Cervical intraepithelial neoplasia (CIN) is a potentially premalignant transformation, which may remain harmlessly or get eliminated( Reference Kim, Kim and Ko 1 ). The likelihood of regression of cervical intraepithelial neoplasia grade 1 (CIN1) is approximately 60 %( Reference Ostor 2 ), and, if not treated, the risk of progression to invasive cervical carcinoma is about 30–50 %( Reference McCredie, Sharples and Paul 3 ). The main risk factors for CIN1 are sexually transmitted diseases and productive human papillomavirus (HPV) infection( Reference Duggan 4 ). In addition, several studies have shown that increased inflammatory cytokines and biomarkers of oxidative stress would result in higher cervicovaginal HPV concentrations( Reference Mitchell, Hitti and Paul 5 , Reference Jiang, Xiao and Khan 6 ), which in turn cause cells to become more vulnerable for the development of cervical cancer( Reference Jiang, Xiao and Khan 6 ).
Recently, dietary supplementation of antioxidants has gained notable interest for the treatment of CIN. Se is an essential trace element, which plays a key role in various major metabolic pathways and protects the body from the poisonous effects of heavy metals( Reference Gupta, Jaworska-Bieniek and Lubinski 7 ). An inverse relationship between serum Se levels and CIN or cervical cancer has been reported( Reference Kim, Kim and Ko 1 ), and the tissue concentrations of Se are significantly lower in cervical cancer tissues compared with paired non-lesion tissues( Reference Cunzhi, Jiexian and Xianwen 8 ). However, another study did not observe any association between serum Se levels and invasive cervical cancer at the typical serum Se levels( Reference Thompson, Patterson and Weinstein 9 ). We have previously reported beneficial effects of Se supplementation on metabolic profiles, biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome (PCOS) and gestational diabetes (GDM)( Reference Jamilian, Razavi and Fakhrie Kashan 10 , Reference Asemi, Jamilian and Mesdaghinia 11 ).
Se intake may reduce the risk of cancer through an increased expression of selenoproteins, protection against oxidative DNA damage and/or enhanced DNA repair processes( Reference Gupta, Jaworska-Bieniek and Lubinski 7 ). These mechanisms might suggest the importance of Se supplementation in the process of malignant transformation and proliferation in cervical carcinogenesis. To the best of our knowledge, no study is available investigating the effects of Se administration on regression, markers of insulin metabolism, lipid concentrations, biomarkers of inflammation and oxidative stress in patients with CIN1. The present study was, therefore, carried out to evaluate the effects of Se supplementation on regression and metabolic status in women with CIN1.
Methods
Participants
The present study was a randomised, double-blind clinical trial in which fifty-eight patients aged between 18 and 55 years with CIN1 diagnosed by colposcopy, biopsy and pathological procedures were included( Reference Jin, Li and He 12 ). Patients attended the Oncology Clinic at the Arak University of Medical Sciences (AUMS), Iran, during August 2014 to February 2015. Patients who had an abnormal Pap smear test, abnormal cervical cytology, abnormal cervical appearance, postcoital bleeding, intermenstrual bleeding and chronic vaginal discharge were invited and asked to undergo colposcopy. The exclusion criteria were as follows: women who had a history of cervical cancer or other cancers of the lower genital tract, a history of hysterectomy or destructive therapy of the cervix and pregnant women. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all the participants. The present study was approved by the ethics committee of AUMS and has been recorded in the Iranian Registry of Clinical Trial (http://www.irct.ir: IRCT201412215623N32).
