The small intestine plays an important role in the digestion and absorption of nutrients. At the same time, it constitutes a physical and immunological barrier against harmful materials including bacteria, viruses, parasites and allergenic macromolecules( Reference Kato and Owen 1 ). Intestinal barrier breakdown can increase intestinal permeability, which allows luminal antigenic agents to ‘leak’ across the mucosa, resulting in initiation or continuation of inflammatory processes and mucosal damage( Reference Hollander 2 – Reference Blikslager, Moeser and Gookin 4 ). Several studies have shown that physical, psychological and chemical stresses cause injury to intestinal barrier function( Reference Samak, Suzuki and Bhargava 5 – Reference Mawdsley and Rampton 7 ).
The mechanisms by which stress causes injury to intestinal barrier function have not been fully elucidated. However, several studies have demonstrated that stress-induced alterations in intestinal barrier function are mediated by the actions of corticotropin-releasing hormone (CRH) and subsequent activation of CRH receptors (CRHR) expressed locally in the gut( Reference Meddings and Swain 8 – Reference Smith, Clark and Overman 10 ). One particular cell type that has been related to CRH/CRHR signalling pathways and stress-induced alterations in intestinal barrier function is the mast cell( Reference Moeser, Klok and Ryan 11 ). Mast cells are haematopoietic-derived immune cells that migrate to peripheral tissues to mature and regulate various effector functions. Mast cells are becoming well known as an important cell type mediating stress-induced intestinal disorders( Reference Hart and Kamm 12 – Reference Santos, Yates and Guilarte 14 ).
Some specific nutrients such as arginine, n-3 PUFA and glutamine have been shown to mitigate intestinal barrier dysfunction at weaning( Reference Zulfikaroglu, Zulfikaroglu and Ozmen 15 , Reference Zhou and Martindale 16 ). In recent years, n-3 PUFA have received considerable attention in both human and animal nutrition. n-3 PUFA such as EPA and DHA, which are rich in fish oil, exhibit potential immunomodulatory and barrier protective effects( Reference Knoch, McNabb and Roy 17 ). Moreover, n-3 PUFA have been shown to stimulate enterocyte differentiation and intestinal maturation, reduce transcellular permeability and stabilise the intestinal barrier function( Reference Vine, Charman and Gibson 18 , Reference Yamagata, Tagami and Takenaga 19 ). However, the exact molecular mechanisms by which n-3 PUFA exert this beneficial effect are poorly understood.
Lipopolysaccharide (LPS) is a membrane component of gram-negative bacteria. A large number of studies have shown that LPS can cause damage to the intestinal barrier function( Reference De Winter, Bredenoord and De Man 20 – Reference Takakura, Hasegawa and Goto 23 ). LPS also is a potent activator of the hypothalamo–pituitary–adrenal (HPA) axis( Reference Beishuizen and Thijs 24 ). LPS exerts this effect principally by stimulating CRH and cortisol secretion( Reference Beishuizen and Thijs 24 , Reference Liu, Chen and Li 25 ). Furthermore, a well-characterised response to stress is activation of the HPA axis resulting in adrenal cortisol release( Reference Becker, Nienaber and Christenson 26 ). Therefore, LPS is a common tool used for inducing acute stress response and studying the effects of dietary regimens( Reference Beishuizen and Thijs 24 , Reference Johnson and von Borell 27 , Reference Dritz, Owen and Goodband 28 ).
Accordingly, we hypothesised that n-3 PUFA would affect intestinal barrier function by regulating CRH/CRHR signalling pathways. In the current experiment, we made use of a well-established model to induce intestinal damage in weanling piglets by administering Escherichia coli LPS. Our objective was to determine whether dietary fish oil supplementation could alleviate the damage to intestinal barrier function caused by LPS through modulation of CRH/CRHR signalling pathways.
Methods
Animals care and experimental design
The experimental protocol used in this study was approved by the Animal Care and Use Committee of Hubei Province, People’s Republic of China. In total, thirty-two pigs (Duroc×Large White×Landrace; barrows; 8·91 (sem 0·74) kg initial body weight (BW), weaned at 28 (sem 3) d of age) were randomly assigned to four treatment groups. Each treatment had eight replicate pens. The pigs were housed in 1·80×1·10-m stainless steel pens (one pig per pen). Pens contained a single-hole feeder and a nipple waterer to allow pigs ad libitum access to feed and water. The weaned pigs were fed a maize–soyabean basal diet with the addition of 5 % of fish oil (menhaden fish oil; Fujian Gaolong Company) or maize oil (Xiwang Food Company). Diets (Table 1) were formulated to meet or exceed NRC( 29 ) requirements for all nutrients. The composition of fatty acids is presented in Table 2.
