Feeding patterns are major determinants of infant health. Indeed, a lack of exclusive breast-feeding during the first 6 months of life is an important risk factor for infant and childhood morbidity and mortality, especially in developing countries(1). Exclusive breast-feeding means that no water or other fluids or food is given to the infant in addition to breast milk. Partial breast-feeding means the infant receives foods or other milk(Reference Moore, Prentice and Coward2, Reference Haisma, Coward and Albernaz3) in addition to breast milk. Based on the findings of Da Costa et al. (Reference Da Costa, Haisma and Wells4), breast-feeding is considered exclusive when non-milk intake is less than 100 g/d, and partial when it is over 100 g/d.
There is a long debate over the adequacy of exclusive breast-feeding until 6 months of age in developing countries. This debate is centred on the ‘weanling's dilemma’, i.e. the choice between the known protective effect of exclusive breast-feeding against infectious morbidity and the theoretical insufficiency of breast milk alone to satisfy the infant's energy and micronutrient requirements beyond 4 months of age. In addition, the most frequent reason given by mothers for solid food introduction before 6 months is that they consider the infant to be hungry and not satisfied by breast milk alone(Reference Fewtrell, Morgan and Duggan5). In this regard, the WHO commissioned expert consultations and a systematic review(6, 7) to assess the scientific evidence of the optimal duration, and the nutrient adequacy of exclusive breast-feeding. Neither endeavour found any objective evidence of ‘weanling's dilemma’ nor any adverse effects of exclusive breast-feeding for 6 months on weight or length gain in infants. Infant growth potential drives milk production(7), and thus the mean intake of human milk provides sufficient energy and protein to meet requirements during the first 6 months. On this basis, exclusive breast-feeding is now recommended by the WHO as the best feeding practice for the infant up to the age of 6 months(1, 8). However, some authors still believe these grounds to be insufficient as a basis for current recommendations, and have hypothesised a gap between the energy provided by breast milk and energy needs for many 6-month-old infants in developed countries(Reference Fewtrell, Morgan and Duggan5, Reference Reilly, Ashworth and Wells9, Reference Reilly and Wells10). All experts have noted a serious lack of data on breast milk composition and breast milk intake of 6-month-old infants in developing countries, and recommended more investigations with standard methods to support current recommendations. The 2H2O tracer, the dose-to-the mother technique, is such a method to assess breast milk intake. Few studies in developing countries using this technique on 6-month-old infants have been reported; however, none of these studies has measured the adequacy of energy intake from breast milk(Reference Butte, Villalpando and Wong11–Reference Galpin, Thakwalakwa and Phuka13). Wells et al. (Reference Wells, Jonsdottir and Hibberd14) calculated 6-month-old infants' energy consumption from breast milk based on the measurement of breast milk intake and the findings of Reilly et al. (Reference Reilly, Ashworth and Wells9), and found that infants meet the WHO recommendations for breast milk intake but not for energy intake. The dose-to-the mother technique was first described by Coward et al. (Reference Coward, Cole and Sawyer15) and involves giving the mother an oral dose of 2H-labelled water, and following the disappearance of 2H from the mother and its appearance in the baby(Reference Cissé, Bluck and Diaham16–18). The major determinant of energy density in breast milk is its fat content. Therefore, an estimate of milk fat concentration can determine its energy value. The creamatocrit method has been used extensively for this estimation since it was validated for human milk by Lucas et al. (Reference Lucas, Gibbs and Lyster19).
In order to assess the adequacy of WHO recommendations, the present comprehensive study was designed to estimate the energy intake from human milk in 6-month-old Senegalese lactating infants. Breast milk intake was measured by the dose-to-the-mother 2H2O turnover method and the fat and energy contents of human milk by creamatocrit.
Methods
Subjects
Mothers of infants aged less than 6 months were identified during routine immunisation sessions at health centres in the neighbourhood of Dakar, the Senegalese capital, and were invited to participate in the study. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethical committee of the University of Cheikh Anta Diop in Dakar. Written informed consent was obtained from all mothers.
