Obesity has become a topic of growing concern given it is associated with various morbid conditions, such as diabetes, hypertension, CVD, dyslipidaemias and atherosclerosis(Reference Upadhyay, Farr and Perakakis1). The aetiology of obesity consists of multifactorial interrelationships that result in accumulation of body fat(Reference Despres and Lemieux2). Obesity is considered a metabolic disorder due to the energy imbalance between high energy intake and low energy expenditure(Reference Mokdad, Ford and Bowman3).
Studies have shown that obesity has an increased inflammatory state and adipocyte hypertrophy is followed by infiltration of macrophages and increased inflammation with production of proinflammatory adipokines, such as TNFα, IL-1β and IL-6(Reference Kintscher, Hartge and Hess4–Reference Alomar, Zaibi and Kepczynska6). Studies performed in humans and rodent obesity models showed a strong correlation between adiposity and markers of systemic oxidative stress(Reference Esposito, Ciotola and Schisano7). Moreover, the mechanisms for these changes seem to be related, in part, to the excessive formation of reactive oxygen species (ROS) and reactive nitrogen species(Reference Sies8). Studies have shown that oxidative stress and lipid dysregulation in obese individuals can result in elevated levels of ROS, which in turn can cause oxidative DNA damage directly or via the formation of reactive lipid peroxidation by-products(Reference Kompella and Vasquez9).
Clinical studies have established correlations between biomarkers and end products of oxidative stress (lipid peroxidation and protein carbonylation) with BMI, supporting this obesity-induced oxidative stress hypothesis in humans(Reference Vincent and Taylor10,Reference Sankhla, Sharma and Mathur11) . Possible contributors to increased ROS in obese individuals include hyperglycaemia(Reference Aronson and Rayfield12), high accumulation of ectopic fat(Reference Beltowski, Wojcicka and Gorny13), vitamin and mineral deficiencies(Reference Ortega, Rodriguez-Rodriguez and Aparicio14), chronic inflammation(Reference Fernandez-Sanchez, Madrigal-Santillan and Bautista15), hyperleptinaemia(Reference Bouloumie, Marumo and Lafontan16), impairment of mitochondrial function(Reference Pennathur and Heinecke17) and enhancement of the NADPH oxidase pathway(Reference DeVallance, Li and Jurczak18). Exogenous and endogenous antioxidant compounds, such as superoxide dismutase (SOD), glutathione peroxidase and catalase (CAT), are in an inverse relationship between body fat and visceral obesity, which induces cell damage in obese individuals(Reference Chrysohoou, Panagiotakos and Pitsavos19). Endogenous antioxidant activity can be reduced by obesity as well as increased systemic oxidative stress(Reference Savini, Catani and Evangelista20).
Modulating oxidative stress and inflammation is a challenge and represents a significant opportunity for a new therapy target to prevent and treat obesity and its co-morbidities. Phytotherapics identified from traditional medicinal plants represent an opportunity for the development of new therapeutic classes and bioactive compounds(Reference Burke, Karlstad and Conley21), as ginger can act positively in the restoration of metabolic balance in obese individuals(Reference Ebrahimzadeh Attari, Malek Mahdavi and Javadivala22). Ginger (Zingiber officinale (ZO) Roscoe) is one of the most widely used spices in the world, belonging to the Zingiberaceae family(Reference Palatty, Haniadka and Valder23). The chemical constituents of ginger can be volatile or non-volatile(Reference Govindarajan24). While volatile components consist mainly of various terpenoids, the non-volatile compounds include the gingerols, shogaols and zingerone(Reference Butt and Sultan25). It has been suggested that in vivo supplementation with ZO exhibits significant anti-inflammatory and antioxidant properties(Reference Isa, Miyakawa and Yanagisawa26). Thus, the present study aims to evaluate in obese animals whether ZO extract may result in an improved systemic metabolic and genotoxic profile.
Methods
Characterisation of animals and diet
Swiss male mice provided by UNESC facilities, aged 2 months old, were housed five per cage with water and food ad libitum. The animals were kept on a 12 h light–12 h dark cycle and maintained at 20 (sd 2)°C. The study was conducted in accordance with the British Journal of Nutrition policy for experimental and clinical studies(Reference Kilkenny, Browne and Cuthill27) and with the Brazilian Guidelines for the Care and Use of Animals for Scientific and Didactic Purposes (DOU 27/5/13; MCTI, p. 7). Animals were randomly divided into two groups: lean control (CNT) rats (n 20), fed on standard rodent chow (53·0 % carbohydrates, 22·0 % proteins, 4 % lipids – relative to energy content, corresponding to approximately 12·13 kJ/g – Puro Lab 22PB), and obese rats (n 20), fed on a high-fat diet (26 % carbohydrates, 14·9 % proteins, 59 % lipids (oil and lard) relative to energy content, corresponding to approximately 20·92 kJ/g – PragSoluções Serviços e Comércio Ltda) (Table 1) for 4 months (n 20). The sample size was based on Avila et al.(Reference Avila, Marques and Luciano28). No animals or data were excluded. After 16 weeks, lean and obese mice were subdivided into: (i) lean CNT mice non-supplemented (CNT + vehicle) (n 10); (ii) lean CNT mice supplemented with ZO (CNT + ZO) (n 10); (iii) obese mice non-supplemented (diet-induced obesity (DIO) + vehicle) (n 10) and (iv) obese mice supplemented with ZO (DIO + ZO) (n 10). The study protocol was approved by the Ethics Committee of the Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil (no. 059/2017-1) and conducted by trained researchers.