Table 1 Ingredient composition of the diets (as-fed basis)

* A compound acidifier including lactic acid and phosphoric acid (Wuhan Fanhua Biotechnology Company).
† A compound mould inhibitor including calcium propionate, fumaric acid, fumaric acid monoethyl ester and sodium diacetate (Sichuan Minsheng Pharmaceutical Co. Ltd).
‡ A compound sweetener including saccharin sodium and disodium 5′-guanylate (Wuhan Fanhua Biotechnology Company).
§ The premix provided the following amounts per kilogram of complete diet: retinol acetate, 2700 μg; cholecalciferol, 62·5 μg; dl-α-tocopheryl acetate, 20 mg; menadione, 3 mg; vitamin B12, 18 μg; riboflavin, 4 mg; niacin, 40 mg; pantothenic acid, 15 mg; choline chloride, 400 mg; folic acid, 700 μg; thiamin, 1·5 mg; pyridoxine, 3 mg; biotin, 100 μg; Zn, 80 mg (ZnSO4·7H2O); Mn, 20 mg (MnSO4·5H2O); Fe, 83 mg (FeSO4·H2O); Cu, 25 mg (CuSO4·5H2O); I, 0·48 mg (KI); Se, 0·36 mg (Na2SeO3·5H2O).
|| Calculated.
¶ Analysed.
Table 2 Fatty acid composition of fish or maize oilFootnote *

* The fatty acid profiles from 4 : 0 to 24 : 1n-9 were analysed in duplicate. Only the most abundant fatty acids are listed. The detection limit was 0·01 mg/g diet for each fatty acid.
† Total n-6 PUFA and total n-3 PUFA corresponded to the sum of all the n-6 or n-3 PUFA detected.
The experiment consisted of a 2×2 factorial arrangement of treatments with diet (5 % maize oil v. 5 % fish oil) and immunological challenge (saline v. LPS). On d 19 of the trial, half of the pigs (n 8) in each dietary treatment were injected intraperitoneally with E. coli LPS (E. coli serotype 055:B5; Sigma Chemical) at 100 μg/kg BW or the equivalent amount of 0·9 % NaCl solution. The dose of LPS was chosen in accordance with our previous experiment( Reference Liu, Chen and Li 25 , Reference Liu, Chen and Odle 30 ).
Collections of intestinal sample
Four hours after administration of LPS or sterile solution, pigs were killed with sodium pentobarbital (80 mg/kg BW). The abdominal cavity was opened and the mesenteric lymph nodes (MLN), liver and spleen were harvested to measure bacterial translocation. The jejunum and ileum were separated and flushed with 0·9 % NaCl solution. A 1–3-cm-long cross-section of intestinal tissue was obtained from the mid-jejunum and mid-ileum and fixed in 4 % paraformaldehyde in PBS for histological analysis. Mucosal samples were obtained by cutting segments lengthwise and scraping mucosa from the connective tissue, were immediately frozen in liquid N2 and then stored at −80°C until measurement of mRNA abundance. Previous studies have shown that LPS caused acute intestinal morphological damage and a breakdown in intestinal barrier function in rats, mice and pigs within 3–6 h after injection( Reference Liu, Huang and Hou 31 – Reference Alscher, Phang and McDonald 33 ). Therefore, the time point of 4 h after LPS or saline injection was chosen for experimental measurements.
Bacterial translocation
Bacterial translocation analysis was based on the method of Yang et al.( Reference Yang, Han and Uchiyama 34 ) and Chen et al.( Reference Chen, Hsu and Wang 35 ) with modifications. The collected MLN, spleen and liver were weighed and homogenised in ten volumes of ice-cold sterile saline. Aliquots of 50 μl of the homogenate from each tissue were plated onto blood and MacConkey’s agar plates and examined after 24 h of aerobic incubation at 37°C. In fact, there are anaerobes and aerobes in the intestine, the former outnumbering the latter. However, most previous investigators have attributed the main role in bacterial translocation to aerobic and facultative gram-negative bacteria( Reference Lichtman 36 – Reference Berg and Itoh 38 ). Therefore, we measured bacterial translocation in aerobic condition. The colonies were counted, and the results are expressed as colony-forming units (CFU) per gram of tissue. For each tissue, positive bacterial translocation was defined as at least four out of eight plates showing bacterial growth with CFU >50/g of tissue.