Study design
At the beginning of the study and after stratification of the participants based on their baseline BMI (<30 and ≥30 kg/m2) and age (<35 and ≥35 years), patients were randomly allocated to take 200 μg Se supplements as Se yeast (n 28) or placebo (n 28) per d, using blocked randomisation method by a trained midwife at the maternity clinic. Se supplements and placebos were provided by Nature Made Co. and Barij Essence Co., respectively. The intervention was carried out for 6 months. The appearance of the placebo tablets (starch), including colour, shape, size and packaging, were identical to the Se tablets. Women were advised not to change their diet and physical activity during the study period. Compliance to the consumption of Se supplements and placebos was evaluated by asking women to bring the medication containers and by sending a short message to the patients’ cell phones as a reminder. All the patients completed three dietary records and three physical activity records (1 weekend day and 2 weekdays) at month 2, 4 and 6 of the intervention. Nutritionist 4 software (First Databank) was used to determine nutrient intakes based on dietary records.
Assessment of variables
Weight and height (Seca) were quantified at the beginning of the study and after 6 months of the intervention in a fasting state without shoes and with minimal clothing by a trained midwife. BMI was determined using the height and weight measurements (weight (kg)/height2 (m2)). At the beginning of the study and at the end, blood samples (10 ml) were collected early morning after an overnight fast. Blood samples were collected according to a standard protocol and were immediately centrifuged (Hettich). Subsequently, the samples were stored at −80°C until analysis at the AUMS reference laboratory.
Primary outcomes
The primary outcome was CIN1, which was determined through colposcopy, cervical biopsy and pathological diagnosis at baseline and 6 months after the intervention. At baseline, in patients who had an abnormal Pap smear test, abnormal cervical cytology, abnormal cervical appearance, postcoital bleeding, intermenstrual bleeding or chronic vaginal discharge, a colposcopy was carried out to diagnose the location and extent of CIN. Colposcopy (Siemens Co.) was carried out with the woman lying on her back, with her legs in stirrups and with the buttocks on the lower edge of the table (a position known as the dorsal lithotomy position). A speculum was placed in the vagina after the vulva was examined for any suspicious lesions. Areas of the cervix that turned dense white after the application of acetic acid or had an abnormal vascular pattern (e.g. mosaicism and punctuation) were considered for a biopsy. Specimens were embedded in formalin solution and then sent for pathological diagnosis. All these procedures were repeated at the end of the study.
Secondary outcomes
The secondary outcomes were as follows: markers of insulin metabolism, lipid profiles, biomarkers of inflammation and oxidative stress. To quantify fasting plasma glucose (FPG), serum TAG, and VLDL, total, LDL and HDL-cholesterol concentrations, we used commercially available kits (Pars Azmun). All inter- and intra-assay CV for FPG and lipid concentrations were <5 %. Serum insulin was measured using an ELISA kit (Monobind). The homeostatic model assessment of insulin resistance (HOMA-IR), homeostatic model assessment of β-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were determined according to suggested formulae( Reference Pisprasert, Ingram and Lopez-Davila 13 ). Serum high-sensitivity C-reactive protein (hs-CRP) was determined using a commercial ELISA kit (LDN). Plasma nitric oxide (NO) was determined by the Giess method( Reference Tatsch, Bochi and Pereira Rda 14 ), total antioxidant capacity (TAC) was determined using the ferric reducing antioxidant power (FRAP) method developed by Benzie & Strain( Reference Benzie and Strain 15 ), total GSH was determined by the method of Beutler & Gelbart( Reference Beutler and Gelbart 16 ) and malondialdehyde (MDA) levels were determined using the thiobarbituric acid reactive substance method( Reference Janero 17 ).
Statistical methods
Normality of variables was evaluated by visual inspection of histograms. Log transformation was applied before statistical analyses, where appropriate. The analyses were performed for all randomised subjects according to the intention-to-treat (ITT) principle. To detect differences in the anthropometric measures as well as dietary intakes between the two groups, we used an independent samples Student’s t test. In addition, paired samples t test was applied to detect a within-group difference. Pearson’s χ 2 test was used for the comparison of categorical variables. To determine the effects of Se administration on markers of insulin metabolism, lipid concentrations, biomarkers of inflammation and oxidative stress, we used one-way repeated-measures ANOVA. To control confounding variables (baseline values, age and BMI at the baseline), we applied ANCOVA. P<0·05 was considered to be statistically significant. All the statistical analyses were carried out using the Statistical Package for Social Science version 18 (SPSS Inc.).
