial, we have shown that this method is more sensitive than the standard deviation for establishing small effects on BP variation( Reference Hodgson, Croft and Woodman 13 ).
Hypertension and type 2 diabetes are associated with elevated oxidative stress( Reference Kumar and Das 14 , Reference Gopaul, Manraj and Hebe 15 ). An increased production of free radicals in the arterial wall may reduce NO bioavailability( Reference Kumar and Das 14 ) and cause endothelial dysfunction( Reference Panza, Quyyumi and Brush 16 ). Intakes of dietary antioxidants including vitamin E, vitamin C and flavonoids have been reported to be associated with less oxidative stress( Reference Davi, Ciabattoni and Consoli 17 – Reference O'Byrne, Devaraj and Grundy 19 ) and reduced CVD risk( Reference Rimm, Stampfer and Ascherio 20 – Reference Mursu, Voutilainen and Nurmi 24 ). However, the results of intervention trials have been less consistent. High-dose vitamin E supplementation may have detrimental effects on BP( Reference Ward, Wu and Clarke 25 ) and cardiovascular outcomes( Reference Miller, Pastor-Barriuso and Dalal 26 ). In contrast, vitamin C( Reference Ward, Hodgson and Croft 27 , Reference Juraschek, Guallar and Appel 28 ) and polyphenols( Reference Hodgson, Puddey and Woodman 29 , Reference Perez-Vizcaino, Duarte and Jimenez 30 ) can reduce BP, but their beneficial effects on cardiovascular events and mortality have not been established.
The trials that we carried out were primarily designed to assess the effects of vitamin E( Reference Ward, Wu and Clarke 25 ), vitamin C and polyphenols( Reference Ward, Hodgson and Croft 27 ) on BP. We found that supplementation with vitamin E resulted in significantly increased BP in individuals with type 2 diabetes( Reference Ward, Wu and Clarke 25 ). We also found that while supplementation with vitamin C alone reduced systolic BP, supplementation with vitamin C and polyphenol combination significantly increased BP( Reference Ward, Hodgson and Croft 27 ). BP data collected from these trials afforded the opportunity to explore for the first time the novel hypothesis that supplementation with dietary antioxidants reduces the rate of BP variation. The results of previous studies suggest that an increased intake of flavonoids, a class of water-soluble dietary antioxidants, can significantly reduce the rate of BP variation( Reference Hodgson, Croft and Woodman 13 , Reference Curtis, Potter and Kroon 31 ) Therefore, the primary objective of the present study was to assess the effects of vitamin E, vitamin C and polyphenols on the rate of BP variation.
Methods
Participants: vitamin E trial
Participants for this trial were recruited from the Perth general population via newspaper advertisements between February and December 2004. A total of fifty-eight men and women with a previous diagnosis of type 2 diabetes, determined via oral glucose tolerance test, or prescribed oral hypoglycaemic therapy were randomised to the trial. The trial was conducted at the University of Western Australia School of Medicine and Pharmacology located at Royal Perth Hospital in Western Australia. At least 3 weeks before study entry and throughout the trial, the participants ceased taking dietary supplements. Usual medication was taken as prescribed for the duration of the trial. Exclusion criteria included the following: BMI >35 kg/m2; use of insulin; prior or current use of vitamin E/tocopherol supplements; type 1 diabetes; previous coronary or cerebrovascular event within the past 6 months; current smoking; premenopausal condition in women; regular use of nitrate medication; use of non-steroidal anti-inflammatory medication; use of oral contraceptives; elevated serum creatinine concentrations (>110 mmol/l in men or >100 mmol/l in women); alcohol intake >40 g/d in men or >30 g/d in women. This trial was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Western Australia Human Research Ethics Committee. Written informed consent was obtained from all participants. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12605000093684).
