There is a growing global epidemic of type 2 diabetes mellitus (T2DM), especially in developing countries such as India( Reference Hu 1 ). Consequently, there is considerable public health and consumer interest in taking steps to reduce this risk. Continuous exposures to higher postprandial glucose (PPG) and postprandial insulin (PPI) responses are believed to be detrimental to health, contributing towards an increased risk for (pre)diabetes( 2 ). There is a wealth of literature showing that reducing PPG (by slowing rates of digestion and reducing bioavailability)( Reference Wachters-Hagedoorn, Priebe and Heimweg 3 ) has benefits for reducing the progression from pre-diabetes to T2DM( Reference Chiasson, Josse and Gomis 4 – Reference Blaak, Antoine and Benton 6 ) and the risk for CVD( Reference Van De Laar, Lucassen and Akkermans 7 , Reference Schnell, Mertes and Standl 8 ).
A lower PPI requirement may also be beneficial in the short and longer term. Consistent with this, the European Food Safety Authority has recognised that the reduction of postprandial glycaemia may be a beneficial physiological effect, but only if PPI is not disproportionally increased( 9 ).
Because of their frequent and consistent use, carbohydrate-rich staple foods are interesting candidates for reducing PPG and PPI exposures( Reference Lafiandra, Riccardi and Shewry 10 ). Wheat-based flatbreads and rice are the two most common carbohydrate-rich staple foods in Southeast Asia( Reference Henry and Kaur 11 ), making them important contributors to the daily glycaemic load. Flatbreads are usually prepared at home from a commercially made whole-wheat flour mix (‘atta’). Chickpea flour (CPF) and bran-fibre flatbreads are especially advised for subjects with T2DM for whom rice is considered less desirable because of its high glycaemic index( Reference Khawaja, Fatima and Mian 12 ). Therefore, commercially viable, efficacious routes to further reduce the PPG response to flatbreads are of interest.
Soluble viscous fibres can lower PPG( Reference Jenkins, Kendall and Axelsen 13 ) by delaying gastric emptying( Reference Marciani, Gowland and Spiller 14 , Reference Woerle, Albrecht and Linke 15 ) and inhibiting the propulsive and mixing effects in the intestine( Reference Blackburn, Redfern and Jarjis 16 , Reference Edwards, Johnson and Read 17 ). In addition, legume flours such as CPF are known to give a lower plasma glucose response than wheat flours( Reference Goni and Valentin-Gamazo 18 ). Previous research has shown that soluble viscous fibres (viz. β-glucan, psyllium and fenugreek) with or without legume flour can lower the PPG( Reference Khawaja, Fatima and Mian 12 , Reference Thondre and Henry 19 , Reference Radhika, Sumathi and Ganesan 20 ) or PPI( Reference Khawaja, Fatima and Mian 12 ) of flatbreads.
The emphasis of this study was on commercially feasible products. We( Reference Boers, MacAulay and Murray 21 ) previously selected and tested various additions of guar gum (GG), konjac or CPF to flatbreads in Caucasian subjects. In that study, the composition with 4 % GG in combination with 15 % CPF resulted in the largest absolute reduction in PPG and could therefore be considered a ‘positive control’ for future studies. However, GG is expensive, and product development work showed that it created a poor product with respect to sensory attributes (dough handling, texture, aroma). Lower amounts of GG (2 or 3 % GG) in combination with 15 % CPF and additions of 3 and 5 % (respectively) barley flour (BF) made more acceptable and affordable products( Reference Mohanan, Nediyedath and Shanmugam 22 ), and these have been used in this trial, alongside the 4 % GG and 15 % CPF. The reason for including different amounts of BF is that higher amounts of GG benefit from more BF in helping to mask the GG flavour and make a more consumer-acceptable product. The present study aims to confirm the efficacy of the combinations with 2 and 4 % GG, which were reported in the previous study (both with a significant effect), and to further extend this work to the Indian population. The level of added BF contains only small amounts of viscous fibres ((≤250 mg) of barley β-glucan) and is unlikely to influence efficacy( Reference Tosh 23 ). The control product was a market standard commercial wheat flour-based product (8 % dietary fibre), whereas the test products were based on an alternative, commercially available, high-fibre atta flour (with 5 % wheat bran included; 12 % dietary fibre). In our previous study, we did not see any effect on PPG between the market standard and high-fibre atta products( Reference Boers, MacAulay and Murray 21 ). For the present study, the higher fibre atta was deemed a more appropriate (commercially realistic) platform for further additions of fibre, and these complete formulations were then compared with the usual, ‘standard’ atta product.