Histology
Fixed intestinal samples were dehydrated with graded ethanol solutions, cleared with xylene and embedded in paraffin. Histological slides were prepared from three cross-sections (4-μm thick) of each intestinal sample and stained with haematoxylin and eosin. The villus height and crypt depth were measured, and the villus:crypt ratio was calculated by dividing villus height by crypt depth. Villus area was quantitated from the perimeter and height of the villi( Reference Nunez, Bueno and Ayudarte 39 ). The ten longest and straightest villi and their associated crypts from each segment were measured. The same villus and crypt were used to determine the number of intraepithelial lymphocytes (IEL) and goblet cells. These variables were expressed per 100 enterocytes.
The number of lamina propria cells, neutrophils and mast cells was counted in histological sections according to cellular and nuclear morphology and toludine blue staining (for mast cells). Cell counts were determined utilising image analysis programme and expressed as number of cells/mm2. All cell counts and intestinal morphological measurements were performed by a histologist who was blinded to the treatments.
Western blot analysis
The method for protein immunoblot analysis in intestinal mucosa was the same as that described in the study by Hou et al.( Reference Hou, Wang and Ding 40 ). In brief, the intestinal samples (100–150 mg) (n 8) were homogenised and lysed in ice-cold lysis buffer. The homogenates were centrifuged at 12 000 g for 15 min at 4°C, and the supernatant was used for Western blot and protein assay. Protein concentration was determined using the bicinchoninic acid protein assay kit (Applygen Technologies Co. Ltd). Intestinal mucosal proteins were separated on a polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 3 % bovine serum albumin in Tris-buffered saline (TBS)-Tween-20 buffer at least for 60 min at room temperature (21–25°C). The membranes were incubated overnight (12–16 h) at 4°C with rabbit anti-claudin-1 (1:1000; no. 51-9000; Invitrogen Technology) or mouse anti-β-actin (1:10 000; no. A2228; Sigma Aldrich). The membranes were washed three times (for 5 min each) with TBS-T (1×Tris-buffered saline including 0·1 % Tween-20) and incubated with goat anti-rabbit (no. ANT020) or mouse (no. ANT019) IgG horseradish peroxidase conjugated secondary antibody (1:5000; AntGene Biotech Co. Ltd) for 120 min at room temperature. Membranes were washed three times with TBS-T over 30 min. Blots were developed with enhanced Chemiluminescence Western blotting kit (Amersham Biosciences), visualised using a Gene Genome bioimaging system and analysed using GeneTools software (Syngene). The relative expression of claudin-1 protein was expressed relatively to β-actin protein.
Intestinal alkaline phosphatase activity analysis
Intestinal alkaline phosphatase (IAP) activity was determined by a microplate reader (SpectraMax M5; Molecular Devices) using a commercial kit (no. A059-2; Nanjing Jiancheng Bioengineering Institute) according to the instructions of the manufacturer. The protein concentrations of intestinal mucosa were determined using Coomassie Brilliant Blue G-250 reagent with bovine serum albumin as a standard. Specific activity of 1 King unit of IAP is defined as the amount of the enzyme that produces 1 mg р-nitrophenol/g of protein for 15 min at 37°C.
mRNA expression analysis by real-time PCR
Total RNA isolation, quantification, RT and real-time PCR were performed as previously described(
Reference Liu, Chen and Odle
30
). The primer pairs used are shown in online Supplementary Table S1. The expressions of the target genes relative to the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were analysed by the
![]() $2^{{{\minus}\Delta \Delta C_{T} }} $
method of Livak & Schmittgen(
Reference Livak and Schmittgen
41
). Our results demonstrated that GAPDH did not display any difference among treatments and tissues. Relative mRNA abundance of each target gene was normalised to the group receiving maize oil and treated with sterile 0·9 % NaCl solution.
$2^{{{\minus}\Delta \Delta C_{T} }} $
method of Livak & Schmittgen(
Reference Livak and Schmittgen
41
). Our results demonstrated that GAPDH did not display any difference among treatments and tissues. Relative mRNA abundance of each target gene was normalised to the group receiving maize oil and treated with sterile 0·9 % NaCl solution.
