Fish is a good dietary source of nutrients such as proteins, fats, and several vitamins and minerals; however, the contents of nutrients in fish varies across fish species(1). Fish consumption is associated with reduced risk of diseases such as type 2 diabetes and CVD(Reference Whelton, He and Whelton2–Reference Zheng, Huang and Yu8), and the health benefits of fish consumption have traditionally been attributed mainly to the effects of n-3 PUFA and vitamin D, especially from fatty fish. Vitamins play essential roles in numerous processes in the body, including metabolism of carbohydrates, fatty acids and amino acids(Reference Brandenburg, Vervloet and Marx9–Reference Allen15). Low serum concentrations of fat-soluble and water-soluble vitamins have been linked to the risk of developing obesity-related co-morbidities including insulin resistance and type 2 diabetes, and low circulating concentration of 25-hydroxyvitamin D3 is associated with increased risk of cardiovascular events(Reference Wang, Song and Manson16,Reference Motiwala and Wang17) . In Norway, vitamin fortification is limited to dairy products (vitamin D) and margarine (vitamins A and D)(18). Cutaneous synthesis of 25-hydroxyvitamin D3 stimulated by sunlight is the major source of vitamin D in humans(Reference Moan, Porojnicu and Dahlback19); therefore, vitamin D-rich foods such as fatty fish, seafood, cod-liver oil, entrails and fortified products are important food sources in northern countries such as Norway in late autumn, winter and early spring when exposure to UVB radiation is low.
We have previously demonstrated that a high salmon intake (750 g/week) for 8 weeks improved post-prandial glucose regulation in study participants with overweight/obesity, whereas high cod intake (750 g/week) did not affect glucose regulation in the same study setting(Reference Helland, Bratlie and Hagen20). In the present study, we wanted to further explore the biological materials from this randomised clinical trial to gain insight into if a high intake of cod or salmon would affect serum vitamin status. The aim of the present study was to investigate effects of high intake of salmon or cod on serum vitamin status. The intervention was conducted in the autumn of 2011 in South-Western Norway (60° north latitude). Our hypothesis was that the high salmon intake would prevent the normal seasonal decline in circulating vitamin D concentration in autumn and that high intake of cod or salmon would improve the general vitamin status.
Methods
Participants, study setting and ethics
The study design, the description of study participants, the study setting and the protocol for study visits have been published in detail previously(Reference Helland, Bratlie and Hagen20). In brief, the study population consisted of adults with overweight or obesity and all participants were of Norwegian ethnic origin (Caucasian) living in the Bergen area in South-Western Norway at 60° north latitude. Inclusion criteria were: BMI ≥ 27 kg/m2, fasting blood glucose ≤ 7·0 mmol/l and age 18–69 years. Exclusion criteria were high habitual fish/seafood intake (>500 g/week), pregnancy, incompatibility with fish consumption (allergies, intolerance and/or dislike), diagnosed diabetes mellitus, heart disease or gastrointestinal diseases, use of medications affecting lipid metabolism or glucose homoeostasis, use of anti-inflammatory medications, use of supplements containing n-3 PUFA, intentional weight loss and large fluctuation in body weight (>3 kg) over the previous 2 months. Participants were interviewed about their fish/seafood intake before they were included in the study, and those with a regular fish intake >1 fish dinner per week were instructed to avoid eating fish for 4 weeks before the baseline visit.