Participants: vitamin C–polyphenol trial
Participants for this trial were recruited from the Perth general population via newspaper advertisements between February 2002 and May 2003. A total of seventy-four men and women with a previous physician diagnosis of hypertension and taking one or more antihypertensive drugs for at least 3 months were randomised to the trial. The trial was conducted at the University of Western Australia School of Medicine and Pharmacology located at Royal Perth Hospital in Western Australia. All participants had a mean 24 h ambulatory systolic BP >125 mmHg and at least one additional CVD risk factor. Additional risk factors included previous coronary or cerebrovascular event >6 months before recruitment, hyperlipidaemia (total cholesterol concentrations >6 mmol/l), use of lipid-lowering therapy, and smoking more than 5 cigarettes/d. At least 3 weeks before study entry and throughout the trial, the participants ceased taking dietary supplements. Usual medication was taken as prescribed for the duration of the trial. Exclusion criteria included the following: BMI >35 kg/m2; previous coronary or cerebrovascular event within the past 6 months; heart failure or unstable disease; premenopausal condition in women; use of nitrate medication; use of oral contraceptives; diagnosed diabetes mellitus; fasting glucose concentrations >7 mmol/l; elevated serum creatinine concentrations (>110 mmol/l in men or >100 mmol/l in women). In addition, throughout the trial, the participants were asked to limit their tea and coffee intakes to three cups/d and cease consuming all red wines and commercial fruit juices for the duration of the trial to limit background polyphenol intake. This trial was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Western Australia Human Research Ethics Committee. Written informed consent was obtained from all participants. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12613000514707).
Design: vitamin E trial
Following a 3-week washout period, the participants were allocated by the study coordinator to a study treatment arm via permuted-block randomisation, using computer-generated random numbers (generated by a biostatistician who was not involved in the conduct of the study) sealed in opaque envelopes. All study personnel and participants were blinded to treatment assignment for the duration of the study. The chief investigator held the code for the capsules in a sealed envelope, which was not broken until the end of the trial. The participants were assigned to receive (1) 500 mg/d of RRR-α-tocopherol; (2) 500 mg/d of mixed tocopherols (60 % γ-tocopherol, 25 % δ-tocopherol and 15 % α-tocopherol); or (3) 500 mg/d of placebo (soyabean oil stripped of tocopherols) for 6 weeks in a double-blind fashion. Capsules were taken twice each day with food as 250 mg of vitamin E. This dose of vitamin E was chosen to reflect that used in previous intervention studies of α-tocopherol. Outcome measures were recorded at the end of the washout (baseline) period and at the end of the 6-week intervention (post).
Design: vitamin C–polyphenol trial
Following a 3-week washout period, the participants were allocated to a study treatment arm via block randomisation, using computer-generated random numbers (generated by a biostatistician who was not involved in the conduct of the study) sealed in opaque envelopes. All study personnel and participants were blinded to treatment assignment for the duration of the study. The chief investigator held the code for the capsules in a sealed envelope, which was not broken until the end of the trial. The participants were assigned to receive (1) 500 mg/d of vitamin C and matched grape-seed polyphenol placebo; (2) 1000 mg/d of grape-seed polyphenols and matched vitamin C placebo; (3) 500 mg/d of vitamin C and 1000 mg/d of grape-seed polyphenols; or (4) matched placebo tablets for both grape-seed polyphenols and vitamin C for 6 weeks in a double-blind fashion. Tablets were taken twice daily during meal as 250 and 500 mg of vitamin C and polyphenols, respectively. The vitamin C, polyphenols and placebo tablets were visually identical. This dose of vitamin C was chosen to reflect that used in previous intervention studies( Reference Juraschek, Guallar and Appel 28 ). This dose of polyphenols was chosen to substantially increase total polyphenol intake. All tablets were supplied by Tarac Technologies. The polyphenols present in each tablet were 20·5 % polymeric compounds, with a mean degree of polymerisation of 2·7, and the rest was made up of monomers, dimers and trimers. Gallic acid was present at 0·05 wt% in each tablet( Reference Ward, Croft and Puddey 32 ). Outcome measures were recorded at the end of the washout (baseline) period and at the end of the 6-week intervention (post).
Blood pressure and its rate of variation
During both trials, BP was assessed as 24 h ambulatory BP with BP and heart rate being measured every 20 min during daytime and every 30 min during night-time( Reference Ward, Wu and Clarke 25 , Reference Ward, Hodgson and Croft 27 ). Ambulatory BP was assessed by a trained researcher who fitted a Spacelabs monitor (Spacelabs Medical, Inc.) and explained its use to the participants. The monitor was fitted to the non-dominant arm approximately 2·5 cm above the antecubital fossa. The participants were instructed to continue their usual daily activities and to avoid vigorous exercise. Measurements showing an error code or those with a pulse pressure of less than 20 mmHg were excluded from the analysis. BP traces were considered complete if more than 80 % of the recordings were valid.