Statistical analysis
The data were analysed by way of variance specific for repeated measurements using mixed procedure of SAS (SAS Institute Inc.). The model included the treatments, gut segment (jejunum and ileum) and their interactions. When a significant treatment×gut segment interaction occurred, comparisons among treatments in each segment (jejunum or ileum) were conducted. If the interaction was not significant, the arithmetic means of the gut segment (jejunum and ileum) were used to compare treatment effects. Effects of LPS and diet were analysed as a 2×2 factorial arrangement by ANOVA using the general linear model (GLM) procedures of SAS. The statistical model included the effects of immunological challenge (saline or LPS), diet (maize oil or fish oil) and their interactions. When significant diet×LPS interaction or a trend for diet×LPS interaction occurred, post hoc testing was performed using Bonferroni’s multiple comparison tests. When variances were heterogeneous, non-parametric ANOVA was conducted. Bacterial translocation (positive animals) data were analysed by one-way ANOVA and Fisher’s exact test. Data are presented as mean values with pooled standard errors. Differences were considered to be significant if P<0·05. Instances where 0·05<P≤0·10 were discussed as trends.
Results
Bacterial translocation
Pigs fed fish oil had lower bacteria translocation incidence (P=0·005) in MLN compared with pigs fed maize oil among LPS-treated pigs, whereas there was no difference among saline-treated pigs (Table 3). Neither LPS nor diet affected bacterial translocation incidence in the spleen and liver. There was no interaction between LPS challenge and diet on bacterial translocation in the MLN, spleen and liver (Table 4). Pigs challenged with LPS had higher translocation micro-organisms in the liver (P=0·004) and tended to have higher translocation micro-organisms in the MLN (P=0·092) than those injected with saline. Moreover, pigs fed fish oil had lower translocation micro-organisms in the MLN (P=0·049) than those fed maize oil. However, neither LPS nor diet affected translocation micro-organisms in the spleen.
Table 3 Effect of fish oil or maize oil supplementation on bacterial translocation incidences in weaned pigs after Escherichia coli lipopolysaccharide (LPS) challenge (n 8 (1 pig/pen))

MLN, mesenteric lymph node.
* P<0·05, Maize oil+LPS v. fish oil+LPS (Fisher’s exact test).
Table 4 Effect of fish oil or maize oil supplementation on translocation micro-organisms of weaned pigs after Escherichia coli lipopolysaccharide (LPS) challengeFootnote * (Means values with their pooled standard errors; n 8 (1 pig/pen))

MLN, mesenteric lymph node; CFU, colony-forming units.
* The values were expressed in log10 (CFU)/g of organ’s weight.
Intestinal morphology
Pigs challenged with LPS displayed intestinal mucosal injury including lifting of epithelium at the tip of the villus and villous atrophy. Fish oil supplementation alleviated intestinal mucosal injury caused by LPS (Fig. 1). There was a treatment×segment interaction observed for villus height (P=0·05). Pigs challenged with LPS had lower villus height in the jejunum (P=0·001) than those injected with saline (Table 5). Pigs fed fish oil had higher villus height in the jejunum (P=0·044) and lower villus height in the ileum (P=0·028) than pigs fed maize oil.

Fig. 1 Intestinal mucosal morphological characterisation of the jejunum (haematoxylin and eosin stained). (a) Pigs fed maize oil and injected with sterile saline. No obvious changes were found. (b) Pigs fed fish oil and injected with sterile saline. No obvious changes were found. (c) Pigs fed maize oil and challenged with lipopolysaccharide (LPS). Morphological changes associated with intestinal mucosal injury, such as lifting of epithelium at the tip of the villus (A) and villous atrophy (B). (d) Pigs fed fish oil and injected with LPS. Intestinal mucosal injury was significantly attenuated. Original magnifications 100×. Scale bars=82·4 μm.
Table 5 Effect of fish oil or maize oil supplementation on the intestinal morphology of weaned pigs after Escherichia coli lipopolysaccharide (LPS) challenge (Means values with their pooled standard errors; n 8 (1 pig/pen))
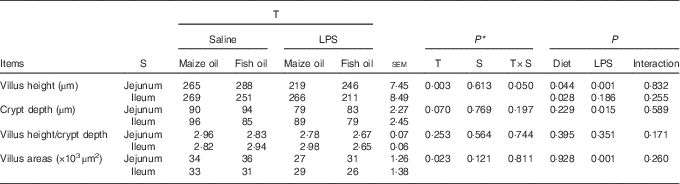
T, treatment; S, segment.