The study was designed as a randomised, controlled intervention study with a parallel group design, with three intervention arms: Atlantic cod (wild-caught Gadus morhua) in weekly doses of 750 g, salmon (farmed Salmo salar) in weekly doses of 750 g and a no-fish group as the Control group. The intervention period was 8 weeks. In all, seventy-six participants were included in the study and were randomly assigned to the Cod group (n 27), Salmon group (n 27) or the Control group (n 22). The participants were randomised into the different groups by the project manager by drawing lots, and the participants were informed about their group allocation during the baseline visit. All examinations were conducted at the Clinical Research Unit at the Haukeland University Hospital, Bergen, Norway. To enhance compliance, the participants were contacted by phone approximately 1 week prior to baseline and endpoint visits, during which they were informed of the schedule and procedures for the following visit. Also, a text message was sent 1–3 d before the 8-week visit, as a reminder of how to prepare for the upcoming visit. For any inquires during the trial period, members of the research group could be reached by email or telephone. Compliance was monitored through interviews; after 1, 4 and 8 weeks intervention, the participants in the fish-eating groups were asked how many dinners with cod/salmon they had not eaten since last contact, instead of asking how well they had complied, to lower the bar for reporting missing intake. Non-compliance was defined as not following the protocol in regard to fish intake (omitting more than three fish dinners in the fish-eating groups), other dietary changes or use of prescription medicine not compatible with the inclusion criteria, or changes in physical activity. As reward for completing the study, participants were offered a dietary consultation with a student dietitian at the last visit and all the results of analyses of blood samples.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Regional Committee for Medical and Health Research Ethics of Western Norway (REC no.: 2011/572). Written informed consent was obtained from all subjects.
Health professionals performing blood sampling, and personnel conducting the laboratory analyses, were all blinded to participants’ identity and group allocation. All data were analysed anonymously. This trial was registered at clinicaltrials.gov as NCT02350595.
Interventions
Cod and salmon fillets were provided to the participants as frozen-skin and boneless-fillet portions (mean weight 150 (sd 10) g; Lerøy Seafood Group ASA), and pallets of fish were chosen at random from Lerøy’s warehouse in Bergen, Norway. The cod and salmon fillets were supplied free of charge to the participants and were distributed at the baseline visit or at any time during the study period, if preferred. Participants in the Cod group were instructed to eat five dinners per week containing 150 g of cod fillet, and participants in the Salmon group were instructed to eat five dinners per week containing 150 g of salmon fillet. The participants in the fish-eating groups were told not to exceed a total amount of 750 g of fish/week, not to consume any other types of fish or seafood during the study period and to maintain their normal eating habits throughout the study period apart from eating the mandatory amount of 750 g fish/week. The Control group was instructed to continue their normal eating habits, except to avoid fish and seafood intake. Subjects in all groups were instructed not to change their physical activity level during the intervention period. The participants’ dietary intake and habitual lifestyle were recorded at baseline and endpoint visits, using food record charts and a questionnaire for reporting physical activity. Reported energy and macronutrient intake and physical activity were not changed within the groups during the study period(Reference Helland, Bratlie and Hagen20).
Protocol for study visits
The total study period was 8 weeks, with baseline visits between 22 August 2011 and 19 September 2011. Examinations were conducted in the morning after an overnight fast. The subjects were instructed not to eat or drink anything except water, and not to use substances containing nicotine after 22 h the previous day, and to avoid physical exercise and alcohol for 24 h before each sampling day.
Body height was measured at the baseline visit, using a wall-mounted stadiometer (Seca 222; Seca). Body weight and body composition were measured in a fasted state using a bioelectrical impedance analysis device (InBody 720; Biospace Co. Ltd) at the baseline and endpoint visits.
Fasting blood samples were collected at baseline and endpoint, in BD Vacutainer SST II Advance gel tubes (Becton, Dickinson and Company) for isolation of serum. The staff complied with a strict protocol for pre-analytical sample handling to ensure high sample quality. Blood samples were centrifuged after 30 min at room temperature, and serum were immediately aliquoted and frozen at −80°C until analyses.
Estimation of vitamin intakes from dietary records
Participants completed dietary records of the 5 preceding days before the baseline visit and the 5 preceding days before the 8-week visit, including at least 1 weekend day. The estimated intakes of vitamins A, B1, B2, B3, B6, B9, C, D and E were calculated from the participants’ dietary records using the ‘Mat på Data 5.1’ software(21), which contains information on the nutrient contents in food items sold in Norway. This food database does not contain information of vitamins B12 and K1 contents in foods. Food records were checked for completeness at both study visits.