Table 1. Diet composition

DIO, diet-induced obesity; CNT, control.
* To convert kcal to kJ, multiply by 4·184.
Zingiber officinale supplementation
Dry extract of ZO was acquired from Essential Nutrition®, presenting 5·2 % gingerols (6-gingerdiol, 6-gingerol, 8-gingerol, 6-shagaol, 10-gingerol). The extract was dissolved in drinking water and supplemented at a dose of 400 mg/kg per d by oral administration for thirty-five consecutive days; this dose is safe for chronic treatment as showed by Nammi et al. In addition, non-supplemented groups (DIO + vehicle and CNT + vehicle) were supplemented daily with potable water via oral administration for 35 d. The size of the cannula was sufficient to reach the oropharynx. During the supplementation period, both groups continued to receive their respective diets.
Intraperitoneal insulin tolerance test and adiposity index
Insulin tolerance tests were performed after 16 weeks of DIO to characterise the model and again 35 d after ZO treatment. Food was withdrawn 6 h before the test, and the first blood collection was equivalent to the zero time of the test. After that, insulin (1 U/kg body weight) was injected intraperitoneally and blood samples were collected from the tail at 0, 5, 10, 15, 20, 25 and 30 min to determine serum glucose by glycosometer.
Adiposity index
After the period of DIO and supplementation with ZO, the animals were euthanised and the epididymal, mesenteric, retroperitoneal and perirenal adipose tissues were extracted for the calculation of the adiposity index (fat/g body weight × 100). In addition, other tissues, such as liver, epididymal adipose tissue and quadriceps were dissected. The samples were processed, aliquoted and stored on ice for further biochemical and molecular analyses.
Histological evaluation
Fragments of hepatic tissue were placed in paraformaldehyde buffered at 4 % for 48 h. The samples were then processed with alcohol at different concentrations (70, 80, 95 and 100 %) and xylol. Subsequently, the tissue was embedded in paraffin blocks and cut into 5 μm sections for further histological analysis. The slides were stained with haematoxylin–eosin to assess tissue histoarchitecture. The images were acquired by optical microscopy (Nikon Eclipse Ti-U).
Serum TAG
TAG (Life Biotechnology) were determined in serum by Trinder enzymatic colorimetric assay according to the manufacturer’s specifications.
Dosage of cytokines
The IL-1β and TNF-α cytokines were measured in the quadriceps, adipose tissue and liver using ELISA, according to the manufacturer’s specifications (Thermo Fisher Scientific).
Species reactivity with difluorescein diacetate
Difluorescein diacetate (DCFH) was measured in the quadriceps, adipose tissue and liver. Reactive species levels were measured based on the oxidation of a 2′,7′-dichlorodihydrofluorescein acetate probe into a 2′,7′-dichlorodihydrofluorescein fluorescent compound as previously described(Reference Hempel, Buettner and O’Malley29). Briefly, samples were incubated with 80 mm 2′,7′-dichlorodihydrofluorescein-diacetate. The production of reactive species was quantified using a standard curve of 2′,7′-dichlorodihydrofluorescein, and the data were expressed as mol 2′,7′-dichlorodihydrofluorescein/mg protein.
Nitric oxide formation indicator
The nitrite levels were measured in the quadriceps, adipose tissue and liver. The production of nitric oxide (NO) was evaluated through stable nitrite dioxide (NO2). The nitrite content was calculated from the standard curve of sodium nitrite (NaNO2) – 0–200 nm. The results were expressed in μmol nitrite/mg protein(Reference Chae, Lee and Kim30).
Superoxide dismutase
SOD activity was measured in the quadriceps, adipose tissue and liver. The activity of the SOD enzyme was estimated by inhibiting the auto-oxidation of adrenaline and reading at a wavelength of 480 nm(Reference McCord and Fridovich31).
GSH/GSSG
The glutathione system was measured in the quadriceps, adipose tissue and liver. GSH levels were measured from the GSH reaction with DTNB (Ellman’s reagent) forming a later-oxidised glutathione-TNB adduct reduced by glutathione reductase in the presence of NADPH with consequent synthesis of GSH. Total GSH concentration was determined using a regression curve generated from various GSH standards. GSSG was measured from the recycling of GSSG reductase with NADPH monitoring spectrophotometrically in the presence of 2-vinylpyridine. The concentration of total GSH and GSSG was determined using a regression curve generated from various GSH standards(Reference Hissin and Hilf32).
Catalase activity
The activity of CAT was measured in the quadriceps, adipose tissue and liver. It was determined by the decay rate of hydrogen peroxide, analysed in a spectrophotometer at 240 nm, as previously described by Aebi. The results were expressed as U/mg protein(Reference Aebi33).