The objectives of this study were to identify one or more flour compositions that give a significant reduction in PPG and PPI after consumption, relative to the market standard product. Exploratory objectives were to estimate the maximum observed glucose response (C max), the time at which the C max was reached (T max) and slope to C max and glucose and insulin concentrations at 3 h. As soluble viscous fibres and CPF are also claimed to increase satiety( Reference Wanders, van den Borne and De 24 , Reference Li, Kendall and De Souza 25 ), an additional exploratory objective was to assess possible effects on appetite-related and mood parameters. To our knowledge, this is the first study testing such commercially feasible combinations of soluble viscous fibres and legume flour in flatbreads on the combined PPG and PPI response in a Southeast Asian population.
Methods
Participants
A total of eighty-seven healthy South-Asian subjects were recruited locally for screening from an existing database of potential participants of Lambda Therapeutic Research, which executed the study. Subjects who met all the inclusion criteria and had none of the exclusion criteria were considered for participation (online Supplementary Table S1). The study was conducted according to the principles of Good Clinical Practice, the Declaration of Helsinki (2008) and according to applicable local laws and regulations concerning studies conducted on human subjects. Ethical approval was obtained from Ethical Committee-Aditya. Each participant provided written informed consent for the study.
Experimental design
This study used a double-blind, randomised, controlled, full-cross-over (within-subject) design. Treatment orders were balanced according to a Williams-type design, and a randomised schedule for allocation to treatment orders was generated with SAS software (version 9.2; SAS Institute Inc.) by a statistician not involved with subject contact or subsequent data analyses. All subjects involved in the study were blinded as to the nature of the test products. Subjects attended the initial screening day, followed by 4 d of test, at least 1 week apart. Participants were instructed to minimise changes in their habitual diet and activity during the study period. On the day prior to each test day subjects were instructed to refrain from physical activity and alcohol consumption and to consume a standardised evening meal. All participants fasted overnight (from 20.00 hours until consumption of the test product) but were allowed to drink water ad libitum. Participants were housed at the test centre the evening before the study day. Between 07.30 and 10.00 hours on each test day, subjects consumed four freshly made flatbreads (100 g flour total) with 250 ml water as breakfast, and completed this within a 15-min period at every visit at the same time and day of the week. They were allowed to drink up to 150 ml water every subsequent hour, to be consumed after venous blood drawings and self-reported appetite and mood ratings. The volume of water consumed was registered.
Test product and preparation
The three wheat flour-based test products constituted a total of 100 g (uncooked flour weight) of an existing commercial, fibre-enriched flatbread high-fibre flour (Annapurna; Hindustan Unilever Ltd), comprising whole-wheat flour plus 5 % bran and the incorporation of 15 g CPF, 2, 3 or 4 g GG and 3 or 5 g BF/100 g in combinations shown in Table 1. A ‘market standard’, Annapurna Atta (100 % wheat flour), was used as the control.
Table 1 Composition of test flatbreads+carbohydrates (carbs) and dietary fibre (g) and water (w/w %)
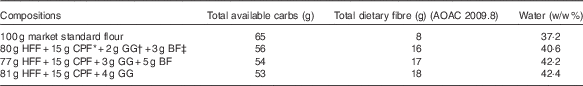
AOAC, Association of Official Analytical Chemists; HFF, high-fibre flour; CPF, chickpea flour; GG, guar gum; BF, barley flour.
* Chickpea flour (Avent Agro Pvt Ltd).
† Guar gum (Ace Gum Industries Pvt Ltd); viscosity cold 1 % in water, measured by a Brookfield RVF viscometer 20-RPM Spindle no. 4 (Brookfield Engineering Laboratories, Inc.), at 30 min: 4500 mPa s, at 2 h 5400 CPS and 24 h: 5500 mPa s.
‡ BF (Cardin Healthcare Pvt Ltd).