The within-visit rates of variation of systolic and diastolic BP, pulse pressure and heart rate were calculated for daytime (08.00–20.00 hours) and night-time (22.00–06.00 hours) periods from the 24 h ambulatory BP traces( Reference Hodgson, Croft and Woodman 13 ). The 24 h rate of BP variation was not considered for analysis because BP usually dips overnight with sleeping and rises rapidly in the morning on waking. The periods with the steepest fall (20.00–22.00 hours) and rise (06.00–08.00 hours) in BP were excluded from the analysis. The within-visit rate of measurement-to-measurement BP and heart rate variation was calculated using the slope of the change in systolic BP, diastolic BP, pulse pressure and heart rate between each reading over time( Reference Hodgson, Croft and Woodman 13 ). Ambulatory BP variability was measured as the standard deviation of BP measurements over 24 h as the weighted 24 h standard deviation according to the method of Bilo et al. ( Reference Bilo, Giglio and Styczkiewicz 33 ). Measures of BP standard deviation provide only a single summary measurement rather than a continuous hourly measurement over 24 h as is the case with the rate of BP variation. For this reason, the power to detect differences is reduced for these measures.
Body weight, biochemistry and compliance
Body weight was recorded with the participants wearing light clothing and no footwear. Height was measured at baseline using a wall-mounted stadiometer. Fasting lipids and glucose were quantified in plasma samples using routine laboratory methods in the PathWest Laboratory at Royal Perth Hospital, Western Australia. Compliance was assessed via post-intervention capsule/tablet counts and analysis of concentrations of serum α- and γ-tocopherol, plasma vitamin C and urinary polyphenol metabolites, including 3-hydroxyphenylpropionic acid and 4-O-methylgallic acid, via HPLC and GC–MS( Reference Ward, Wu and Clarke 25 , Reference Ward, Hodgson and Croft 27 ).
Statistical analyses
Sample size for the two trials was calculated using BP as the primary outcome. The sample size calculated for each trial provided >80 % power to detect a 5 mmHg difference in systolic BP( Reference Ward, Wu and Clarke 25 , Reference Ward, Hodgson and Croft 27 ). The BP data collected and the sample size calculated for each trial also provided sufficient power to explore effects on the rate of BP variation. An effect size ≥ 15 % was considered to be potentially clinically relevant. Hypertensive subjects have a higher rate of daytime and night-time systolic BP variation (approximately 15 %)( Reference Zakopoulos, Tsivgoulis and Barlas 6 ). Using previously published data( Reference Hodgson, Croft and Woodman 13 ), it was estimated that a sample size of sixteen or more participants per group would provide at least 80 % power to detect a 4 mmHg/h difference (approximately 15 %) in the rate of daytime systolic BP variation (based on twelve hourly BP measurements, a standard deviation of 10 mmHg, and within-subject and within- and between-visit correlations of 0·2) and a 3 mmHg/h difference (approximately 15 %) in the rate of night-time systolic BP variation (based on eight hourly BP measurements, a standard deviation of 8 mmHg, and within-subject and within- and between-visit correlations of 0·3).
The primary analysis was per-protocol. This population was defined as participants who completed the intervention. Descriptive statistics are presented as means and standard deviations. Categorical variables are summarised by number in each category. A type 1 error rate of P< 0·05 was the level of significance used for hypothesis testing. Log transformation was performed on variables not normally distributed, as assessed using normal probability plots. At baseline, the characteristics of participants of each trial were compared using ANOVA with Tukey's HSD post hoc test for between-group differences.