The protocol was designed as a comprehensive study, and was part of an initial evaluation powered to test the most appropriate population indicator of vitamin A deficiency in 225 mother/infant pairs. Of the mother/infant pairs from the initial sample, one-quarter were randomly selected using a computer-generated block randomisation scheme (Epi Info) for inclusion in the present study. Finally, fifty-nine breast-feeding mothers and their 6-month-old infants were included for the study. The sample size required to detect a 100 g/d significant difference in milk intake between exclusively and partially breast-fed infants, assuming a sd of 130 g/d, a statistical power of 80 % and an α of 5 %, was fifty-four mother/infant pairs.
Mothers' socio-economic status was evaluated using a questionnaire to know whether the mother was a homeowner or renting, had running water and electricity at home, to know the type of fuel used for cooking, the type of toilets used, the equipment used in the house and also whether the mother had possession of animals and/or farms for agriculture.
The recruitment was based on the fact that the infant was still breast-feeding, and was not acutely severely malnourished (a mid-upper arm circumference ≤ 11·5 cm and/or a weight-for-length ≤ − 3 z-score were used to define severe and acute malnutrition). Before the recruitment, mothers' Hb was screened using a HemoCue Hb 201 analyser (HemoCue AB), and those with severe anaemia (Hb < 70 g/l) were excluded from the study and referred to the hospital for appropriate clinical care. Mothers with twins and pregnant women were also excluded from the study.
Measurement of breast milk intake
Infant milk intake was determined using the dose-to-the mother 2H2O turnover method first described by Coward et al. (Reference Coward, Cole and Sawyer15, Reference Coward, Whitehead and Sawyer20) and later reported by Haisma et al. (Reference Haisma, Coward and Albernaz3) and the International Atomic Energy Agency(18). Briefly, baseline saliva samples of both mothers and infants were collected on day 0 (pre-dose) before an oral dose of 30 g 2H2O (Cambridge Isotope Laboratories, Inc.) was given to the mother. The dose was weighed to the nearest 0·0001 g using an analytical scale (OHAUS Corporation). On days 1, 2, 3, 4, 13 and 14, six saliva samples (post-dose) were collected from both mothers and infants and the samples were stored at − 20°C until measurement. 2H enrichment of the saliva samples was determined by a Fourier transform IR spectrometer (Nicolet iS10; Thermo Scientific). The technique is reflective of the infant's water intake from milk and non-milk water intake over 14 d(18). Anthropometric measurements (weight and height) of both mothers and infants were recorded on the 1st and 14th day.
Calculations
Breast milk and non-milk intakes were calculated by fitting 2H enrichment data to a model of water turnover in mothers and infants using the Solver function of Microsoft Excel, as described by Coward et al. (Reference Coward, Cole and Sawyer15) and Haisma et al. (Reference Haisma, Coward and Albernaz3).
Determination of the mother's body composition
Body composition (lean body mass and fat mass) of mothers was calculated from the total-body water component as part of the dose-to-the-mother method. Total body water was assumed to be equal to 2H dilution space divided by 1·04(Reference Racette, Schoeller and Luke21). Fat-free mass was calculated as total body water/0·73(Reference Prentice22). Body fat was computed as body weight minus fat-free mass.
Determination of the infant's breast-feeding pattern
Breast-feeding patterns were defined according to the amount of non-milk intake estimated from tracer studies. Based on the WHO definitions, exclusive breast-feeding was defined as the infant receiving only human milk without any additional food or drink, not even water for 6 months of life. Predominant breast-feeding consisted of breast milk as the infant's predominant source of nourishment, but the infant could also receive water and water-based drinks, fruit juice and other fluids in limited quantities. Partial breast-feeding was defined as the infant receiving foods or other milk in addition to breast milk. Haisma et al. (Reference Haisma, Coward and Albernaz3) found no difference in breast milk intake between exclusively and predominantly breast-fed infants. However Haisma's classification was based on mothers' self-report, which is questionable(Reference Moore, Prentice and Coward2). For this reason, we chose to use actual non-milk water intake as a means to classify infants by the feeding pattern. Da Costa et al. (Reference Da Costa, Haisma and Wells4), in a multi-centre analysis of data from twelve countries using a standardised stable isotope method, have shown a decrease of 45 g/d of human milk intake for each additional 100 g/d of non-milk water intake. Based on this result, we considered breast-feeding to be exclusive when non-milk intake is less than 100 g/d, and partial when non-milk intake is equal to or more than 100 g/d. Therefore, two groups were formed according to the infants' breast-feeding pattern: Ex group (exclusive breast-feeding infants) and Part group (partial breast-feeding infants).