Results
Among patients in the Se group, three subjects were excluded. Three subjects from the placebo group were also excluded. Finally, fifty participants (Se (n 25) and placebo (n 25)) completed the trial (Fig. 1). Based on the ITT protocol, all fifty-six patients (twenty-eight in each group) were included in the final analysis. On average, the rate of compliance in our study was high, such that >90 % of tablets were taken by the subjects throughout the study in both the groups. Se supplementation in CIN1 patients showed no side-effects throughout the study.

Fig. 1 Summary of the patient flow diagram.
Mean age and height of the participants were not significantly different between the Se supplement group and the placebo group. Baseline weight and BMI as well as their means after 6 months of the intervention were not significantly different between the two groups (Table 1). Moreover, after 6 months of Se supplementation, greater percentage of women in the Se group had regressed CIN1 (88·0 v. 56·0 %; P=0·01) compared with the placebo group.
Table 1 General characteristics of the study participants (Mean values and standard deviations)
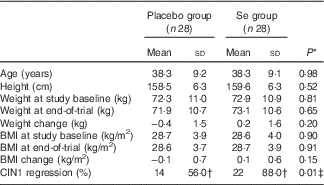
CIN1, cervical intraepithelial neoplasia grade 1.
* Obtained from the independent t test.
† The analysis was carried out on twenty-five patients, three subjects were excluded.
‡ Obtained from Pearson’s χ 2 test.
Comparison of dietary intakes of the study participants throughout the study revealed no significant differences in macronutrient and micronutrient intakes, including energy, carbohydrates, proteins, fats, SFA, PUFA, MUFA, cholesterol, total dietary fibre, vitamin E, vitamin C, Mg, Mn and Se between the two groups (data not shown).
Long-term Se supplementation, compared with the placebo, resulted in significant decreases in FPG (−0·37 (sd 0·32) v. +0·07 (sd 0·63) mmol/l; P=0·002), serum insulin levels (−28·8 (sd 31·2) v. +13·2 (sd 40·2) pmol/l; P<0·001), HOMA-IR (−1·3 (sd 1·3) v. +0·5 (sd 1·4); P<0·001), HOMA-B (−14·9 (sd 20·4) v. +9·8 (sd 30·7); P<0·001) as well as a significant elevation in QUICKI score (+0·03 (sd 0·03) v. −0·01 (sd 0·01); P<0·001) (Table 2). In addition, patients who received Se supplements had significantly decreased serum TAG levels (−0·14 (sd 0·55) v. +0·15 (sd 0·38) mmol/l; P=0·02) and VLDL-cholesterol levels (−2·6 (sd 10·0) v. +2·9 (sd 7·1) mg/dl; P=0·02) and had significantly increased serum HDL-cholesterol levels (+0·13 (sd 0·21) v. −0·01 (sd 0·15) mmol/l; P=0·003). In addition, compared with the placebo group, there were significant elevations in plasma TAC (+186·1 (sd 274·6) v. +42·8 (sd 180·4) mmol/l; P=0·02) and GSH (+65·0 (sd 359·8) v. −294·2 (sd 581·8) μmol/l; P=0·007) and a significant decrease in MDA levels (−1·5 (sd 2·1) v. +0·1 (sd 1·4) μmol/l; P=0·001) among those who were supplemented with Se. We did not observe any significant effect on other lipid profiles and inflammatory markers following the administration of Se supplement.
Table 2 Metabolic profiles, biomarkers of inflammation and oxidative stress at study baseline and after 6 months of intervention in women with cervical intraepithelial neoplasia grade 1 (CIN1) who received either selenium supplements or placebo (Mean values and standard deviations)

FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-B, homeostatic model assessment of β-cell function; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde.
* Obtained from paired samples t test.
† Obtained from repeated-measures ANOVA test.
‡ To convert FBG in mg/dl to mmol/l, multiply by 0·0555.