Carbonylation of protein
The oxidative damage in proteins was measured in the quadriceps, adipose tissue and liver using the determination of carbonyl groups based on the reaction with dinitrophenylhydrazine, as previously described by Levine. The carbonyl content was determined spectrophotometrically at 370 nm using the 22 000 M coefficient(Reference Levine, Garland and Oliver34).
Formation of malondialdehyde
The oxidative damages in lipids were measured in the quadriceps, adipose tissue and liver. As an index of lipoperoxidation, malondialdehyde formation measured by HPLC (Prominence, Shimadzu, JP) was then quantified on an Ascentis® C18 (250 × 2·1 mm, 5 μm, Supelco Sigma-Aldrich) column(Reference Grotto, Santa Maria and Boeira35).
Alkaline kite test
The comet assay (EC) was used to analyse genomic lesions such as single- and double-stranded DNA breaks, alkali labile sites and specific oxidative lesions that are amenable to repair(Reference Tice, Agurell and Anderson36).
Statistical analysis
The results were expressed as means and standard deviations. The obtained data were tested for normality (Shapiro–Wilk test) and equality of variance (Levene’s test) and analysed statistically by one-way ANOVA test. This was followed by post hoc analysis using Tukey’s test. In order to evaluate the effect of diet or ZO alone, a Student’s t test was used within the same diet group using GraphPad Prism 6.0 software. Differences between groups were considered significant when P < 0·05.
Results
Body composition, insulin sensitivity and serum TAG
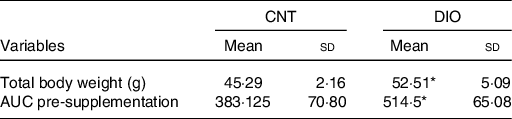
The DIO increased body weight and insulin resistance compared with control animals before ZO supplementation (Table 2).
Table 2. Effects of diet-induced obesity (DIO) before Zingiber officinale supplementation on body composition and insulin sensitivity†
(Mean values and standard deviations)
CNT, control.
* P < 0·05 v. CNT + vehicle.
† Averages obtained from serum samples from six animals per group. To evaluate the effect of diet alone, Student’s t test was used within the same diet group.
Supplementation with 400 mg/d of ZO extract improved the insulin sensitivity of the DIO + ZO group compared with the DIO + vehicle group (Fig. 1(a)). The body weight and adiposity index were higher in DIO + vehicle and DIO + ZO groups compared with CNT + vehicle and CNT + ZO animals (Fig. 1(b) and (c), respectively). Supplementation with ZO reduced the serum TAG levels of the DIO + ZO group compared with the CNT + ZO and DIO + vehicle groups (Fig. 1(d)).

Fig. 1. Body composition, insulin sensitivity and serum TAG. (a) AUC after supplementation; (b) total body weight; (c) adiposity index; (d) TAG. One-way ANOVA followed by Tukey’s post hoc was used. Data are expressed as means and standard deviations. † P < 0·05 v. control (CNT) + vehicle; ‡ P < 0·05 v. CNT + Zingiber officinale (ZO); * P < 0·05 v. diet-induced obesity (DIO) + vehicle (n 5–8 animals per group). ![]() , Vehicle;
, Vehicle; ![]() , ZO. § To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
, ZO. § To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
Inflammatory parameters, production of oxidants, antioxidant defence system and oxidative damage of the adipose tissue
The TNF-α and IL-1β concentrations were decreased in the CNT + ZO group compared with the CNT + vehicle group. In addition, TNF-α and IL-1β levels were increased in the DIO + ZO group compared with the CNT + ZO group (Fig. 2(a) and (b), respectively).