All experimental flour mixes were formulated at the pilot plant of Unilever R&D, Bangalore, India, and flatbreads were prepared fresh at the test site. For each single test serving, 100 g flour was kneaded to a soft and uniform consistency with the addition of approximately 77 ml water and allowed to rest for 30 min, and then divided into four equal balls of 40 g each and rolled to 2–3 mm thickness. More water was added and absorbed when fibres or legume flour was incorporated (see Table 1). The flatbreads were subsequently baked and kept warm until consumption within 30 min of cooking.
Blood collection and glucose and insulin measurements
Venous blood was collected in tubes containing sodium fluoride for plasma glucose analysis and in plain tubes (without any additive) for serum insulin samples. Baseline samples were collected at −15 min (two baseline measurements) prior to the test meals, followed by samples at 15, 30, 45, 60, 90, 120 and 180 min postprandially. Two consecutive samples were collected at each time-point for plasma glucose and serum insulin analyses. All serum samples were centrifuged (192 g for 10 min at 4°C) prior to immediate analysis or storage at −20°C. Plasma glucose concentrations were measured on VITROS® 5,1 FS, Ortho Clinical Diagnostics (Johnson & Johnson) (intra- (within-) day %CV: 0·6 % and between- (inter-) day %CV: 0·7 %). Insulin was measured using an immulite 1000® analyzer (Siemens Diagnostics) (intra- (within-) day %CV: 2·6 % and between- (inter-) day %CV: 4·3 %).
Measurement of appetite and mood
Self-ratings of appetite feelings and mood (‘are you feeling hungry?’, ‘do you desire to eat?’, ‘are you feeling energetic?’ and ‘are you feeling happy and contented?’) were made at baseline (before consumption) and 25, 40 and 135 min postprandially. The questions were asked in a language that the subjects understood. These were scored on 100 mm visual analogue scales anchored at the low and high end with ‘not at all’ and ‘extremely’( Reference Blundell, De and Hulshof 26 ).
Statistical methods
The primary outcome variable was positive incremental AUC2 h (+iAUC2 h): that is, the area of PPG response lying above the baseline concentration. A power calculation indicated that a minimum of forty-four subjects would be required to test for the significance of a 40 mmol×min/l difference in PPG+iAUC2 h for each test product relative to the control. This assumed an sd of 50 (based on a previous study in the UK( Reference Boers, MacAulay and Murray 21 ) adjusted for venous v. capillary blood), at α 0·05 and β 0·90, where α is conserved over the three comparisons with the control by Dunnett’s test. Considering the Williams design for four treatments and the experience of dropouts at the test site, twelve additional subjects were included to make the initial sample size of n 56.
The +iAUC2 h was calculated using the trapezium rule, and linear interpolation was used between time points where PPG crossed the baseline value to establish the time of crossing. Statistical comparisons were made using a mixed-model ANOVA, with subject as a random effect, product as a fixed effect and baseline (fasting score) as a covariate. The order of product testing, sex and body weight were all included as covariates. Comparisons were only made between the control and other test products, and Dunnett’s test was used to adjust the multiple comparisons to an overall significance level of 0·05. All analyses were performed with SAS version 9.2. The secondary variable was total AUC2 h (tAUC2 h) for serum insulin. Exploratory variables included mean value at 3 h for serum insulin, maximum post-meal plasma glucose concentration (C max), time when this was reached (T max), and mean value at 3 h for plasma glucose, tAUC2 h glucose and self-reported scores on the two appetite- and two mood-related measures. The AUC for appetite- and mood-rating scales were calculated using the trapezium rule and expressed as the original scale units by dividing with the length of time measured. There were no pre-planned statistical analyses of exploratory measures and therefore only descriptive statistics are presented for these.
Results
Subject baseline characteristics
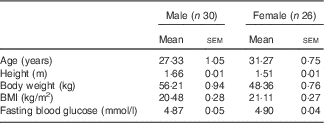
From an initial eighty-seven subjects screened for participation, fifty-six were enrolled and fifty (twenty-five male and twenty-five female) completed the study, with all dropouts occurring prior to the start or after the first test session (Fig. 1). The baseline characteristics of participants are shown in Table 2(a) and separately by sex in Table 2(b). Differences between results of the intention to treat (ITT) and per protocol (PP) analysis were small regarding both effect sizes and statistical significance. However, the PP results are shown and discussed here, as the current study was a proof-of-principle study (study of efficacy). For completeness and transparency, the ITT data are available in the online Supplementary material.