Analyses of serum samples
Vitamin B1 (thiamine and thiamine monophosphate), vitamin B2 (riboflavin and flavin mononucleotide), vitamin B3 (nicotinic acid, nicotinamide and N1-methylnicotinamide), vitamin B6 (pyridoxal 5′-phosphate, pyridoxal, pyridoxine and 4-pyridoxic acid) were analysed in serum by Bevital AS (http://www.bevital.no) using liquid chromatography combined with tandem MS, as previously described(Reference Midttun, Hustad and Ueland22). Thiamine, thiamine monophosphate, nicotinic acid, nicotinamide and N1-methylnicotinamide with corresponding isotope labelled internal standards were added to the previously published assay(Reference Midttun, Hustad and Ueland22). Vitamin A (all-trans retinol), vitamin D (25-hydroxyvitamin D2 and 25-hydroxyvitamin D3), vitamin E (α-tocopherol and γ-tocopherol) and vitamin K1 (phylloquinone) were measured by liquid chromatography–tandem MS(Reference Midttun, McCann and Aarseth23), and vitamin B12 (Reference Kelleher and Broin24) and folate(Reference Molloy and Scott25) by microbiological assays. Serum concentration of vitamin K1 was below the level of detection (0·33 nmol/l) for sixteen samples, for these we used the value level of detection divided by 2. Serum concentrations of 25-hydroxyvitamin D2, nicotinic acid and pyridoxine were below level of detection in all samples.
Outcome measurements
The primary outcome of the present study was changes in serum vitamin D concentration after a weekly intake of 750 g fillet from either salmon or cod for 8 weeks. Secondary outcomes were changes in serum concentrations of other fat-soluble and water-soluble vitamins and changes in estimated dietary intakes of vitamins within the groups over time.
Sample size estimation
The sample size calculation for this trial was originally conducted with the aim to investigate the effects of high intake of cod or salmon on post-prandial glucose regulation after a standardised breakfast in participants with overweight or obesity(Reference Helland, Bratlie and Hagen20). We estimated that it was necessary to include seventy-six participants divided into three groups to ensure that twenty participants in each group completed the trial with satisfactory compliance, with a power of 80 % and α of 0·05, and of these sixty-five participants were included in statistical analyses(Reference Helland, Bratlie and Hagen20). In the present study, we wanted to further explore the biological materials from this randomised clinical trial to investigate if a high intake of cod or salmon would affect serum vitamin status. The primary aim of the present study was to investigate the effects of high intake of salmon or cod on changes in serum vitamin D concentration in autumn at 60° north latitude. From two of the participants, we did not have a sufficient amount of blood serum left for analyses; thus, we had serum samples from sixty-three subjects available for laboratory and statistical analyses.
This is the first study to investigate the effects of 8 weeks of high intake (750 g/week) of cod or salmon on serum vitamin status in adults with overweight/obesity living in South-Western Norway; therefore, no data are available for sample size calculation for the present study. However, sample size estimation using data from a pilot study conducted in spring in Bergen, Norway, with normal-weight adults consuming 750 g/week of cod, salmon or chicken for 4 weeks showed that thirteen participants in each group were sufficient to detect differences between groups for changes in vitamin D, with a power of 80 % and α of 0·05. Based on this, the present study where 19–22 adults with overweight/obesity consume 750 g/week of cod, salmon or fish-free diet for 8 weeks should have the sufficient statistical strength for comparing changes in vitamin D in autumn at 60° north latitude between groups consuming cod, salmon or fish-free diet.
Statistical analyses
Statistical analyses were conducted using SPSS Statistics 25 (SPSS, Inc., IBM Company). Subjects who did not complete the study were excluded from the statistical analyses. For analytes in serum and for estimated intake of vitamins from dietary records, most data were not normally distributed according to the Shapiro–Wilk test and non-parametric tests were used to investigate changes within groups (Wilcoxon signed-rank test). For these non-parametric data, the Kruskal–Wallis test was used to compare values between the three groups at baseline. Changes within the groups were compared using univariate ANCOVA with adjustment for baseline values after log transformation, followed by the Tukey’s honestly significant difference test whenever between-group differences were detected. Data are expressed as medians and 25th, 75th percentiles. Categorical data were compared using Pearson’s χ 2 test. All comparisons were two-sided, and P < 0·05 was considered statistically significant.