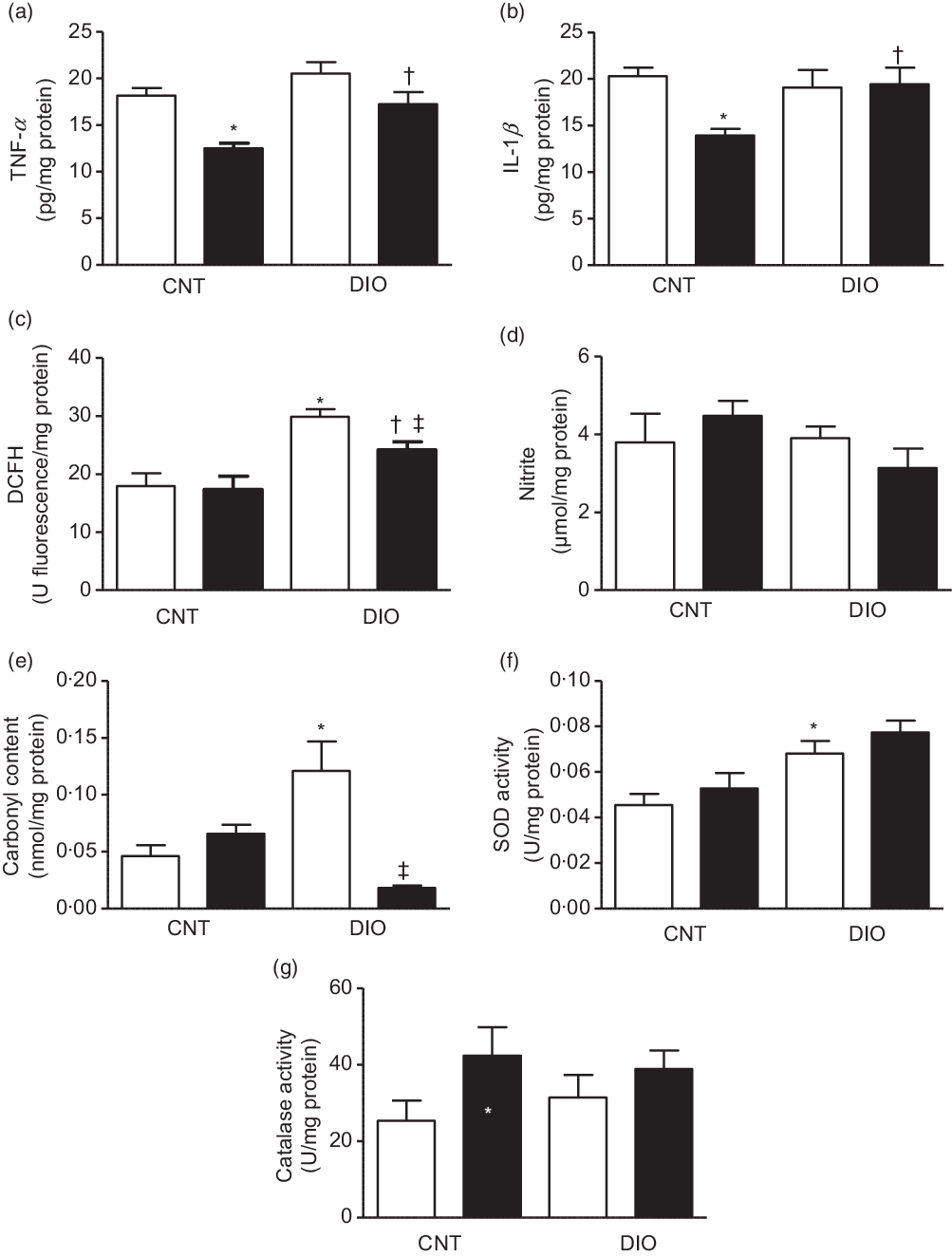
Fig. 2. Production of oxidants, antioxidant defence system and oxidative damage on the tissue adipose epididymal. (a) TNF-α; (b) IL-1β; (c) difluorescein diacetate (DCFH); (d) nitrite; (e) carbonyl content; (f) superoxide dismutase (SOD) activity and (g) catalase activity. One-way ANOVA followed by Tukey’s post hoc was used. Data are expressed as means and standard deviations (n 6–9 animals per group). * P < 0·05 v. control (CNT) + vehicle; † P < 0·05 v. CNT + Zingiber officinale (ZO); ‡ P < 0·05 v. diet-induced obesity (DIO) + vehicle. ![]() , Vehicle;
, Vehicle; ![]() , ZO.
, ZO.
The oxidative damage was evaluated by ROS and NO metabolites. The oxidation of DCFH was increased in the DIO + vehicle group when compared with the CNT + vehicle and DIO + ZO groups. Moreover, the DIO + ZO group exhibited higher oxidation of DCFH than did the CNT + ZO group (Fig. 2(c)). Nitrite levels did not change at all (Fig. 2(d)). A protein oxidation marker was analysed by carbonyl content increase in the DIO + vehicle animals compared with the CNT + vehicle group (Fig. 2(e)). SOD activity was higher in the DIO + vehicle group than in the CNT + vehicle group (Fig. 2(f)). Regarding CAT activity, there were no differences between groups (Fig. 2(g)).
Production of oxidants, antioxidant defence system, oxidative damage and morphology of the hepatic tissue
In hepatic tissue, the levels of proinflammatory cytokines TNF-α and IL-1β were decreased in DIO + ZO animals compared with DIO + vehicle and CNT + ZO animals (Fig. 3(a) and (b)). The levels of oxidation of DCFH and the nitrite levels were increased in the DIO + vehicle group compared with CNT + vehicle. Supplementation with ZO decreased DCFH and nitrite levels in the CNT + ZO and DIO + ZO groups compared with the CNT + vehicle and DIO + vehicle groups (Fig. 3(c) and (d)). The malondialdehyde levels were increased in the DIO + vehicle group compared with the CNT + vehicle and DIO + ZO groups (Fig. 3(e)). The groups DIO + vehicle and DIO + ZO presented greater carbonyl content in relation to the CNT + vehicle and CNT + ZO groups. The carbonyl groups were decreased in the DIO + ZO group compared with the DIO + vehicle group (Fig. 3(f)). SOD activity was increased in the DIO + vehicle group compared with the CNT + vehicle group (Fig. 3(g)). No statistically significant differences were observed in CAT activity (Fig. 3(h)). The GSH:GSSG ratio was bigger in the DIO + ZO group compared with the DIO + vehicle group (Fig. 3(i)).