Outcome variables were analysed using linear mixed models in STATA (StataCorp). The STATA ‘xtmixed’ and ‘margins’ commands were used to determine baseline-adjusted between-group differences at 6 weeks. All analyses were carried out by group (rather than by main effects) because we had previously observed a significant interaction between vitamin C and polyphenols that affected BP( Reference Ward, Hodgson and Croft 27 ). Subject was included as a random factor in each model with either only a random intercept or random intercept and random slope for hour according to a comparison of model fit assessed using a likelihood ratio test. Fixed effects included visit (baseline or post), treatment group, hour, and treatment group × hour interaction. The overall effect of treatment was established using the global significance test for the treatment group term, which is based on 3 df. The baseline-adjusted differences between each active treatment group and placebo group were also assessed for significance and reported individually. Individual treatment effects were considered significant if the P value was less than 0·0167 (0·05/3), in addition to a P value < 0·05 for the global test. Differences between the groups were also adjusted for potential confounding factors, which were considered as covariates in separate models.
Results
Baseline characteristics
Vitamin E trial
The vitamin E trial was completed by fifty-five of the fifty-eight participants randomised to the trial (Fig. 1). The baseline characteristics of the participants according to treatment group are given in Table 1. The mean compliance estimated using capsule counts was 97 % and did not differ between the groups. Serum α-tocopherol concentrations increased substantially following treatment with α-tocopherol and serum γ-tocopherol concentrations increased substantially following treatment with mixed tocopherols( Reference Ward, Wu and Clarke 25 , Reference Clarke, Ward and Wu 34 ). The type and dose of antihypertensive medication used remained unaltered during the trial.

Fig. 1 Vitamin E study design and flow of participants (adapted from Ward et al. ( Reference Ward, Wu and Clarke 25 )).
Table 1 Baseline characteristics of participants of the vitamin E trial according to treatment group* (Mean values and standard deviations; number of participants and percentages)
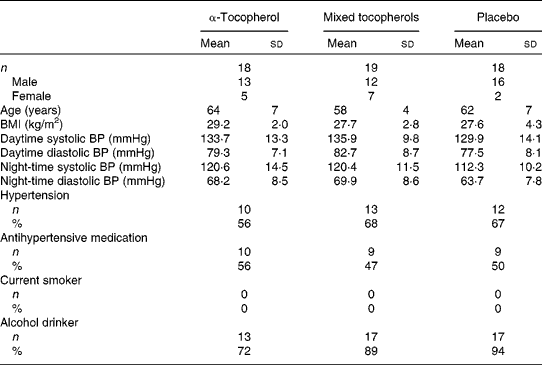
α-Tocopherol, 500 mg/d of RRR-α-tocopherol; Mixed tocopherols, 500 mg/d of mixed tocopherols (60 % γ-tocopherol, 25 % δ-tocopherol and 15 % α-tocopherol); Placebo, 500 mg/d of placebo (soyabean oil stripped of tocopherols); BP, blood pressure; Hypertension, use of antihypertensive medication or 24 h ambulatory systolic BP >125 mmHg; Current smoker, smoking more than 5 cigarettes/d; Alcohol drinker, consuming at least one standard drink of alcohol (10 g/week).
* There were no significant differences in characteristics of participants between treatment groups.
Vitamin C–polyphenol trial
The vitamin C–polyphenol trial was completed by sixty-nine of the seventy-four participants randomised to the trial (Fig. 2). The baseline characteristics of the participants according to treatment group are given in Table 2. The mean compliance estimated using tablet counts was 96 % and did not differ between the groups. Plasma vitamin C concentrations increased substantially following treatment with vitamin C and urinary excretion of a polyphenol metabolite (3-hydroxyphenylpropionic acid) increased substantially following treatment with polyphenols( Reference Ward, Hodgson and Croft 27 ). The type and dose of antihypertensive medication used remained unaltered during the trial.

Fig. 2 Vitamin C–polyphenol study design and flow of participants (adapted from Ward et al. ( Reference Ward, Hodgson and Croft 27 )).
Table 2 Baseline characteristics of participants of the vitamin C–polyphenol trial according to treatment group (Mean values and standard deviations; number of participants and percentages)

Vitamin C, 500 mg/d of vitamin C; Polyphenols, 1000 mg/d of grape-seed polyphenols; Placebo, matched vitamin C placebo or grape-seed polyphenol placebo; BP, blood pressure; Hypertension, use of antihypertensive medication or 24 h ambulatory systolic BP >125 mmHg; Current smoker, smoking more than 5 cigarettes/d; Alcohol drinker, consuming at least one standard drink of alcohol (10 g/week).