Results
Participant characteristics
In total, seventy-six participants were included in the study and completed the first study visit, and sixty-eight participants completed the trial. One participant (a woman in the Salmon group) was excluded from statistical analysis after analyses of post-prandial blood glucose that revealed she had prediabetes, and two participants (one woman in the Cod group and one man in the Salmon group) were withdrawn from analysis because they did not comply with the protocol. From two of the participants (one man in the Salmon group and one man in the Control group), we did not have a sufficient amount of blood serum for analyses; therefore, these two subjects are excluded from all analyses in the present paper. In total, sixty-three subjects (twenty-seven men and thirty-six women) were included in the statistical analyses. The flow of participants in the study is presented in Fig. 1.

Fig. 1. Flow diagram displaying the progress of participants during the study period. Participants who did not comply with the study protocol were excluded from statistical analysis. Non-compliance was defined as not following the protocol in regard to fish intake (omitting more than three fish dinners in the fish-eating groups), other dietary changes or use of prescription medicine not compatible with the inclusion criteria, or changes in physical activity.
Groups were similar at baseline in regard to sex distribution, age, BMI, percentage body fat and percentage muscle mass (Table 1), with median age 45·6 (25th, 75th percentiles 37·1, 53·9) years and median BMI 32·3 (25th, 75th percentiles 29·6, 35·7) kg/m2. After 8 weeks, no changes were seen in any of the groups for BMI, percentage body fat or muscle mass (data not presented).
Table 1. Participant characteristics at baseline
(Medians and 25th, 75th percentiles)
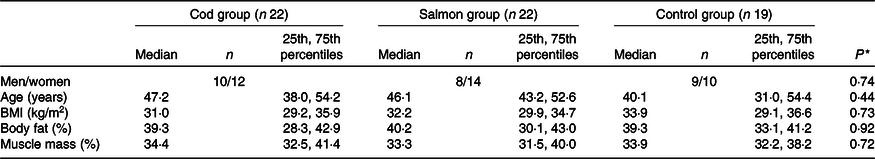
* Groups were compared at baseline using Pearson’s χ 2 (categorical data) or the Kruskal–Wallis test (continuous data).
Estimated dietary intake of vitamins
The participants registered food intake for the last 5 d before the endpoint visit. The Cod group reported that 71 % of dinners contained cod, whereas 16 % of the meat for dinners was sausages, minced meat, hamburger or pizza/lasagne with meat, 4 % of dinners contained chicken, 4 % contained lamb, with only one reported meat-free dinner. In the Salmon group, 71 % of dinners contained salmon, whereas 15 % of dinners contained meat from sausages, minced meat, hamburger or pizza/lasagne, 6 % of dinners contained pork, 4 % contained chicken, and no meat-free dinners were reported. Participants in the Control group, who were not allowed to eat fish or seafood during the intervention period, preferred to include meat as part of their dinners; of the ninety-five dinners registered by the nineteen participants in the Control group, only one of the reported dinners did not contain meat. The most popular types of meat for dinner in the Control group were sausages, minced meat, hamburger and pizza/lasagne with meat (a total of 48 % of the dinners), followed by pork (17 % of dinners) and chicken (12 % of dinners). None of the participants in any of the three groups reported intake of liver or kidney in their food diaries before the baseline and endpoint visits.
The estimated median intakes of vitamins A, B1, B2, B3, B6, B9, C, D and E were similar between the groups at baseline (Kruskal–Wallis test, P > 0·05). The estimated vitamin D intake was significantly increased from baseline to 8 weeks in the Salmon group when compared with both the Cod group and the Control group (Table 2). For the other vitamins, no changes were seen in estimated daily intake within any of the groups from baseline to 8 weeks.