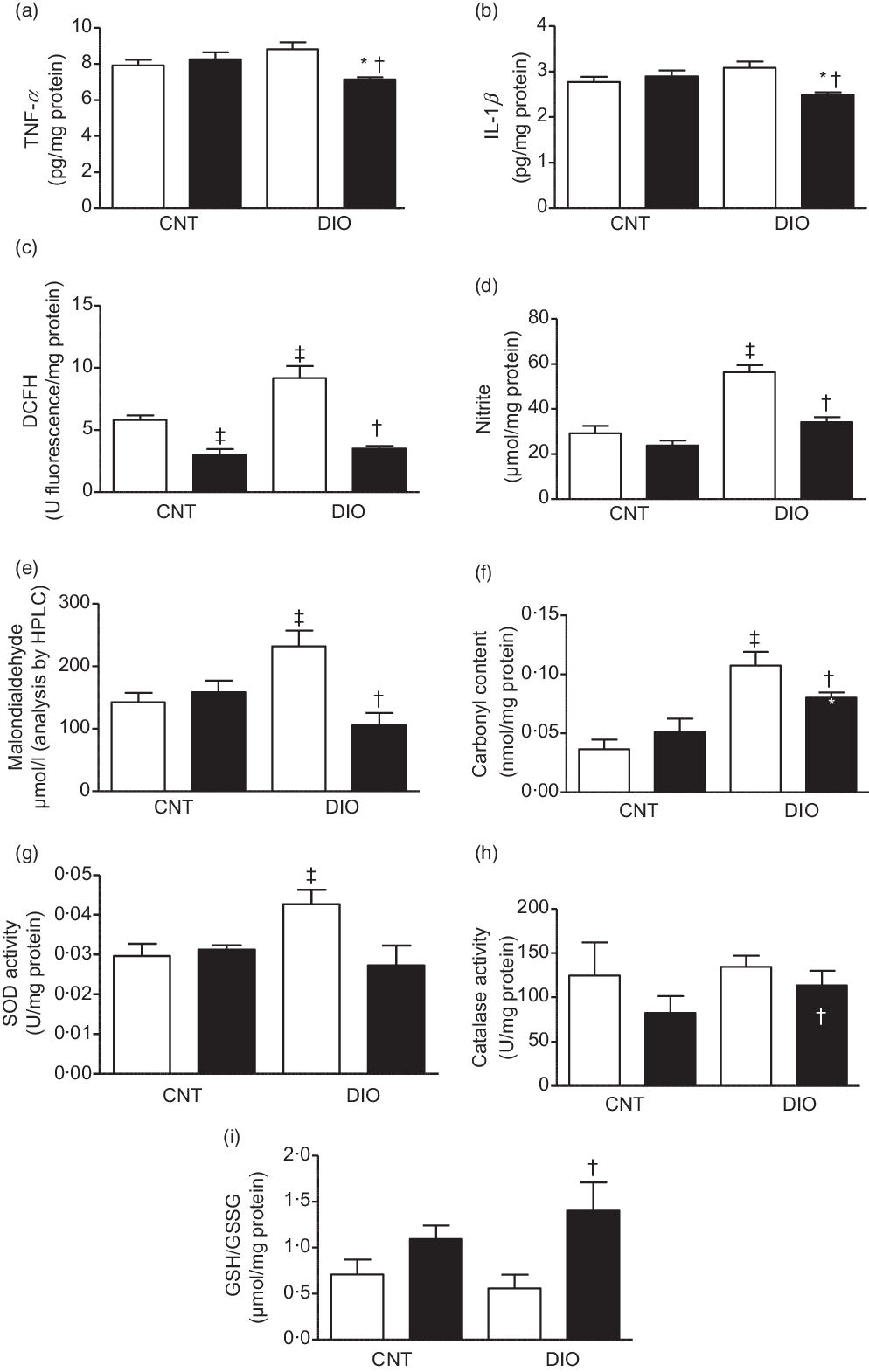
Fig. 3. Production of oxidants, antioxidant defence system and oxidative damage on the hepatic tissue. (a) TNF-α; (b) IL-1β; (c) difluorescein diacetate (DCFH); (d) nitrite; (e) malondialdehyde; (f) carbonyl content; (g) superoxide dismutase (SOD) activity; (h) catalase activity and (i) GSH/GSSG. One-way ANOVA followed by Tukey’s post hoc was used. Data are expressed as means and standard deviations (n 6–9 animals per group). ‡ P < 0·05 v. control (CNT) + vehicle; * P < 0·05 v. CNT + Zingiber officinale (ZO); † P < 0·05 v. diet-induced obesity (DIO) + vehicle. ![]() , Vehicle;
, Vehicle; ![]() , ZO.
, ZO.
A normal architecture was observed in the histology of hepatic tissue in the CNT + vehicle, CNT + ZO and DIO + ZO groups. In contrast, when animals become obese, there is lipid droplet formation indicative of hepatic steatosis (Fig. 4(a)–(d)).