§ To convert insulin in μIU/ml to pmol/l, multiply by 6·945.
|| To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
¶ To convert VLDL-cholesterol, total cholesterol, LDL-cholesterol and HDL-cholesterol in mg/dl to mmol/l, multiply by 0·0259.
Baseline levels of TAG, VLDL-cholesterol and NO were significantly different between the two groups. Therefore, we adjusted the analyses for baseline values, age and BMI at baseline (Table 3). After further adjustment, no significant changes in our findings occurred except for the levels of TAG (P=0·23), VLDL-cholesterol (P=0·23) and total cholesterol (P=0·04).
Table 3 Adjusted changes in metabolic variables between groups after 6 months of intervention in women with cervical intraepithelial neoplasia grade 1 (CIN1) who received either selenium supplements or placebo (Mean values with their standard errors)
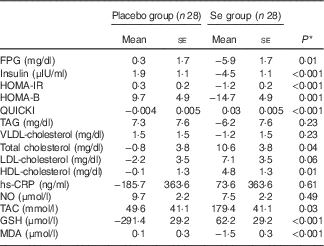
FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-B, homeostasic model assessment of β-cell function; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde.
* Obtained from ANCOVA test. Values are adjusted for baseline values, age and baseline BMI.
Discussion
In the present study, we evaluated the effects of long-term Se administration on the regression and metabolic status of patients with CIN1. Our study demonstrated for the first time that administration of 200 μg Se/d for 6 months in patients with CIN1 resulted in its higher regression, improved homeostatic glucose parameters, decreased serum TAG and VLDL-cholesterol levels, increased HDL-cholesterol levels and decreased oxidative stress compared with the placebo group.
We also found that taking Se supplements daily for 6 months among CIN1 women resulted in its regression. To the best of our knowledge, the effects of Se administration in patients with CIN have not been evaluated so far. In a study by Kim et al.( Reference Kim, Kim and Ko 1 ), it was observed that Se levels were significantly lower in patients with CIN or cancer compared with controls. Although an inverse association was found between Se levels and the results of other types of cancer studies, it is suggested that Se has protective effects( Reference Lu and Jiang 18 ). Administration of a mixture of antioxidant supplements, including 50 mg Se, 60 mg vitamin C, 10 mg vitamin E and 303 mg vitamin A, significantly lowered levels of apoptosis and lipid peroxides among cervical cancer patients undergoing postoperative radiotherapy compared with patients who did not receive supplements( Reference Ismail, Amer and Wahba 19 ). Several epidemiological studies have also shown the significant associations between Se intake and decreased risk of breast( Reference Harris, Bergkvist and Wolk 20 ) and prostate( Reference Brinkman, Reulen and Kellen 21 ) cancers. Previous studies have reported that the cancer-preventive action of Se may be due to the antioxidant effects of Se–cysteine–glutathione peroxidase (GSHPx)( Reference Michiels, Raes and Toussaint 22 , Reference Saydam, Kirb and Demir 23 ). Moreover, Se may result in increased expression of selenoproteins, which in turn may decrease the risk of various types of cancer( Reference Gupta, Jaworska-Bieniek and Lubinski 7 ).
We found that Se administration for 6 months in patients with CIN led to significant reductions in FPG, serum insulin concentrations, HOMA-IR, HOMA-B, TAG, VLDL-cholesterol concentrations as well as significant elevations in QUICKI score and HDL-cholesterol concentrations compared with the placebo group, but it did not affect other lipid concentrations. Our previous study among PCOS women demonstrated that 200 μg/d Se supplementation for 8 weeks had beneficial effects on markers of insulin metabolism, TAG and VLDL-cholesterol concentrations, but did not influence other lipid profiles( Reference Jamilian, Razavi and Fakhrie Kashan 10 ). These findings were in agreement with results obtained by Alizadeh et al.( Reference Alizadeh, Safaeiyan and Ostadrahimi 24 ) who noted significant decreases in serum insulin levels and HOMA-IR score following Se administration among pre-menopausal women with central obesity for 6 weeks. In another study, supplementation with 100 μg Se/d in pregnant women from the first trimester of pregnancy until delivery did not affect lipid profiles( Reference Tara, Maamouri and Rayman 25 ). Se intake may improve homeostatic glucose parameters and TAG levels by regulating the expressions of genes responsible for the synthesis of the enzymes involved in the carbohydrate metabolism, increased uptake of the glucose by tissues( Reference Chen, Qiu and Zou 26 ) and the inhibition of inflammatory cytokines including TNF-α and IL-1( Reference Brigelius-Flohe, Banning and Kny 27 ). The lack of a significant effect of Se intake on other lipid concentrations in the present study might be explained by the distinct trial design, various dosages of Se supplementation used and subjects of the study.