Table 2. Estimated daily dietary intake of vitamins based on 5 d dietary records at baseline and after 8 weeks*
(Medians and 25th, 75th percentiles)
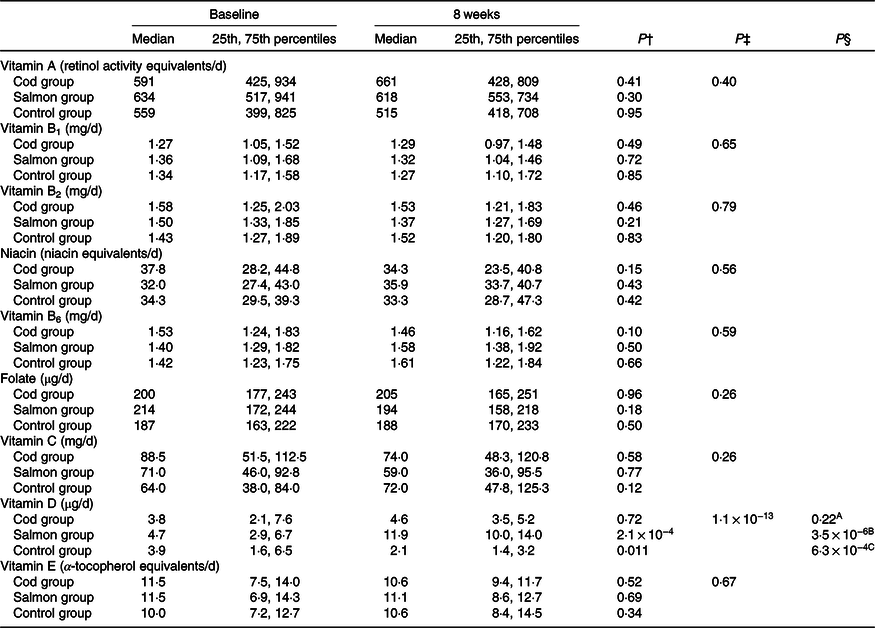
* No differences were seen between the groups at baseline (Kruskal–Wallis test). Results are presented for twenty-two participants in the Cod group, twenty-two participants in the Salmon group and nineteen participants in the Control group.
† Within-group changes were tested using Wilcoxon’s signed-rank test.
‡ Changes within the Cod group, Salmon group and Control group were compared using ANCOVA with adjustment for baseline values after log transformation.
§ Changes within the Cod group were compared with the Control group (A), changes within the Salmon group were compared with the Control group (B), changes within the Cod group were compared with the Salmon group (C) using Tukey’s honestly significant difference test when the ANCOVA test showed differences between the groups.
Vitamins in serum
Concentrations of all-trans retinol, thiamine, thiamine monophosphate, riboflavin, flavin mononucleotide, nicotinamide, N1-methylnicotinamide, pyridoxal 5′-phosphate, pyridoxal, 4-pyridoxic acid, folate, cobalamin, 25-hydroxyvitamin D3, α-tocopherol, γ-tocopherol and phylloquinone in serum were similar between the groups at baseline (Kruskal–Wallis test, P > 0·05) (Table 3).
Table 3. Serum concentrations of vitamins at baseline and after 8 weeks*
(Medians and 25th, 75th percentiles)
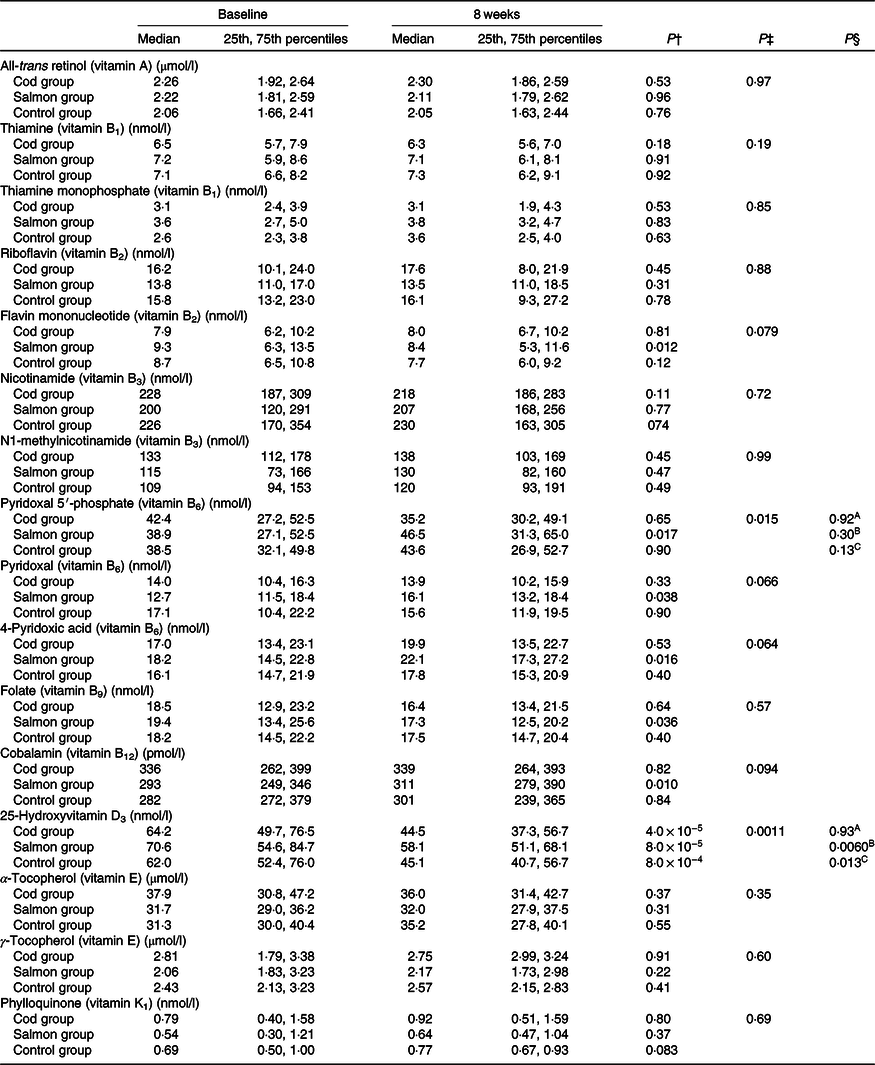
* No differences were seen between the groups at baseline (Kruskal–Wallis test). Results are presented for twenty-two participants in the Cod group, twenty-two participants in the Salmon group and nineteen participants in the Control group.