* P-values obtained using treatment as the main effect and analysing data from the jejunum and ileum as repeated measurements.
No significant treatment×segment interaction was observed for crypt depth, villus height/crypt depth and villus areas. Overall, pigs challenged with LPS had lower villus area (P=0·001) and crypt depth (P=0·015) than those injected with saline. Moreover, pigs fed fish oil tended to have lower villus height/crypt depth (P=0·080) and had higher crypt depth (P=0·006). There was no LPS challenge×diet interaction observed for crypt depth, villus height/crypt depth and villus areas.
Intestinal tight junction protein claudin-1 expression
Claudin-1 tended to be higher in the ileum than in the jejunum (P=0·067) (Table 6). A trend for treatment×segment interaction was observed for claudin-1 expression (P=0·078). Pigs challenged with LPS had lower claudin-1 expression (P=0·042) in the ileum than those injected with saline. Pigs fed fish oil had higher claudin-1 expression (P=0·034) in the ileum and trended to have higher claudin-1 expression in the jejunum compared with those fed maize oil (P=0·053). There was no LPS challenge×diet interaction observed for claudin-1 expression in the jejunum and ileum.
Table 6 Effect of fish oil or maize oil supplementation on intestinal tight junction protein expressions, intestinal alkaline phosphatase (IAP) activity and gene expression in weaned pigs after Escherichia coli lipopolysaccharide (LPS) challenge (Means values with their pooled standard errors; n 8 (1 pig/pen))

T, treatment; S, segment.
* P-values obtained using treatment as the main effect and analysing data from the jejunum and ileum as repeated measurements.
Intestinal alkaline phosphatase activity and mRNA expression
IAP activity was higher in the ileum than that in the jejunum (P=0·014) (Table 6). No treatment×segment interaction was observed for IAP activity and mRNA expression. Overall, pigs challenged with LPS had lower IAP activity (P=0·012) and mRNA expression (P=0·031) than those injected with saline. There was no LPS challenge×diet interaction observed for IAP activity and mRNA expression.
Intestinal immune cells and lamina propria cells
The numbers of IEL (P=0·019) and lamina propria cells (P=0·007) were lower in the jejunum than those in the ileum (Table 7). There was an interaction (P=0·002) between treatment and segment on mast cells. Pigs challenged with LPS had higher mast cell number in the ileum (P<0·001) than those injected with saline (Fig. 2). A LPS×diet interaction (P=0·002) was observed for mast cell number in the jejunum, with pigs fed fish oil having lower mast cell number (P<0·001) compared with pigs fed maize oil among LPS-treated pigs, whereas there was no difference among saline-treated pigs.

Fig. 2 Photomicrographs of pig jejunal mucosa showing mast cells stained with toluidine blue. (a) Pigs fed maize oil and injected with sterile saline. (b) Pigs fed fish oil and injected with sterile saline. (c) Pigs fed maize oil and challenged with lipopolysaccharide (LPS). (d) Pigs fed fish oil and injected with LPS. Arrows indicate toluidine blue-positive mast cells. Pigs fed fish oil had lower mast cell numbers (P<0·05) compared with pigs fed maize oil among LPS-treated pigs, whereas there was no difference among saline-treated pigs. Original magnifications 400×. Scale bars=22·4 μm.
Table 7 Effect of fish oil or maize oil supplementation on immune cells and lamina propria cells in the intestine of weaned pigs after Escherichia coli lipopolysaccharide (LPS) challenge (Means values with their pooled standard errors; n 8 (1 pig/pen))

T, treatment; S, segment; IEL, intraepithelial lymphocyte.
* P-values obtained using treatment as the main effect and analysing data from jejunum and ileum as repeated measurements.