* Percentage value was significantly different between the polyphenols and the vitamin C+ polyphenols groups (P= 0·035).
Rate of blood pressure variation
Vitamin E trial
As has been reported previously, treatment with α-tocopherol or mixed tocopherols resulted in significantly higher BP (approximately 2–7 mmHg) relative to placebo in individuals with type 2 diabetes( Reference Ward, Wu and Clarke 25 ). The mean rate of daytime and night-time BP variation at baseline and at the end of the 6-week intervention (post) in each treatment group is summarised in Table 3. Compared with placebo, treatment with α-tocopherol or mixed tocopherols did not significantly alter the rate of daytime or night-time BP variation (Fig. 3). Adjustment for BP at the same time points did not alter the interpretation of the findings. The rate of heart rate variation was not significantly altered (data not presented).
Table 3 Rate of daytime and night-time blood pressure (BP) variation at baseline and at the end of the 6-week intervention (post) in the α-tocopherol, mixed tocopherol and placebo treatment groups (Mean values and standard deviations)

α-Tocopherol, 500 mg/d of RRR-α-tocopherol; Mixed tocopherols, 500 mg/d of mixed tocopherols (60 % γ-tocopherol, 25 % δ-tocopherol and 15 % α-tocopherol); Placebo, 500 mg/d of placebo (soyabean oil stripped of tocopherols); bpm, beats/min.

Fig. 3 Differences in the rate of blood pressure (BP) variation during daytime (a) and night-time (b) following treatment with α-tocopherol (α-Toc) and mixed tocopherols (Mixed toc) relative to placebo. Values are means, with their standard errors represented by vertical bars.
Compared with placebo, treatment with α-tocopherol or mixed tocopherols did not significantly alter the weighted 24 h systolic or diastolic BP standard deviation (see online supplementary Fig. S1).
Vitamin C–polyphenol trial
As has been reported previously, relative to placebo, treatment with vitamin C alone resulted in lower systolic BP (approximately 2 mmHg), but treatment with the vitamin C and polyphenol combination resulted in higher systolic and diastolic BP (approximately 3–5 mmHg) in treated hypertensive individuals( Reference Ward, Hodgson and Croft 27 ). The mean rate of daytime and night-time BP variation at baseline and at the end of the 6-week intervention (post) in each treatment group is summarised in Table 4. Compared with placebo, treatment with vitamin C alone or polyphenols alone did not significantly alter the rate of daytime or night-time BP variation. However, treatment with the vitamin C and polyphenol combination resulted in a higher rate of BP variation: the rate of night-time systolic BP (P= 0·022) and pulse pressure (P= 0·0036) variation was significantly higher and there was a borderline increase in the rate of daytime systolic BP variation (P= 0·056) (Fig. 4). Adjustment for BP did not alter the interpretation of the findings. The rate of heart rate variation was not significantly altered (data not presented). The rate of BP variation exhibited a diurnal pattern, with the nadir between 02.00 and 04.00 hours, a rapid increase between 06.00 and 08.00 hours, and a peak between 08.00 and 11.00 hours. The pattern of rate of systolic BP variation over 24 h at baseline and at the end of the 6-week intervention following treatment with placebo and the vitamin C and polyphenol combination is shown in Fig. 5.
Table 4 Rate of daytime and night-time blood pressure (BP) variation at baseline and at the end of the 6-week intervention (post) in the vitamin C, polyphenol, vitamin C+polyphenol and placebo treatment groups (Mean values and standard deviations)

Vitamin C, 500 mg/d of vitamin C; Polyphenols, 1000 mg/d of grape-seed polyphenols; Placebo, matched vitamin C placebo or grape-seed polyphenol placebo; bpm, beats/min.

Fig. 4 Differences in the rate of blood pressure (BP) variation during daytime (a) and night-time (b) following treatment with vitamin C (VC), polyphenols (Poly) and vitamin C+polyphenols (VC+Poly) relative to placebo. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from those observed following treatment with placebo (P< 0·05).