Breast milk energy content and infant's energy intake
On day 14, a full milk sample was collected from each mother with a manual breast pump. Milk samples were collected from one breast that had not been used to feed the infant for at least 2 h while the infant was suckling the other one. They were well mixed and immediately after, three capillary tubes were filled for creamatocrit determination with Creamatocrit Plus™ (Separation Technology, Inc.) for creamatocrit determination. Creamatocrit has been mostly used in clinical settings and could also be used in research and epidemiological studies. In addition, it has been validated by Meier et al. (Reference Meier, Engstrom and Zuleger23) against laboratory techniques for performing creamatocrit such as the standard laboratory centrifuge with a haematocrit reader and the standard laboratory centrifuge with digital calipers. Creamatocrit Plus™ is a small centrifuge with an embedded reader system that automatically calculates the amount of fat and energy (kcal) in milk from creamatocrit values(Reference Lucas, Gibbs and Lyster19). The following equations are used by the device:
Infants' energy intake from breast milk was calculated as a product of breast milk intake and the energy content of the mother's milk.
Statistical analysis
Characteristics of infants and mothers are tabulated as means and standard deviations. Breast milk intake was expressed as g/d and energy intake as kJ/d and kJ/kg body weight per d. They are tabulated as means and standard deviations and 95 % CI. Statistical analysis was carried out with PASW Statistics 17 (SPSS, Inc.) and Microsoft Office Excel 2003 (Microsoft). P values < 0·05 were considered as significant. ANOVA and the t test were used to compare means between the groups. Multivariate linear regressions were used to assess the association between breast milk intake and the characteristics of mothers and infants.
Results
Mothers in the study were defined as relatively poor. All the fifty-nine mother–infant pairs initially enrolled in the study completed the dose-to-the-mother 2H dilution technique and fifty-five completed the creamatocrit measurements (Fig. 1). Of the fifty-nine mother–infant pairs, fifteen infants (25·4 %) had non-milk intakes < 100 g/d and were then considered as the Ex group, while forty-four (74·6 %) had non-milk intakes ≥ 100 g/d and were considered as the Part group.
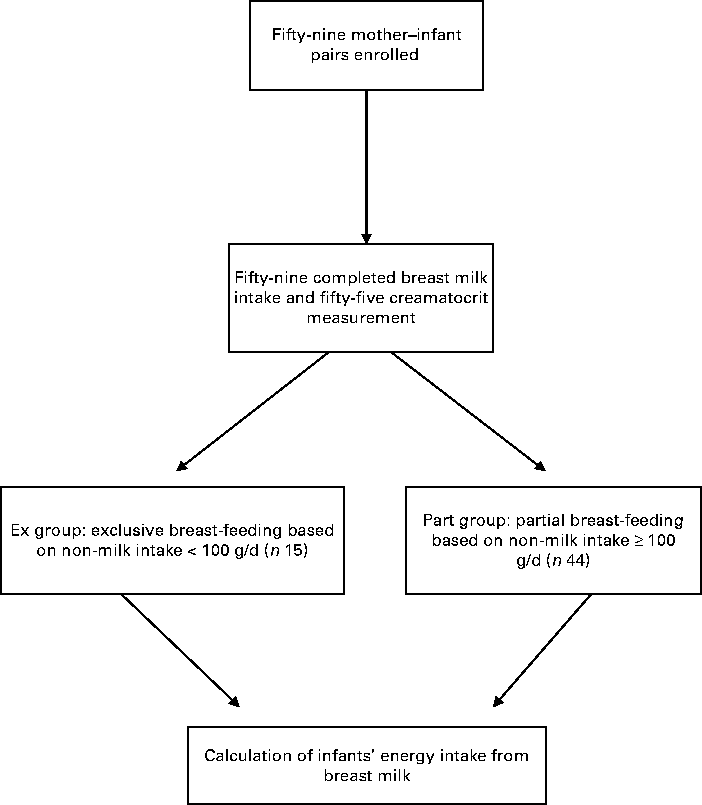
Fig. 1 Study profile.