No treatment×segment interaction was found for IEL, goblet cell, lamina propria cell and neutrophil numbers. Overall, pigs challenged with LPS had lower IEL (P<0·001) and lamina propria cell numbers (P<0·001) and higher neutrophil number (P<0·001) than those injected with saline. There was an interaction (P=0·046) between LPS challenge and diet on IEL number. Pigs fed fish oil had higher IEL number (P=0·034) compared with pigs fed maize oil among LPS-treated pigs, whereas there was no difference among saline-treated pigs. No LPS challenge×diet interaction was observed for goblet cells, lamina propria cells and neutrophils. Pigs fed fish oil had lower neutrophil number (P=0·002) (Fig. 3) compared with those fed maize oil. Neither LPS nor diet affected goblet cell number in the jejunum and the ileum.

Fig. 3 Representative photomicrographs of pig jejunal mucosa showing neutrophils (haematoxylin and eosin stained). (a) Pigs fed maize oil and injected with sterile saline. (b) Pigs fed fish oil and injected with sterile saline. (c) Pigs fed maize oil and challenged with lipopolysaccharide (LPS). (d) Pigs fed fish oil and injected with LPS. Arrows indicate neutrophils. Pigs challenged with LPS had higher numbers of jejunal neutrophils (P<0·05) than those injected with saline. Pigs fed fish oil had lower neutrophil numbers in the jejunum (P<0·05) compared with those fed maize oil (P=0·058). Original magnifications 400×. Scale bars=22·4 μm.
Corticotropin-releasing hormone receptor 1, corticotropin-releasing hormone, glucocorticoid receptor and tryptase mRNA expressions
The mRNA abundance of CRHR1 in the jejunum was higher compared with the ileum (P=0·003, Table 8). There was a treatment×segment interaction (P=0·03) observed for the mRNA abundance of CRHR1. Pigs challenged with LPS had higher mRNA abundance of jejunal (P=0·002) and ileal CRHR1 (P<0·001) than those injected with saline. Fish oil supplementation tended to decrease the mRNA abundance of CRHR1 in the ileum (P=0·053).
Table 8 Effect of fish oil or maize oil supplementation on mRNA expressions of corticotropin-releasing hormone (CRH), glucocorticoid receptors (GR), tryptase and corticotropin-releasing hormone receptor 1 (CRHR1) in the intestine of weaned pigs after Escherichia coli lipopolysaccharide (LPS) challengeFootnote * (Means values with their pooled standard errors; n 8 (1 pig/pen))
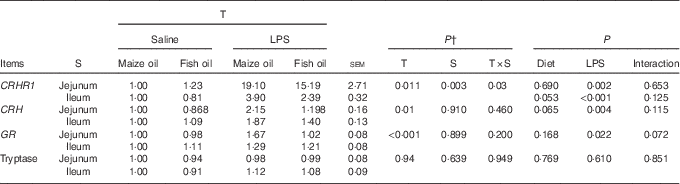
* All the data were acquired using real-time PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the housekeeping gene, and the pigs fed the maize oil diet and injected with saline comprised the calibrator samples.
† P-values obtained using treatment as the main effect and analysing data from the jejunum and ileum as repeated measurements.
There was no treatment×segment interaction observed for CRH, glucocorticoid receptors (GR) and tryptase. Overall, pigs challenged with LPS had higher mRNA abundance of CRH (P=0·004) and GR (P=0·022) than those injected with saline. There was a trend for LPS challenge×diet interaction observed for GR (P=0·072). Pigs fed fish oil had lower mRNA abundance of GR compared with pigs fed maize oil among LPS-treated pigs, whereas there was no difference among saline-treated pigs. There was no LPS challenge×diet interaction observed for CRH and tryptase. However, pigs fed fish oil tended to have lower mRNA abundance of CRH (P=0·065) compared with those fed maize oil. Neither LPS nor diet affected tryptase mRNA abundance.
Discussion
Our previous studies have demonstrated that fish oil supplementation alleviated LPS-induced activation of the HPA axis and intestinal injury in a weaned piglet model( Reference Liu, Chen and Li 25 , Reference Liu, Chen and Odle 30 ). In the current experiment, our aim was to investigate the effect of dietary fish oil supplementation on the intestinal barrier function in weaned pigs.