† Within-group changes were tested using Wilcoxon’s signed-rank test.
‡ Changes within the Cod group, Salmon group and Control group were compared using ANCOVA with adjustment for baseline values after log transformation.
§ Changes within the Cod group were compared with the Control group (A), changes within the Salmon group were compared with the Control group (B), changes within the Cod group were compared with the Salmon group (C) using Tukey’s honestly significant difference test when the ANCOVA test showed differences between the groups.
Serum 25-hydroxyvitamin D3 concentration was significantly reduced within all groups from baseline to endpoint; however, the reduction in vitamin D3 in the Salmon group was significantly smaller when compared with both the Cod group and the Control group (Fig. 2). Otherwise, no changes were seen in serum concentrations for the other vitamins during the intervention period (Table 3).
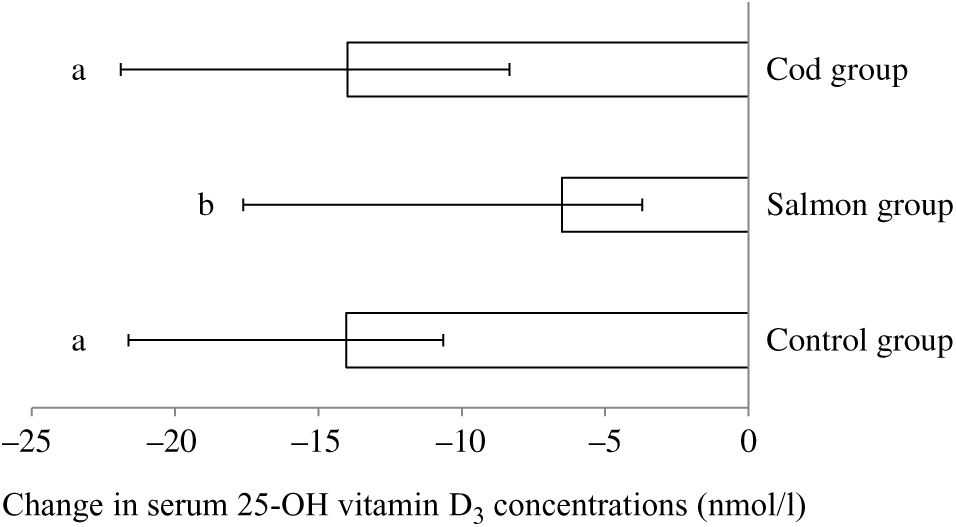
Fig. 2. Change in serum concentrations of 25-hydroxyvitamin D3 from baseline to 8 weeks. Results are presented for twenty-two participants in the Cod group, twenty-two participants in the Salmon group and nineteen participants in the Control group and are presented as medians and 25th, 75th percentiles. Changes within the Cod group, Salmon group and Control group were compared using ANCOVA with adjustment for baseline values after log transformation followed by Tukey’s honestly significant difference test. a,b Bars with different letters are significantly different (P < 0·05).