Subjects' characteristics
The characteristics of the fifty-nine mother–infant pairs are indicated in Table 1. Mothers' age, BMI and fat mass in the Ex group were not different from those in the Part group. However, their fat-free mass was significantly higher than that of the Part group mothers (P= 0·021). Of the fifty-nine subjects, 7 % (n 4) of mothers had energy deficiency (BMI < 18·5 kg/m2) and 22 % (n 13) were overweight (BMI >25 kg/m2). Infants' age, weight, weight-for-length, length-for-age and weight gain on the 14th day were similar in both groups.
Table 1 Characteristics of Senegalese lactating mothers and their 6-month-old breast-feeding infants (Mean values and standard deviations)
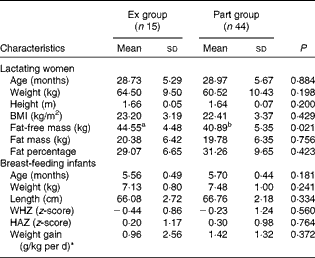
Ex group, exclusively breast-fed infants; Part group, partially breast-fed infants; WHZ, weight-for-length; HAZ, length-for-age.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Calculated from the weight at the beginning of the study and at day 14: weight gain = 1000 × (weight at day 14–weight at day 0)/(weight at day 0 × 14).
Breast milk and non-milk intake
The overall breast milk intake (n 59) was 870 (sd 215) g/d. It was significantly higher for infants in the Ex group (P< 0·01). Inversely, non-milk intake was significantly higher for infants in the Part group (P< 0·001; Table 2). Multiple linear regression showed that breast milk intake was positively associated with the infant's weight (β = 0·548, P <0·001) and negatively associated with non-milk intakes (β = − 0·485, P <0·001) and breast milk fat content (β = − 0·262, P <0·05).
Table 2 Infants' breast milk and non-milk intakes in the two groups (Mean values and standard deviations)
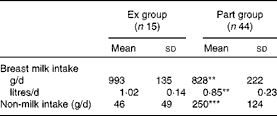
Ex group, exclusively breast-fed infants; Part group, partially breast-fed infants.
Mean values were significantly different from those of the Ex group: ** P< 0·01, *** P< 0·001.
Energy intake from breast milk
The means for creamatocrit and fat content were 4·5 (sd 1·5) % and 31 (sd 9) g/l, respectively, and were comparable in both groups (P>0·05). Breast milk energy content was not different between the groups (P>0·05) and the overall mean was 2661 (sd 351) kJ/l (636 (sd 84) kcal/l) (Table 3). Energy intake from breast milk (derived from the energy content of breast milk and breast milk intake) was significantly higher in the Ex group than in the Part group (P< 0·01). When corrected for the infant's body weight, the difference became more significant (P <0·001). There was no difference between the energy intake of girls and boys within the same group (Table 4).
Table 3 Breast milk creamatocrit, fat and energy content (Mean values and standard deviations)
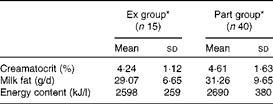
Ex group, exclusively breast-fed infants; Part group, partially breast-fed infants.
* There were no significant differences between the groups for each variable (P>0·05).