Fig. 1 Flow diagram of participants throughout the study. CPF, chickpea flour; GG, guar gum; BF, barley flour.
Table 2a Subject baseline demographic characteristics (Mean values and standard errors of the mean)

Table 2b Subject baseline demographic characteristics by sex (Mean values and standard errors of the mean)
Postprandial plasma glucose concentrations
The PPG response curves are shown in Fig. 2, percentage differences in plasma glucose +iAUC2 h v. control are shown in Fig. 3, and the absolute values and percentage difference are given in Table 3. All three test products resulted in a reduction in the postprandial +iAUC2 h, relative to the control market standard flatbread, and this difference was statistically significant for samples containing 3 and 4 % GG (both P<0·01). The data suggest a general dose–response reduction in +iAUC2 h with 2, 3 and 4 % GG relative to control; however, this was not tested statistically as no comparisons between treatments were made.

Fig. 2 Effect of flatbread consumption with different amounts of guar gum (GG) (2–4 %) and 15 % chickpea flour (CPF) on postprandial plasma glucose. Values are means and standard errors of the mean. ![]() , Market standard;
, Market standard; ![]() , high-fibre flour (HFF)+15 g CPF+2 g GG+3 g barley flour (BF);
, high-fibre flour (HFF)+15 g CPF+2 g GG+3 g barley flour (BF); ![]() , HFF+15 g CPF+3 g GG+5 g BF;
, HFF+15 g CPF+3 g GG+5 g BF; ![]() , HFF+15 g CPF+4 g GG.
, HFF+15 g CPF+4 g GG.

Fig. 3 Percentage change (mean values and standard errors of the mean) in postprandial glucose (positive incremental AUC2 h (+iAUC2 h)) of flatbreads with different amounts of guar gum (GG) (2–4 %) and 15 % chickpea flour (CPF) and P value relative to the control. HFF, high-fibre flour; BF, barley flour.
Table 3 Glucose positive incremental AUC0-2h (+iAUC0-2h) (per protocol data) (Mean absolute and percentage difference from control; mean values +/− SEM)
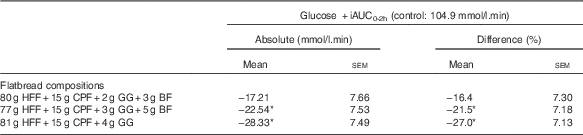
HFF, high fibre; CPF, chickpea flour; GG, guar gum; BF, barley flour.
Statistically significant: *P<0.01.
Postprandial serum insulin concentrations
Data for serum insulin (tAUC) can be found in Fig. 4 and 5 and Table 4. All three test flatbreads significantly and markedly lowered postprandial total PPI tAUC2 h compared with the control flatbread (P<0·0001 for all). In addition, the data suggest a dose–response effect, but this was not tested statistically.

Fig. 4 Effect of flatbread consumption with different amounts of guar gum (GG) (2–4 %) and 15 % chickpea flour (CPF) on postprandial serum insulin. Values are means and standard errors of the mean. ![]() , Market standard;
, Market standard; ![]() , high-fibre flour (HFF)+15 g CPF+2 g GG+3 g barley flour (BF);
, high-fibre flour (HFF)+15 g CPF+2 g GG+3 g barley flour (BF); ![]() , HFF+15 g CPF+3 g GG+5 g BF;
, HFF+15 g CPF+3 g GG+5 g BF; ![]() , HFF+15 g CPF+4 g GG.
, HFF+15 g CPF+4 g GG.

Fig. 5 Percentage change (mean values and standard errors of the mean) in postprandial insulin (total AUC2 h (tAUC2 h)) of flatbreads with different amounts of guar gum (GG) (2–4 %) and 15 % chickpea flour (CPF) and P value relative to the control. HFF, high-fibre flour; BF, barley flour.
Table 4 Insulin total AUC0-2h (tAUC0-2h) (per protocol data) (Mean absolute and percentage difference from control; mean values +/− SEM)

HFF, high fibre; CPF, chickpea flour; GG, guar gum; BF, barley flour.
Statistically significant: **P≤0.0001.