Discussion
In the present study we investigated the effects of high intake of cod or salmon fillets on serum concentrations of a comprehensive panel of water-soluble and fat-soluble vitamins. We observed that a weekly intake of 750 g salmon fillet curbed but was not sufficient to prevent the expected seasonal decrease in serum 25-hydroxyvitamin D3 concentration in the autumn in Norway.
We have previously demonstrated that a high salmon intake (750 g/week) improved post-prandial glucose regulation in these study participants, whereas high cod intake (750 g/week) did not affect glucose regulation after 8 weeks intervention(Reference Helland, Bratlie and Hagen20). The estimated intakes of energy and macronutrients (protein, fat and carbohydrates) as well as estimated physical activity were similar between the experimental groups at baseline and did not change from baseline to endpoint(Reference Helland, Bratlie and Hagen20). In the present paper, we extended the statistical analyses of estimated intakes and show that no differences were seen between the groups for estimated intakes of vitamins A, B1, B2, B3, B6, B9, C, D and E at baseline, and of these vitamins, only vitamin D estimated intake increased from baseline to endpoint in the Salmon group when compared with the Cod group and the Control group. The presented findings regarding estimated intakes of energy, macronutrients(Reference Helland, Bratlie and Hagen20) and vitamins based on food diaries give a good indication that the participants did not change their dietary habits during the study period, except for the inclusion of cod or salmon in the fish-eating groups.
In humans, cutaneous synthesis of 25-hydroxyvitamin D3 stimulated by sunlight is the major source of vitamin D(Reference Moan, Porojnicu and Dahlback19). The participants in the present study were living in Bergen, in South-Western Norway, at 60° north latitude. The baseline visits were in the last week of August and the three first weeks of September, with the endpoint visits starting in the middle of October and lasting throughout the first third of November. The study was thus conducted in a period when Bergen usually experiences a lot of overcast weather with little sun, and the inhabitants have little chance of getting direct sunshine on their skin for cutaneous synthesis of vitamin D3. The autumn of 2011, when the present study was conducted, was no exception with 4 d of fair weather and 64 rainy days with a total of 783 mm rainfall during the 80 d of the study period(26). A reduction in circulating vitamin D is expected in the autumn this far north of the Equator since the intensity of UVB radiation is too low for subcutaneous production of vitamin D(Reference Webb, Kline and Holick27,Reference Degerud, Hoff and Nygard28) , unless the vitamin D intake from food or supplements is increased. The Nordic Council of Ministers recommends a daily intake of 10 µg vitamin D for the adult Nordic population (<75 years) and recommends circulating 25-hydroxyvitamin D concentrations ≥50 nmol/l(Reference Brustad and Meyer29). At baseline, fifty of the sixty-three participants (84 %) had serum 25-hydroxyvitamin D3 concentration >50 nmol/l, but 8 weeks later, this was seen in 49 % of the participants (thirty-one participants). Fatty fish such as salmon is regarded to be a good dietary source of vitamin D3 (1,21) , and we expected that the high intake in the Salmon group of the present study would be sufficient to sustain the serum 25-hydroxyvitamin D3 concentration throughout autumn. However, although the median daily vitamin D intake was estimated to be 11·9 µg (interquartile range 10·0, 14·0 µg) in the Salmon group, which was above the recommendation of 10 µg vitamin D per d, the serum 25-hydroxyvitamin D3 concentration decreased in this group, albeit to a lesser degree when compared with both the Cod group and the Control group. Thus, a weekly intake of 750 g salmon was insufficient to prevent the seasonal reduction in serum 25-hydroxyvitamin D3 in the autumn in this group of healthy adults with overweight or obesity living in South-Western Norway.