Table 4 Infants' daily energy intake from breast milk* (Mean values and standard deviations)
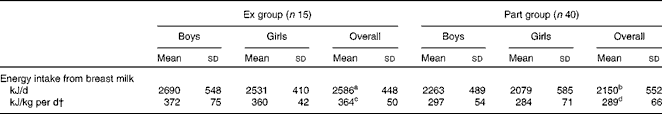
Ex group, exclusively breast-fed infants; Part group, partially breast-fed infants.
a,b,c,dMean values within a row with unlike superscript letters were significantly different: a>b (P< 0·01), c>d (P< 0·001).
* There were no difference between boys and girls in the same group.
† Obtained by dividing the infants' daily energy intake by their weight.
Discussion
In the present study, a standard method to measure breast milk intake, the dose-to-the mother 2H2O turnover method, was used. It is more accurate than test weighing. It does not interfere with the infant's feeding patterns and is easier to achieve in the field(18, Reference Infante, Lara and Vio24, Reference Savenije and Wells25). This technique allows the determination of the infant's non-milk intake, the mother's body composition and the infant's mean breast milk intake over a 14 d period(Reference Haisma, Coward and Albernaz3, Reference Fewtrell, Morgan and Duggan5). Infants were classified by the feeding pattern on the basis of their actual non-milk intake according to Da Costa et al. (Reference Da Costa, Haisma and Wells4). A non-milk intake cut-off of 100 g/d was used to differentiate between the Ex and Part groups. The present results show that the infant's feeding patterns have more consequences on breast milk intake. Indeed, the infants' breast milk intake in the Ex group was significantly higher than that of infants in the Part group. The WHO estimations of breast milk intake for developing countries are 804 g/d for exclusively breast-fed infants and 611 g/d for partially breast-fed infants(7). These values appear to be significantly lower than those obtained in the present study. In fact, the WHO values were based on a compilation of data generally obtained by test weighing. Breast milk intakes measured by the dose-to-the-mother 2H2O turnover method are generally higher than the WHO values(Reference Da Costa, Haisma and Wells4). The overall mean milk intakes in the present study are comparable with published values using the same technique on 6-month-old breast-feeding infants in Mexico (869 g/d)(Reference Butte, Villalpando and Wong11), Chile (863 g/d)(Reference Alvear, Salazar and Berlanga12), Malawi (910 g/d)(Reference Galpin, Thakwalakwa and Phuka13) and Iceland (901 g/d)(Reference Wells, Jonsdottir and Hibberd14). These results indicate that exclusively breast-fed infants consume a sufficient amount of breast milk. However the cut-off proposed by Da Costa et al. (Reference Da Costa, Haisma and Wells4) remains arbitrary and more studies are needed to investigate the effect of non-milk intake on breast milk intake.
As defined by the WHO, energy requirements for 6-month-old infants are 325 kJ/kg body weight per d (78 kcal/kg body weight per d) for boys and 330 kJ/kg body weight per d (79 kcal/kg body weight per d) for girls(26, Reference Butte27). When applied to the present study, it would equate to 2328 kJ/d (556 kcal/d) for the average Ex group infants and to 2443 kJ/d (583 kcal/d) for the average Part group infants.
The energy content of breast milk was estimated in the present study by creamatocrit, an accurate technique, which was validated on breast milk samples in different conditions(Reference Lucas, Gibbs and Lyster19, Reference Meier, Engstrom and Zuleger23, Reference Lemons, Shreiner and Gresham28–32). Yet, since breast milk fat content varies widely from one breast to another but also according to the collection time, to test properly the nutritional adequacy of a mother's milk, a representative (24 h collection or mid-morning and mid-afternoon collection or total breast expression) sample, which has been well mixed, is needed(Reference Lemons, Shreiner and Gresham28, 33, Reference Rocquelin, Tapsoba and Mbemba34). Given all these factors and the difficulty of sampling 24 h milk among our subjects, we chose to collect full milk samples, which consist of a total expression of a breast that was not suckled for at least 2 h. The fat content values that we found are similar to published data on Gambian 24 h breast milk samples (39 g/l)(Reference Prentice, Prentice and Whitehead29) and Senegalese mid-morning and mid-afternoon well-mixed samples (31 (sd 10) g/l)(35). Breast milk energy content did not differ between the groups and was in the range of the WHO published values for developing countries(33). Indeed, according to the WHO, energy content varies between 2516 kJ/l (601 kcal/l) and 3245 kJ/l (775 kcal/l) with a mean of 2717 kJ/l (649 kcal/l)(7).