Exploratory outcomes
tAUC, C max, T max, 3 h plasma glucose data and slope to C max data are shown in the online Supplementary Tables S2(a) and (b). All treatments resulted in lower mean C max and slope to C max values compared with the control, whereas T max and plasma glucose at 3 h were in general little different from that of the control. Data for serum insulin (+iAUC) can be found in the online Supplementary Fig. S1. Serum insulin concentrations at 3 h were consistently lower for all three experimental products relative to the control (see online Supplementary Table S3). No reliable effect of the experimental treatments could be discerned for the appetite or mood measures (online Supplementary Fig. S2(a) and (b), S3(a) and (b)).
Adverse events
One adverse event (AE) was reported during the conduct of the study. A female subject vomited after consuming the product with 4 % GG and 15 % CPF on her 1st test day. This AE was resolved within 30 min without requiring any medical treatment, and the subject was excluded from the rest of the study.
Discussion
This study clearly shows that a commercially feasible, high-fibre formulation including the addition of small amounts of GG in combination with CPF lowers the PPG and PPI responses to flatbreads in an Asian population, relative to a commercial ‘market standard’ flour mix. This finding builds on the outcome of our initial exploratory study with Caucasian subjects( Reference Boers, MacAulay and Murray 21 ) in which four combinations of flour and fibre mixes decreased the +iAUC by >30 %. The composition with 4 % GG and 15 % CPF from that study was carried along as ‘positive control’, as it resulted in the largest absolute reduction in PPG( Reference Boers, MacAulay and Murray 21 ). However, this amount of GG has adverse impacts on cost, dough handling and aroma, and therefore lower levels of GG were needed. Our research is in line with reports demonstrating that other staple flatbread foods in India (chapattis, naan, rotis) containing dietary fibres and/or legume flours lower the PPG response after a meal( Reference Khawaja, Fatima and Mian 12 , Reference Thondre and Henry 19 , Reference Radhika, Sumathi and Ganesan 20 ). A novel result is the demonstrated efficacy both on PPG and PPI reduction of the combination of CPF with lower levels of GG and BF in Asians, as efficacy of this combination previously had been shown only in Caucasians and only on PPG reduction( Reference Boers, MacAulay and Murray 21 ). This suggests the potential for affordable, efficacious formulations with lower levels of GG.
Although there was a trend for a dose–response effect observed for the additions of 2, 3 and 4 % of GG alone on PPG (+iAUC2 h), the effects were statistically significant only for 3 and 4 % GG compared with the control product. It seems that addition of 2 % GG may be too low to have a consistently meaningful effect related to the effect size on PPG in this food format. Brennan( Reference Brennan 27 ) calculated that 2·5 % GG lowered the predicted glycaemic index (PGI) in bread by 4 %, whereas 5 % GG reduced the PGI by 13 %. There was also an indication of a dose–response effect on the C max. Other studies have shown the efficacy of GG (ranging from 3·8 to 14·8 g GG) incorporated into bread for lowering PPG in Caucasians, and these showed a greater reduction in PPG than the outcomes here( Reference Ekstrom, Bjorck and Ostman 28 – Reference Wolf, Wolever and Lai 31 ).
GG has been found to exhibit viscous characteristics throughout gastric and small intestinal simulation( Reference Brennan 27 ), leading to reductions in the rate of gastric emptying and starch digestion and absorption in the intestine, resulting in a lower PPG and insulin response( Reference Jenkins, Kendall and Axelsen 13 ). GG and some other fibres can even directly inhibit digestive enzymes( Reference Slaughter, Ellis and Jackson 32 , Reference Dhital, Gidley and Warren 33 ). On the product level, viscous fibres can also alter the rheological and/or microstructural properties of the food, resulting in reduced ability of the starch to gelatinise during cooking( Reference Symons and Brennan 34 ). Scanning electron microscopy has shown that GG included in bread forms a dense, continuous network in which protein, dietary fibre and starch are embedded( Reference Brennan 27 ). This dense network was found to be retained after 300 min of in vitro digestion, while the majority of the structure of a control bread (without GG) had been degraded( Reference Brennan 27 ).
It is possible that the small amounts of added BF might also have influenced the outcomes of this study. However, it appears that 4 g of processed barley β-glucan is the minimum required dosage to get a meaningful PPG-lowering effect with respect to effect size( Reference Tosh 23 ). The dosages used in this study (3 and 5 g BF containing 5 % (≤250 mg) of barley β-glucan) are therefore very unlikely to have meaningfully affected the efficacy.