Fig. 4. Histological photomicrographs of hepatic tissue stained with haematoxylin–eosin from obese mice induced by diet treated with Zingiber officinale (ZO). (a) Control (CNT) + vehicle group: histological images compatible with normal hepatic tissue. Presence of hepatocytes in polyhedral formations with strongly coloured nuclei, oriented radially and separated by connective tissue (black arrow). (b) CNT + ZO group: areas compatible with normal hepatic tissue, maintaining histoarchitecture and liver cells of normal morphology, including areas separated by connective tissue and blood vessels (black arrow). (c) Diet-induced obesity (DIO): images showing a large presence of cell infiltrates with adipocyte morphology, interwoven with hepatic tissue (black arrow), suggesting accumulation of fat cells in the liver, compatible with hepatic steatosis in a generalised way. (d) DIO + ZO: images showing decreased adipocytes infiltrated in hepatic tissue (black arrow), and areas with liver cells of normal appearance. All images were acquired under optical microscope in 20× objective (n 3 per group).
Production of oxidants, antioxidant defence system and oxidative damage of the quadriceps tissue
The levels of TNF-α and IL-1β were increased in the DIO + vehicle group compared with other groups (Fig. 5(a) and (b)). The DCFH levels were increased in the DIO + vehicle animals compared with the other groups (Fig. 5(c)). The nitrite content was higher in the DIO + vehicle and DIO + ZO groups compared with the CNT + vehicle and CNT + ZO groups (Fig. 5(d)). The malondialdehyde levels showed no significant difference between the experimental groups (Fig. 5(e)). The carbonyl levels were increased in the DIO + vehicle group compared with the CNT + vehicle group. The DIO + ZO group had reduced carbonyl levels in relation to the DIO + ZO group (Fig. 5(f)). The DIO + ZO animals showed a decrease in SOD activity compared with the CNT + ZO group (Fig. 5(g)). CAT activity was increased in the CNT + ZO and DIO + ZO groups compared with the CNT + vehicle and DIO + ZO groups (Fig. 5(h)). The GSH:GSSG ratio did not show a significant difference between the groups (Fig. 5(i)).

Fig. 5. Production of oxidants, antioxidant defence system and oxidative damage on the quadriceps tissue. (a) TNF-α; (b) IL-1β; (c) difluorescein diacetate (DCFH); (d) nitrite; (e) malondialdehyde; (f) carbonyl content; (g) superoxide dismutase (SOD) activity; (h) catalase activity and (i) GSH/GSSG. One-way ANOVA followed by Tukey’s post hoc was used. Data are expressed as means and standard deviations (n 6–9 animals per group). * P < 0·05 v. control (CNT) + vehicle; ‡ P < 0·05 v. CNT + Zingiber officinale (ZO); † P < 0·05 v. diet-induced obesity (DIO) + vehicle. ![]() , Vehicle;
, Vehicle; ![]() , ZO.
, ZO.
Effects of Zingiber officinale supplementation on DNA damage assessed by the comet test
DIO-induced damage to the DNA was evaluated by two parameters of the comet assay, damage index and damage frequency, in both liver tissue and blood (Fig. 6(a)–(d)). When the ZO groups were examined with respect to their antigenotoxicity potential towards the DIO groups, a significant reduction in DNA damage was observed in the liver and blood (Fig. 6(a)–(d)).

Fig. 6. Effects of Zingiber officinale (ZO) supplementation on DNA damage assessed by the comet assay. (a) Damage index (liver); (b) damage frequency (liver); (c) damage index (blood) and (d) damage frequency (blood). One-way ANOVA followed by Tukey’s post hoc was used. Data are expressed as means and standard deviations (n 5–8 animals per group). * P < 0·05 v. control (CNT) + vehicle; † P < 0·05 v. diet-induced obesity (DIO) + vehicle. ![]() , Vehicle;
, Vehicle; ![]() , ZO.
, ZO.
Discussion
Despite recent advances in pharmacological treatments for obesity, adjuvant non-pharmacological therapies are needed. In this sense, natural medicinal compounds appear as an effective non-pharmacological alternative for metabolic changes associated with obesity(Reference Hassan and El-Gharib37). We showed that ZO could be useful as an adjuvant in obesity treatment since it modulates different tissues at the molecular level in DIO mice.
The exact mechanisms of the anti-obesity action of ginger are not fully known. However, increasing the activity of hormone-sensitive lipase activated by the sympathetic nervous system inducing lipolysis is one postulated effect of ZO(Reference Misawa, Hashizume and Yamamoto38,Reference Mansour, Ni and Roberts39) . The results on body weight and the epididymal fat pad agree with the DIO model(Reference Matsuzawa-Nagata, Takamura and Ando40); however, the ZO treatment groups did not show any difference in body weight or fat pads (Fig. 1). Ginger is proposed as a functional dietary agent for weight control and prevention of metabolic disorders(Reference Chrubasik, Pittler and Roufogalis41–Reference Okamoto, Irii and Tahara44); 6-gingerol supplementation reduces body weight(Reference Saravanan, Ponmurugan and Deepa45,Reference Naidu, Uddandrao and Naik46) . Moreover, supplementation with 500 mg/kg per d of ZO decreased food intake, body weight and fat accumulation(Reference Kaur and Kulkarni47), and, in a clinical trial, a dose of 2000 mg/kg per d of ginger supplementation caused a decrease in hip circumference(Reference Honarvar, Zarezadeh and Khorshidi48). Gingerone A(Reference Suk, Kwon and Lee49) and ginger essential oil(Reference Lai, Lee and Lin50) were effective in avoiding an increase in body weight when used concomitantly with the DIO experiment. The discrepancy between these results and those shown in our work could be explained by differences in the methodology of supplementation, the concentration of bio compounds and dose used. Furthermore, the animals in our study were already obese when ZO treatment began, unlike other studies cited, in which treatment with ZO was concomitant with obesity induction.