The current study revealed that Se intake for 6 months had no significant effect on serum hs-CRP and plasma NO concentrations among CIN1 women compared with the placebo group. Supporting our study, 200 μg Se administration/d for 3 months did not influence any significant effect on CRP levels among patients with chronic kidney disease( Reference Omrani, Rahimi and Nikseresht 28 ). Furthermore, no significant difference in hs-CRP concentrations was observed following the administration of 200 μg/d Se among haemodialysis patients for 12 weeks( Reference Salehi, Sohrabi and Ekramzadeh 29 ). However, in our previous study, Se supplementation among GDM women for 6 weeks resulted in a significant decrease in hs-CRP levels( Reference Asemi, Jamilian and Mesdaghinia 11 ). The baseline characteristics of the study patients as well as the dosage of Se supplements along with the study duration might result in some explanations for the different findings.
We found that taking Se supplements for 6 months was associated with significant rises in plasma TAC, GSH concentrations and a significant decrease in MDA concentrations in women with CIN compared with the placebo group. In agreement with our study, the erythrocyte and plasma total antioxidant status (TAS) and erythrocyte GSH levels had significantly increased following the supplementation of Se (twice daily with 0 1 mg doses) for 45 d in patients with epilepsy and refractory epilepsy( Reference Yurekli and Naziroglu 30 ). In addition, 200 μg/d Se supplementation among GDM patients for 6 weeks resulted in a significant increase in plasma GSH and a significant decrease in plasma MDA levels( Reference Asemi, Jamilian and Mesdaghinia 11 ). However, other studies did not observe such effects of Se administration on biomarkers of oxidative stress. For instance, 200 μg/d Se supplementation for 3 weeks did not influence TAS and GSH levels in normal weight and overweight healthy subjects( Reference Savory, Kerr and Whiting 31 ). Se administration may reduce oxidative stress through participation along with selenoproteins and in the GSHPx system( Reference Ozturk, Batcioglu and Karatas 32 ) and by inhibiting the production of reactive oxygen species/reactive N species( Reference Zeng, Zhou and Huang 33 ).
To interpret our findings, some limitations need to be considered. We did not to evaluate the effects of Se administration on HPV. Unfortunately, due to limited funding, we did not examine the effects of Se supplementation on serum or urine Se concentrations, Se-dependent antioxidant enzymes including glutathione peroxidase isoforms, thioredoxin reductase and the signalling pathway involved in CIN.
In conclusion, taking Se supplements for 6 months by women with CIN1 led to its regression and had beneficial effects on the markers of insulin metabolism, TAG, VLDL and HDL-cholesterol concentrations as well as biomarkers of oxidative stress.
Acknowledgements
The authors thank the staff of Kosar Oncology Clinic (Arak, Iran) for their assistance to the current project. The authors gratefully acknowledge Fariba Kolahdooz who reviewed the manuscript and offered critical comments.
The present study was supported by a grant from the Vice-Chancellor for Research, Arak University of Medical Sciences, Iran.
Z. A. contributed to the conception, design, statistical analysis and drafting of the manuscript. M. K., S. N., A. Z. and Z. V. contributed to the conception, data collection and manuscript drafting. The final version of the manuscript was read and approved by all the authors for submission.
None of the authors has any personal or financial conflicts of interest.