It is also possible that the addition of CPF contributed to the lower PPG. Replacement of wheat flour by CPF can lower the PPG because of its higher content of resistant starch( Reference Jukanti, Gaur and Gowda 35 ) and high concentration of slowly digestible starch( Reference Nestel, Cehun and Chronopoulos 36 ). Zafar et al.( Reference Zafar, Al-Hassawi and Al-Khulaifi 37 ) showed that supplementation of whole-wheat bread with at least 35 % CPF significantly reduced the glycaemic response of whole-wheat bread, whereas Johnson et al.( Reference Johnson, Thomas and Hall 38 ) observed this in white bread.
A lower PPI requirement may also be beneficial in the short and longer term. In the short term, a lower insulin response prevents hypoglycaemia and inappropriate increases of free fatty acids and stress hormone concentrations( Reference Juntunen, Laaksonen and Autio 39 – Reference Kallio, Kolehmainen and Laaksonen 41 ), which are often seen during the late postprandial period after consumption of refined carbohydrates( Reference Ludwig 42 ). Regular consumption of diets with a low PPI response – for example, rye-pasta diets – may also benefit individuals with impaired first-phase insulin secretion (first 10–30 min) by allowing β-cell function to recover, leading to improved pancreatic β-cell function in the long term( Reference Laaksonen, Toppinen and Juntunen 43 ). Higher PPI and fasting insulin concentrations are positively associated with CVD risk factors such as blood pressure, total cholesterol and LDL-cholesterol( Reference Fang, Tian and Li 44 ). In addition, insulin resistance and postprandial hyperinsulinaemia are related to impaired arterial relaxation, which is an independent predictor of CVD( Reference Greenfield, Samaras and Chisholm 45 ).
We found that all three test flatbread flour mixes significantly and markedly reduced PPI levels relative to the control. The reason for the lower PPI response of the test flatbreads with fibre and flour mix is probably a slower glucose absorption in the blood, resulting in a reduced stimulation of the entero-insular axis, notably the incretin gastric inhibitory polypeptide (GIP), the secretion of which is directly related to the rate and site of absorption of glucose( Reference Morgan, Flatt and Marks 46 – Reference Wachters-Hagedoorn, Priebe and Heimweg 48 ). It has also been observed that GG in bread directly lowered the GIP response( Reference Gatenby, Ellis and Morgan 29 ). Khawaja et al.( Reference Khawaja, Fatima and Mian 12 ) showed that flatbread containing bran resulted in a lower PPI response than flatbread without bran (19·3 and 8·6 g dietary fibre, respectively) in healthy Asians as well as in Asians with T2DM, but our products did contain lower amounts of bran (8 and 12 g for the market standard and high fibre, respectively). Previous reports have demonstrated that supplementation of bread with GG results in a substantially lower PPI( Reference Ekstrom, Bjorck and Ostman 28 – Reference Wolever, Jenkins and Nineham 30 , Reference Ellis, Dawoud and Morris 49 ).
The contribution of CPF to the reduced PPI is not clear, as studies on the effect of chickpeas on PPI have yielded mixed results. Nestel et al.( Reference Nestel, Cehun and Chronopoulos 36 ) showed in healthy subjects a serum insulin reduction of 55 % at 30 and 60 min after consumption of mashed chickpeas compared with a wheat-based meal( Reference Nestel, Cehun and Chronopoulos 36 ), whereas Johnson et al. ( Reference Johnson, Thomas and Hall 38 ) reported an insulin increase of +iAUC of 32 % when 24 % of the wheat flour was replaced by CPF( Reference Johnson, Thomas and Hall 38 ). This discrepancy can be explained by the fact that fine grinding of legumes (as is the case for CPF) disrupts the cell structure and renders starch more readily accessible for digestion( Reference Jarvi, Karlstrom and Granfeldt 50 ).
No reliable effect on self-reported appetite ratings was apparent for the fibre and CPF mixes used here or in our previous study( Reference Boers, MacAulay and Murray 21 ). Other research suggests that changes in plasma glucose( Reference Aston, Stokes and Jebb 51 , Reference Peters, Ravestein and Van Der Hijden 52 ) or insulin per se ( Reference Peters, Ravestein and Van Der Hijden 52 , Reference Wikarek, Chudek and Owczarek 53 ) may have limited effects on appetite. In addition, Clark & Slavin( Reference Clark and Slavin 54 ) concluded in a systematic review that most fibres do not reduce appetite in acute study designs. Nevertheless, there are several reports of enhanced satiety effects associated with the addition of legumes or specific fibres to foods and beverages( Reference Wanders, van den Borne and De 24 , Reference Li, Kendall and De Souza 25 ). Unfortunately, the levels of viscous fibres needed to influence appetite may be incompatible with desired sensory attributes of many products( Reference Ekstrom, Bjorck and Ostman 55 ).