The intestinal mucosal barrier is the first line of defence against a hostile environment within the intestinal lumen( Reference Smith, Clark and Overman 10 ). The breakdown of this barrier may result in the crossing of viable bacteria and their products to MLN and more distant sites – a process known as bacterial translocation( Reference Gatt, Reddy and MacFie 42 ). In the present study, we showed that bacteria translocation incidences and translocation micro-organisms in the MLN and the liver increased or trended to increase in the pigs with LPS challenge, which indicates that intestinal obstruction really led to an increase in intestinal permeability. We also found that LPS caused the decrease of IAP activity and mRNA expression. In fact, IAP plays important roles in LPS dephosphorylation, reduction of LPS-induced intestinal inflammation and restriction of bacterial translocation( Reference Goldberg, Austen and Zhang 43 , Reference Lallès 44 ). Therefore, the decrease in IAP activity and mRNA expression reduced intestinal capacity to detoxify LPS, which in turn may lead to intestinal barrier dysfunction and inflammation. Fish oil reduced bacterial translocation incidences and translocation micro-organisms in the MLN. However, fish oil did not affect IAP activity and mRNA expression. In contrast with our findings, Nieto et al.( Reference Nieto, Torres and Ríos 45 ) reported that 10 % fish oil supplementation decreased IAP activity in ulcerative rats compared with 10 % olive oil.
The intestinal barrier function is partly dependent on the mucosal structure of the intestine. Our histological study showed that LPS caused a significant morphological injury to the mucosa of the intestine, such as epithelium lifting and villous atrophy. Consistent with bacterial translocation, fish oil alleviated villous epithelium lifting caused by LPS. Similarly, Whiting et al.( Reference Whiting, Bland and Tarlton 46 ) reported that a diet enriched with n-3 PUFA (fish oil) enhanced epithelial barrier function and ameliorated several chronic inflammatory diseases.
The intestinal barrier function is maintained and regulated by the tight junctions between intact epithelial cells( Reference Anderson and Van Itallie 47 ). The formation of tight junctions requires the assembly of several proteins anchored directly or indirectly to the actin-based cytoskeleton. Tight junction proteins include occudin and members of a large class of proteins called claudins( Reference Yang, Han and Uchiyama 34 ). In the present study, fish oil increased claudin-1 expression in the ileum and tended to increase claudin-1 expression in the jejunum. In agreement with our finding, Li et al.( Reference Li, Zhang and Wang 48 ) reported that n-3 PUFA improved tight junction formation and reduced transcelluar permeability. In the intestine, the dynamic renewal of the epithelium is characterised by cell production in the crypts followed by cell maturation and cell migration to the tip of the villi( Reference Boudry, Douard and Mourot 49 ). Many reports have shown that n-3 PUFA stimulate differentiation, support intestinal maturation and reduce transcellular permeability( Reference Vine, Charman and Gibson 18 , Reference Yamagata, Tagami and Takenaga 19 , Reference Pscheidl 50 ). Our results indicate that fish oil protected the intestinal integrity and maintained barrier function partially by improving intestinal morphology and the expressions of tight junction proteins.
The main sites of the mucosal immune system in the intestine are gut-associated lymphoid tissue and immuno-associated cells such as IEL, mast cells and goblet cells( Reference Wang, Ma and She 51 ). IEL play an important role in maintaining the intestinal barrier function and as such form the first line of defence against infectious agents or allergens. It is well known that LPS causes gut barrier dysfunction and induces inflammatory response( Reference Deventer, Cate and Tytgat 52 , Reference Webel, Finck and Baker 53 ). In the present study, LPS increased neutrophil infiltration and decreased IEL numbers in the intestine. However, fish oil increased IEL numbers and decreased neutrophil infiltration in the jejunum. Maeshima et al.( Reference Maeshima, Fukatsu and Moriya 54 ) reported that adding fish oil to parenteral nutrition partially reversed parenteral nutrition-induced IEL loss. In addition, dietary n-3 PUFA have been reported to protect intestinal epithelial cells from pro-inflammatory insults, alleviate the intestinal inflammatory response and accelerate recovery from inflammation( Reference Liu, Chen and Odle 30 , Reference Shen, Rex Gaskins and McIntosh 55 ). Moreover, previous studies in mice have shown that the decrease in IEL number was associated with decreasing tight junction integrity in the intestine( Reference Kudsk 56 ). This finding supports the current finding that fish oil maintained epithelial barrier integrity by inducing the proliferation of IEL and alleviating the intestinal inflammatory response.