Fig. 5 Diurnal pattern of the rate of systolic blood pressure (BP) variation following treatment with placebo (![]() ) and the vitamin C and polyphenol combination (
) and the vitamin C and polyphenol combination (![]() ). Values are unadjusted (raw) means, calculated as the 3 h moving average, for each hour over 24 h according to treatment at baseline (a) and 6 weeks (post, b). The periods with the steepest fall (20.00–22.00 hours) and rise (06.00–08.00 hours) in BP were excluded from the analysis.
). Values are unadjusted (raw) means, calculated as the 3 h moving average, for each hour over 24 h according to treatment at baseline (a) and 6 weeks (post, b). The periods with the steepest fall (20.00–22.00 hours) and rise (06.00–08.00 hours) in BP were excluded from the analysis.
Compared with placebo, treatment with vitamin C alone or polyphenols alone or the vitamin C and polyphenol combination did not significantly alter the weighted 24 h systolic or diastolic BP standard deviation (see online supplementary Fig. S2).
Discussion
In the present study, two randomised, double-blind, placebo-controlled trials were conducted to investigate the effects of supplementation with major dietary antioxidants such as vitamin E, vitamin C and polyphenols on the rate of daytime and night-time ambulatory BP variation. We had previously reported that supplementation with both α-tocopherol and mixed tocopherols resulted in significantly higher BP in individuals with type 2 diabetes( Reference Ward, Wu and Clarke 25 ). Despite the substantial increases in BP, we found no evidence for an effect of either α-tocopherol or mixed tocopherols on the rate of BP variation in the present study. We had also previously reported that while supplementation with vitamin C alone reduced systolic BP, supplementation with the vitamin C and polyphenol combination resulted in significantly increased BP in the study participants with treated hypertension( Reference Ward, Hodgson and Croft 27 ). The present study did not provide any evidence for an effect of either vitamin C or polyphenols alone on the rate of BP variation or other measures of BP variability, but demonstrated that the vitamin C and polyphenol combination increased BP variability. Adjustment for BP did not alter the interpretation of the results.
High BP is a major risk factor for cardiovascular and total mortality( Reference Kearney, Whelton and Reynolds 35 ). BP level, measured in the office/clinic, home or ambulatory setting, is the primary indicator of individual risk( Reference Rothwell 1 ). However, other measures derived from the measurement of BP, including night-time BP, day-to-night BP dip, and measures of within- and between-day BP variability, may also contribute to CVD risk. All these measures are believed to be associated with CVD risk by worsening hypertensive end-organ damage. However, it is yet to be established that an intervention to alter BP variability, or other measures derived from the measurement of BP, can reduce the risk of CVD. In addition, certain classes of antihypertensive medications, which can reduce BP and CVD risk, may increase BP variation( Reference Webb, Fischer and Mehta 11 ) without apparent detrimental effects. Therefore, the importance of the measurement of BP variability, in addition to BP levels, for prediction of individual risk is not clear.
There is increasing evidence that measures of BP variation represent an independent risk factor for CVD( Reference Rothwell 3 , Reference Eguchi, Ishikawa and Hoshide 8 , Reference Muntner, Shimbo and Tonelli 9 ). In large prospective studies with event and mortality outcomes, BP variation is most often assessed as BP variability measured using the within- or between-visit standard deviation. The results of recent studies have demonstrated that the effects on both BP and BP variability determine the ultimate beneficial effects of antihypertensive medications on cardiovascular risk( Reference Ohkubo 10 ). In addition, BP variability may be as important as BP in determining hypertensive end-organ damage( Reference Rothwell 1 , Reference Parati 2 ).