It is known that being overweight is one of the main factors in the development of insulin resistance, impaired glucose metabolism, type 2 diabetes, oxidative and nitrosative stress(Reference Engin51) and elevated inflammatory levels(Reference Lee and Lee52). In diabetic rats, ZO improved insulin sensitivity(Reference Akhani, Vishwakarma and Goyal53), as in our study, pointing to glucose metabolism as a key factor in the mechanism of ZO protection from obesity(Reference de Las Heras, Valero-Munoz and Martin-Fernandez54). It is well established that insulin resistance in peripheral tissues is closely associated with elevated levels of circulating lipids and the accumulation of tissue lipids(Reference McGarry55). The TAG levels of obese animals were decreased by ZO, a known effect of ginger(Reference Nammi, Sreemantula and Roufogalis56). Moreover, high TAG levels are associated with heart disease(Reference Spartalis, Spartalis and Tzatzaki57). The effect of ginger TAG reduction (Fig. 1) may be due to the presence of niacin, since this causes inhibition of very LDL secretion, which, in turn, reduces TAG levels and increases hepatic uptake of LDL(Reference Durrington58).
Ginger presents anti-inflammatory, antiemetic, antimutagenic and hypoglycaemic effects(Reference Wang, Ke and Bao59). Ginger extract and 6-gingerol down-regulated the TNF-α levels in the adipose tissue of DIO animals(Reference Brahma Naidu, Uddandrao and Ravindar Naik60). ZO supplementation decreased the levels of TNF-α in control and DIO mice. Moreover, in control animals, it decreased the IL-1β, suggesting that ZO has an anti-inflammatory effect on adipose tissue even without obesity (Fig. 2). Ginger and its main components, gingerols and shogaols, could inhibit synthesis of several proinflammatory cytokines, including IL-1, TNF-α and IL-8; it could also inhibit prostaglandin and leukotriene synthesis enzymes(Reference Grzanna, Lindmark and Frondoza61) and encode cytokines, chemokines and the inducible enzyme cyclo-oxygenase-2 genes(Reference Grzanna, Phan and Polotsky62).
Moreover, obesity could induce oxidative stress in adipose tissue by increased lipid peroxidation and H2O2 production, whereas expression and activity of antioxidant enzymes, such as SOD, glutathione peroxidase and CAT, were down-regulated(Reference Furukawa, Fujita and Shimabukuro63). In addition, DIO-induced oxidative stress is correlated with increased inflammatory markers and insulin resistance(Reference Matsuzawa-Nagata, Takamura and Ando40,Reference Furukawa, Fujita and Shimabukuro63) . DIO increased ROS and protein carbonylation and ZO reverted this damage, while in both DIO groups, the SOD activity was increased. These results indicated that ZO modulated the intracellular oxidative status of adipose tissue (Fig. 2). Furthermore, ginger extract, containing gingerol, the most active compound in the plant, was able to reduce adipocyte differentiation and increase uptake of insulin-sensitive glucose(Reference Tzeng, Liou and Chang64). Ginger extracts, aqueous and methanolic, induced antioxidant, antidiabetic and hypolipidaemic effects without any toxicity signal(Reference Bekkouch, Harnafi and Touiss65,Reference Tramontin, Luciano and Marques66) .
The results showed no changes in proinflammatory cytokines in the livers of the DIO mice. Although IL-6, one of the most important cytokines in obesity development(Reference Vincent and Taylor10), was not evaluated, which is one limitation of our work, the ZO supplementation decreased both cytokines in the liver, which could indicate that ZO could improve the inflammatory mechanism (Fig. 3). Oxidative stress induces non-alcoholic steatohepatitis(Reference Sumida, Niki and Naito67) and oxidative damage(Reference Amirkhizi, Siassi and Djalali68). The results showed oxidative damage to the liver without a complete compensation antioxidant mechanism in DIO mice; however, in ZO-supplemented mice, the antioxidant mechanism was improved (Fig. 3). ZO supplementation is known to increase the activity of antioxidant enzymes(Reference Sani, Belani and Sin69); moreover, gingerol decreased the oxidative stress and peroxynitrite-mediated oxidation mediated by LPS on macrophages(Reference Ippoushi, Azuma and Ito70). Ginger extract showed antioxidant effects in human chondrocyte cells in an IL-1β-induced oxidative stress(Reference Hosseinzadeh, Bahrampour Juybari and Fatemi71). Moreover, it induced the expression of several antioxidant enzymes and reduced the generation of ROS and lipid peroxidation(Reference Ansari, Bhandari and Pillai72). In stressed rat heart homogenates, ginger extract decreased the content of malondialdehyde, which was related to lipid peroxidation(Reference Akinyemi, Ademiluyi and Oboh73). Ginger and its bioactive compounds (such as 6-shogaol) exhibited antioxidant activity via the nuclear factor erythroid 2-related factor 2 signalling pathway(Reference Peng, Yao and Liu74). The results of the present study showed a decrease in lipid droplets on the liver induced by ZO in DIO animals, suggesting that the modulation of ZO on liver metabolism and mitochondria could induce reduced deposition of TAG on the liver, reducing the non-alcoholic steatohepatitis (Fig. 4).