Expressed as kJ/d or kJ/kg body weight per d, energy intake from breast milk in the Ex group infants was significantly higher than that in the Part group infants. When their energy intakes were compared with their requirements, boys and girls in the Ex group met their energy needs by breast milk alone, whereas energy intake from breast milk was low for infants in the Part group. Reilly et al. (Reference Reilly, Ashworth and Wells9) have proposed that the metabolisable energy content of breast milk at the age of 6 months is 260·0 kJ/100 g (62·1 kcal/100 g). Using these estimates, the infants' energy intake from breast milk would be 2582 kJ/d (616 kcal/d) in the Ex group and 2153 kJ/d (514 kcal/d) in the Part group, which are not different from the values obtained by creamatocrit. These results are not very different from those found by Wells et al. (Reference Wells, Jonsdottir and Hibberd14) in 6-month-old exclusively and partially breast-fed infants in Iceland. Infants had good nutritional status in both groups and exhibited normal growth. These results suggest that partially breast-fed infants (Part group) also meet their energy requirements by breast milk and non-milk intakes that are high for these infants.
Maternal nutritional status did not affect breast milk intake. In fact, no association was found between breast milk intake and the mother's BMI and body composition (fat-free mass and fat mass). However, the present study is consistent with published results showing that infants' characteristics affect their milk intake. A positive association was found between breast milk intake and the infant's weight. Breast milk intake was negatively associated with non-milk intake. In the present study, 48 % of breast milk intake variation can be explained by the infant's intake of water and other foods. This result corroborates previous studies(Reference Da Costa, Haisma and Wells4, Reference Heinig, Nommsen and Peerson36, Reference Islam, Peerson and Ahmed37) about the effect of complementary feeding on breast milk intake. The present findings are consistent with the WHO's conclusion indicating that early introduction of other liquid and/or foods in infant feeding should be avoided in order to optimise the intake of breast milk, which is the most suitable food for the infant(38). Furthermore, recent cohort studies have shown that the late introduction of complementary foods (at 6 months) would protect the infant against adulthood obesity(Reference Nommsen-Rivers and Dewey39, Reference Shack-Nielsen, Sorensen and Mortensen40).
The present study demonstrates that 6-month-old Senegalese exclusively breast-fed infants consume sufficient amounts of breast milk. They receive an adequate amount of energy from breast milk to meet their energy requirements. Although partially breast-fed infants cover their energy needs as shown from their growth data, early introduction of complementary foods by this age reduces the consumption of breast milk. Since breast milk is the most complete food for infants, and uncontrolled complementary foods are potential risks of infections, advocacy of exclusive breast-feeding should be strengthened in countries where safe water intake is an issue.
Acknowledgements
The present study was supported by the International Atomic Energy Agency (IAEA), TC project SEN60/16, and the University of Dakar, Senegal. We would like to thank the mothers and infants who participated in the study, the health centre's staff, the nurses, the fieldworkers and the laboratory of Nutrition UCAD staff (students and technicians) for their support and contributions. We also thank Dr Les Bluck for the initial reviews and feedback on the manuscript. S. W., A. A.-D. and K. M. K. designed the study and implemented the protocol. A. A.-D. and K. M. K. completed the field, clinical data collection and analyses. A. D., N. I.-D. and A. T. G. provided the training. S. W., K. M. K. and A. A.-D. wrote the manuscript. All authors reviewed and approved the final manuscript. The authors have no conflicts of interest to declare.