Comparison of the PPG results with those from our previous study( Reference Boers, MacAulay and Murray 21 ) shows that the addition of CPF+4 % GG reduced PPG by approximately 28 % in this study and by approximately 35 % in the previous study against slightly different control products; however, the effect here for addition of 2 % GG (approximately 16 %) was much lower than previously observed (approximately 33 %). One of the key differences between the two studies is the use of venous blood here, in contrast to capillary blood in the previous study. Venous blood gives a lower PPG response compared with capillary blood (factor 0·67)( Reference Kuwa, Nakayama and Hoshino 56 , Reference Larsson-Cohn 57 ) and this was reflected in the absolute effect size for which the study was powered. The lower PPG values in this study could also be due to a better handling of the glycaemic carbohydrate load in this Indian population (e.g. higher insulin sensitivity), possibly related to the rather low BMI and age range of the subjects. Nevertheless, all the test mixes in the current study gave at least 15 % reduction in PPG+iAUC, an effect size that we believe to be a reasonable benchmark for a physiologically meaningful effect in a general population. Although this is a subjective judgement, there does not seem to be any threshold for beneficial effects of lowering PPG in reducing CVD risk( Reference Ceriello 58 ). Furthermore, there are intervention studies with the PPG-lowering drug acarbose indicating that this level of reduction is possible and linked to a more effective endogenous insulin secretion effect( Reference Kageyama, Nakamichi and Sekino 59 ).
While this research confirms the effect of specific, commercially feasible flour/fibre mixes in decreasing the PPG and PPI responses, further research should focus on the mechanism of action (MoA). One of the supposed MoA is a delayed entry of glucose in the systemic circulation originating from starch, largely due to the viscosity generated by GG in the gastrointestinal tract. However, the slow influx of glucose can only be determined by the dual( Reference Eelderink, Moerdijk-Poortvliet and Wang 60 ) or triple stable isotope technique( Reference Rizza, Toffolo and Cobelli 61 ). In addition, incretin and glucagon measurements should be included in future studies to better characterise the overall nature of the physiological responses to these kinds of fibre and flour mixes.
Conclusions
Together with our previous research( Reference Boers, MacAulay and Murray 21 ) we have demonstrated that flatbread flour mixes incorporating a combination of GG and CPF can produce statistically significant reductions in PPG and PPI responses. The data suggest a dose–response effect at low levels of GG addition, although this would need to be confirmed. However, these additions do not appear to influence postprandial appetite or mood-related parameters. The results suggest that these additions to commercial flatbread flour mixes could be an efficacious and feasible approach to achieve reduced PPG and PPI responses to such starchy staples in both Caucasian and Southeast Asian populations.
Acknowledgements
The authors are grateful to Carole Verhoeven, Anne-Roos Hoogenraad and Anton Porcu (Unilever Clinicals, Vlaardingen) and to Tanvi Kadam and Anisha Pargal (Unilever Clinicals, Mumbai, India) for facilitating the clinical studies. The authors are also grateful to Ramitha K., Suman Majumder and Chandrika Mohanan and her design team at Unilever Bangalore for providing the flours and to Jack Seijen ten Hoorn for formulating the different flatbreads and to Lambda Therapeutic Research Ltd, Ahmedabad (India), for executing the clinical studies.
This research was entirely funded by Unilever.
H. M. B., K. M., M. A. M. S., P. M. and D. J. M. designed the research; M. A. M. S. and R. D. facilitated execution of the study; P. M. performed statistical analysis. H. M. B. wrote the manuscript with significant contributions from D. J. M., K. M., P. M. and R. D. H. M. B. and D. J. M. had primary responsibility for final content. All authors read and approved the final version of the manuscript.
All authors are employees of Unilever, which manufactures and markets consumer food products, including the flour used for the flatbreads in this study.
Supplementary materials
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000277