The method that we used to measure BP variation involves determining the slope for the change in BP between each reading over time. It provides an estimate of the rate or speed at which BP changes between readings during daytime and night-time. This provides a continuous hourly measurement of the rate of BP variation( Reference Hodgson, Croft and Woodman 13 ), rather than a single-value within-visit standard deviation. The calculation of hourly measurements of the rate of BP variation over 24 h is a major advantage of this method because it increases the power to detect smaller differences compared with the use of a single summary measurement of variability such as the standard deviation. Furthermore, the standard deviation does not adequately capture the measurement-to-measurement variability. Highly variable reading-to-reading changes can have the same standard deviation as a gradual change over several hours. The rate of BP variation has been reported to be positively associated with hypertension, carotid atherosclerosis( Reference Zakopoulos, Tsivgoulis and Barlas 6 ) and left ventricular mass( Reference Zakopoulos, Tsivgoulis and Barlas 7 ). It has also been reported to be associated with end-organ damage in hypertensive patients( Reference Manios, Tsagalis and Tsivgoulis 5 ) and adverse outcomes in acute stroke patients( Reference Zis, Vemmos and Spengos 12 ). The rate of BP variation is yet to be linked to the risk of cardiovascular events or death. These studies are needed to establish whether the rate of BP variation is a predictor of CVD outcomes.
Hypertension is the leading risk factor for cardiovascular and total mortality. It affects one-quarter of the world's population and is projected to affect one-third of the world's population within 20 years (approximately 1·5 billion people)( Reference Kearney, Whelton and Reynolds 35 ). Changing diet and lifestyle is the initial primary means of addressing hypertension. Several diet and lifestyle factors have been proven to lower BP. These include engaging in regular moderate physical activity( Reference Whelton, Chin and Xin 36 ), maintaining a healthy body weight or weight loss( Reference Kim, Lee and Jee 37 ), limiting alcohol consumption( Reference Xin, He and Frontini 38 ), reducing salt (Na) intake( Reference He and MacGregor 39 ), and consuming a diet rich in plant foods( Reference Appel, Moore and Obarzanek 40 , Reference Beilin, Rouse and Armstrong 41 ). Intakes of the major dietary antioxidants, including vitamin E, vitamin C and polyphenols, are increased on consuming plant food-rich diets. There is evidence that these dietary constituents contribute to the lowering of BP( Reference Juraschek, Guallar and Appel 28 – Reference Perez-Vizcaino, Duarte and Jimenez 30 , Reference Egert, Boesch-Saadatmandi and Wolffram 42 ), by reducing oxidative stress( Reference Davi, Ciabattoni and Consoli 17 – Reference O'Byrne, Devaraj and Grundy 19 ), enhancing NO status and improving endothelial function( Reference Ras, Zock and Draijer 43 – Reference Ashor, Lara and Mathers 45 ). However, the benefit of supplementation with these constituents is less clear.
High-dose supplementation with vitamin E may increase BP( Reference Ward, Wu and Clarke 25 ) and CVD risk( Reference Miller, Pastor-Barriuso and Dalal 26 ). Despite a substantial increase in BP with vitamin E supplementation, we found no evidence for an effect on the rate of BP variation. We estimated that a sample size of sixteen participants per group would provide at least 80 % power to detect a 15 % difference between the groups. A post hoc power calculation based on the observed standard deviation and within-subject and between-visit correlations indicated that the study had at least 80 % power to detect a 16 % difference in the rate of daytime systolic BP variation and an 18 % difference in the rate of night-time systolic BP variation. Observed differences in the rate of daytime systolic BP variation were less than 5 % and those in the rate of night-time systolic BP variation were approximately 15 %. Therefore, the results of the present study do not rule out a smaller effect of less than 16 and 18 % in the rate of daytime and night-time systolic BP variation, respectively.
The results of the present study indicate that mechanisms involved in the regulation of BP and BP variation may differ. The differential effects of antihypertensive medications that lower BP on BP variation are consistent with this suggestion. Ca-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and β-blockers lower BP. However, while Ca-channel blockers may reduce BP variability, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and β-blockers may increase BP variability( Reference Webb, Fischer and Mehta 11 ). Several potential mechanisms responsible for these detrimental effects on BP observed in the present study were investigated previously. However, the investigations did not provide evidence for effects on mechanisms linked to elevated BP, including increased vasoconstriction, increased inflammation and increased oxidative stress( Reference Ward, Wu and Clarke 25 , Reference Clarke, Ward and Wu 34 , Reference Wu, Ward and Indrawan 46 ).