The mechanisms by which stress causes breakdown in intestinal barrier function are not been fully understood. Several studies have demonstrated that stress-induced alterations in intestinal barrier function are mediated by the release of central and peripheral stress mediators such as CRH and adrenal glucocorticoids( Reference Meddings and Swain 8 – Reference Smith, Clark and Overman 10 ). Subsequent activation of CRHR and GR has been shown to trigger disturbances in the intestinal barrier( Reference Santos, Saunders and Hanssen 57 , Reference Teitelbaum, Gareau and Jury 58 ). Our previous studies also demonstrated that LPS-induced activation of the HPA axis, whereas fish oil attenuated the activation of the HPA axis( Reference Liu, Chen and Li 25 ). In the present study, we observed that LPS increased mRNA abundance of CRHR1, CRH and GR in the intestine. Fish oil supplementation tended to alleviate the increase in mRNA abundance of CRH and GR caused by LPS. Moeser et al.( Reference Moeser, Klok and Ryan 11 ) reported that CRH might be a more sensitive stress indicator of stress-induced intestinal dysfunction. Smith et al.( Reference Smith, Clark and Overman 10 ) found that weaning-induced mucosal barrier dysfunction could be prevented by a CRH receptor antagonist, which suggests that CRH plays an important role in intestinal mucosal barrier integrity. CRH mediates its effects by binding to two CRH receptors (CRHR1 and CRHR2), which are expressed on multiple intestinal cell types, including enteric neurons, lamina propria immune cells and epithelial cells( Reference Liu, Gao and Gao 59 – Reference Von Mentzer, Murata and Ahlstedt 61 ). Moeser et al.( Reference Moeser, Klok and Ryan 11 ) have shown that peripheral CRHR activation mediates intestinal mucosal disturbances induced by early weaning. On the basis of the CRHR antagonist experiment, Smith et al.( Reference Smith, Clark and Overman 10 ) indicated that CRHR1 likely mediated impaired intestinal barrier dysfunction and hypersecretion in early-weaned pigs. Similarly, our study showed that LPS increased mRNA abundance of CRHR1 in the intestine. Fish oil supplementation alleviated the increase of ileal mRNA abundance of CRHR1 caused by LPS. However, fish oil did not affect jejunal mRNA abundance of CRHR1. The reason might be that the different segments of the intestine had different responses to fish oil supplementation. These results suggest that fish oil might improve intestinal barrier function and inhibit the CRH/CRHR1 signalling pathway.
In addition, mast cells are recognised as an important cell type mediating stress-related intestinal disorders( Reference Hart and Kamm 12 , Reference Barbara, Stanghellini and De Giorgio 62 , Reference Downing and Miyan 63 ). Mast cells participate in the regulation of intestinal motility, gut barrier function and mucosal immune function( Reference Santos, Guilarte and Alonso 64 ). Mast cells express CRHR. Mast cells can be activated either directly by CRH or indirectly by neuropeptides released from the CRH-stimulated neural process( Reference Santos, Benjamin and Yang 65 ). Our histological study showed that LPS increased mast cell number in intestinal mucosa. Mast cell number increased along with mast cell activation in the jejunum of diarrhoea-predominant irritable bowel syndrome patients( Reference Guilarte, Santos and de Torres 66 ). Mast cell activation leads to degranulation and release of several mediators such as protease, histamine, eicosanoids, cytokines and chemokines, which results in injury to the intestinal structure and increase in intestinal permeability( Reference Metcalfe, Baram and Mekori 67 ). Our data showed that fish oil alleviated the increase in mast cell number induced by LPS. Similarly, Wang & Marianna( Reference Wang and Marianna 68 ) found that n-3 PUFA inhibited mast cell activation by disruption of FcεRI localisation and shuttling into lipid rafts. In present study, fish oil alleviated LPS-induced increase in mast cell number, which might decrease the release of protease, histamine, eicosanoids and cytokines from mast cells, thereby maintaining gut barrier function.
In conclusion, fish oil supplementation attenuates disruption of intestinal barrier function and inflammatory response induced by LPS. It suggests that fish oil supplementation improves intestinal barrier function and inhibits CRH/CRHR1 signalling pathways and mast cell tissue density.
Acknowledgements
This study was supported by National Science Foundation of China (31422053 and 31372318) and the project of the Hubei Province Department of Education (T201508).
The authors’ contributions are as follows: Y. L., H. Z. and S. C. designed the study; H. Z., Y. L., S. C., X. W., D. P. and W. L. conducted the study; H. Z., Y. L., D. P. and W. L. analysed the data; H. Z., Y. L., D. P. and W. L. wrote the paper; Y. L. had primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001100