Obesity increased the TNF-α levels in quadriceps, which contributes to diacylglycerol and protein kinase C-induced lipotoxicity(Reference Salles, Tardif and Landrier75). DIO induced an increase in cytokines and supplementation with ZO decreased the TNF-α and IL-1β levels in the quadriceps of the DIO mice (Fig. 5). The decrease in TNF-α was already shown in muscle cells of DIO animals(Reference Tzeng, Liou and Chang64). Among the anti-inflammatory effects, ZO supplementation increased antioxidant activity in quadriceps and reverted the oxidative stress induced by DIO (Fig. 5). Although the activity of SOD is high in DIO animals, the non-alteration of CAT activity prevents the neutralisation of hydrogen peroxide, which was high in DIO animals, resulting in higher levels of oxidative damage to lipids and proteins (Fig. 5). In the heart muscle, ginger protects against isoproterenol-induced oxidative damage(Reference Ansari, Bhandari and Pillai72). The carbonylation of proteins was increased by DIO and ZO supplementation reverted this effect in muscle cells (Fig. 5). Oxidative stress has been implicated in the pathogenesis of various diseases, including non-alcoholic hepatic steatosis and diabetes(Reference Sumida, Niki and Naito67). The chronic treatment of mice with a hyperglycaemic and hyperlipidaemic diet resulted in DNA damage in peripheral blood and liver (Fig. 6). Higher levels of genetic damage in obese animals studies are observed(Reference Setayesh, Nersesyan and Misik76). These results, regarding the genotoxicity, can be associated with increased levels of oxidative stress and concomitant depletion of antioxidants and provide clearer answers, that is, positive associations between excess body weight and comet formation. In this study, we evaluated the antigenotoxic activity of ZO on animals on the obesogenic diet. The antigenotoxicity effects of this extract were evaluated by comet assay in two tissue samples. Our results showed that ZO extract given in combination with the obesogenic diet led to a significant reduction of damage in the peripheral blood and liver (Fig. 6). Ginger consists of two major active components, 6-gingerol and 6-shogaol, which are essential for preventing oxidative stress and inflammation(Reference Mohd Sahardi and Makpol77). These bioactive compounds are able to reduce oxidative stress levels, and the intake of ZO extract can therefore induce antigenotoxic effects. In fact, the main evidence that suggests its antigenotoxic potential has emerged due to its antioxidant capacity. Some other studies have already underlined the important role ginger extracts can play in the protection of DNA against oxidative damage(Reference Lopez-Romero, Izquierdo-Vega and Morales-Gonzalez78).
Conclusion
ZO treatment after DIO was able to restore insulin sensitivity and oxidative markers in adipose tissue; furthermore, in the liver and muscle, ZO treatment decreased the levels of TNF-α and IL-1β, modulated the oxidative status and re-established the normal levels of lipids in the liver. It also reverted DNA damage in the blood. Although other parameters could be analysed such as other cytokines, pointing to some limitations of our work, these results in the rat model may indicate ZO as an adjuvant on obesity treatment.
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico, e Tecnológico (CNPq)-Instituto Nacional de Neurociência Translacional-INNT no. 465346/2014-6, Projeto CNPq UNIVERSAL 2018, Fundação de Amparo a Pesquisa do Estado de Santa Catarina (FAPESC)-PPSUS-2016 e Universidade do Extremo Sul Catarinense (UNESC).
Authors’ contributions were as follows: T. F. L.: conceptualisation, data curation, investigation, methodology, project administration, supervision, writing original draft; C. T. d. S.: conceptualisation, data curation, investigation, methodology, resources, writing original draft; R. A. P.: conceptualisation, data curation, formal analysis, resources, supervision; S. d. O. M.: data curation, formal analysis, investigation, methodology; G. P. L.: formal analysis, investigation, methodology; N. d. S. T.: formal analysis, investigation, methodology; P. C. L. d. S.: formal analysis, investigation, methodology, resources; V. M. d. A.: formal analysis, investigation, methodology, resources; A. P. M.: conceptualisation, data curation, investigation, methodology, project administration, supervision, writing original drafting, resources, writing the original draft, writing – review and editing.
The authors alone are responsible for the content and writing of this paper.
The authors report no conflicts of interest.