Treatment with the vitamin C and polyphenol combination resulted in higher BP( Reference Ward, Hodgson and Croft 27 ), higher rate of BP variation, and higher estimates of BP variability. The magnitudes of differences in the rate of BP variation during daytime and night-time were similar, but were significant only for systolic BP and pulse pressure during night-time. These results suggest that supplementation with the vitamin C and polyphenol combination results in detrimental effects on BP and BP variability. Several potential mechanisms responsible for these detrimental effects on BP observed in the present study were investigated previously. The investigations explored effects on markers of oxidative stress, vasoactive fatty acid metabolites and markers of inflammation. These factors were not significantly altered during the study and therefore do not provide evidence for effects on these mechanisms( Reference Ward, Hodgson and Croft 27 ). The possibility that the combination of vitamin C and grape-seed polyphenols may be interfering with the metabolism of antihypertensive drugs has not been ruled out( Reference Cowpland, Su and Murray 47 ).
Indirect evidence suggests that dietary polyphenols may contribute to the lowering of the rate of BP variation. A component of black tea solids, which are rich in polyphenols, was found to reduce the rate of systolic BP variation during night-time by up to 16 %( Reference Hodgson, Croft and Woodman 13 ), and a supplement containing polyphenols found in chocolate and soya was found to reduce the rate of pulse pressure variation during daytime by approximately 20 %( Reference Curtis, Potter and Kroon 31 ). The observed differences between the effects of polyphenols alone and those of placebo on the rate of BP variation in the present study were generally less than 5 %. Post hoc analysis of power based on the observed standard deviation and within-subject and between-visit correlations indicated that the study had at least 80 % power to detect a 15 % difference in the rate of daytime systolic BP variation and a 19 % difference in the rate of night-time systolic BP variation. Therefore, we cannot rule out smaller effects of polyphenols.
There is evidence that polyphenols derived from tea( Reference Hodgson, Puddey and Woodman 29 , Reference Ras, Zock and Draijer 43 ), chocolate( Reference Desch, Schmidt and Kobler 48 , Reference Egan, Laken and Donovan 49 ) and soya( Reference Beavers, Beavers and Miller 50 , Reference Taku, Lin and Cai 51 ) can enhance endothelial function and lower BP. Although the present study does not support a role for the polyphenols in the lowering of BP variation, the structure of the polyphenols may influence bioactivity. Grape-seed polyphenols are primarily polymeric proanthocyanidins that are metabolised to smaller-molecular-weight phenolic acids, with unknown bioactivity, in the large intestine( Reference Ward, Croft and Puddey 32 ). There is stronger evidence that the monomeric flavonoids found in tea, chocolate and soya can be absorbed and have direct effects on vascular function( Reference Hodgson and Croft 52 ) by enhancing NO status( Reference Loke, Hodgson and Proudfoot 44 ).
In conclusion, vitamin E, vitamin C and polyphenols did not significantly alter the rate of daytime or night-time BP variation. However, treatment with the vitamin C and polyphenol combination resulted in higher BP variation. Although mechanisms responsible for this are not known, the results of the present study do suggest that the combination of high doses of vitamin C and polyphenols could be detrimental to treated hypertensive individuals.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514002542
Acknowledgements
The present study was funded by the National Health and Medical Research Council of Australia (grant no. 139067 and 254568). J. M. H. and G. A. H. were supported by the National Health and Medical Research Council Fellowships. N. C. W. was supported by a Medical Research Foundation/University of Western Australia Postdoctoral Fellowship. The supplements used in the study were provided by Cognis Nutrition and Health, Cardinal Health and Tarac Technologies.
The authors’ contributions are as follows: J. M. H., K. D. C., I. B. P., J. H. Y. W., L. J. B. and N. C. W. designed the study; J. M. H., K. D. C., I. B. P., C. P. B., J. H. Y. W., L. J. B. and N. C. W. carried out the study; J. M. H., R. J. W., C. P. B., E. V. L., G. A. H. and N. C. W. analysed the data; J. M. H. and R. J. W. conducted the statistical analyses; J. M. H., K. D. C., R. J. W., I. B. P., C. P. B., J. H. Y. W., L. J. B., E. V. L., G. A. H. and N. C. W. wrote the manuscript. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.