The contents of vitamin A, vitamin B1, niacin and vitamin B6 are higher per wet weight in salmon fillet compared with cod fillet, whereas amounts of vitamin E, vitamin B2, folate and vitamin C are comparable in cod and salmon fillets(21). We found no differences in the estimated dietary intakes of these eight vitamins when comparing the food diaries from the Cod group, the Salmon group and the Control group. The participants had a varied diet throughout the study period and regularly reported that they consumed common foodstuffs that are good sources of different vitamins, such as dairy products (vitamins A and B2, folate), eggs (vitamins A, B1, B2, E and folate), fruits (vitamin C, folate), vegetables (vitamins A and C, folate), meat (vitamins A, B1, B2 and B6, niacin, folate), grains (vitamins B1, B2 and B6, folate, niacin), nuts (vitamins B1, B2, B6 and E, niacin, folate), legumes (vitamins A, B1, B2 and C, folate) and berries (vitamins C and E, folate)(21). In line with this, no changes in serum concentrations of vitamins A, B1, B2, B6, E, and niacin and folate were found within or between any of the experimental groups.
Vitamin K1 and cobalamin are not included in the food database (Mat på data) used for estimations of dietary intakes in the present study; therefore, we were not able to report estimated intake of these vitamins. Little information is available on the content of vitamin K1 in various foods, but this vitamin is found in highest amounts in plants and vegetable oils(30), and no changes were seen in serum vitamin K1 concentration in any of the groups during the intervention. Only a few food types are rich in vitamin B12, such as fish, meat, egg and dairy products(30) which were regularly consumed by our participants, in addition to liver and kidney(30) which were not reported in the food diaries by any of the participants in the present study. The bioavailability of vitamin B12 is also an issue when considering food sources of this vitamin, and it has been reported that for humans, the bioavailability is several times higher from milk, fish and meat compared with eggs(Reference Watanabe31). The cobalamin content in farmed Atlantic salmon is approximately three times that in cod(1), but still no differences were observed between the groups for changes in serum cobalamin concentrations after 8 weeks intervention.
The present study has some strengths and limitations. Strengths include the measurement of both water-soluble and fat-soluble vitamins and the use of 5-d dietary registrations at baseline and endpoint for estimation of vitamin intake. A possible limitation of the present study is that the results obtained may not be valid for individuals with different demographics such as BMI, living at different latitudes and different lifestyles than those in the present work; thus, additional studies are needed to address these factors. Also, the effects of a longer intervention period on the serum micronutrient concentrations should be investigated. The vitamin D serum concentration for the participants that habitually consumed >1 fish dinner per week may have been lower in the beginning of study than normal since these participants had a 4 week fish-free period before the baseline visit. The cod and salmon fillets used in the present study were randomly chosen from different batches in Lerøy’s warehouse in Bergen and were representative for cod and salmon sold in Norwegian grocery stores. We did not conduct our own analyses of vitamin contents in these batches, but instead we used average values from the official Norwegian food database.
Conclusion
A high intake of salmon curbed but was not sufficient to prevent a decrease in serum 25-hydroxyvitamin D3 concentration in autumn in South-Western Norway in adults with overweight/obesity. We also found that high intake of cod or salmon did not affect serum concentrations of the other investigated vitamins. The Norwegian Directorate of Health recommends a weekly intake of 300–450 g fish, of which at least 200 g should be fatty fish, for the general public(32); however, findings in the present study suggest that such intake will not be sufficient to prevent the decline in serum vitamin D concentration in autumn at 60° north latitude. This result suggests that it is difficult to sustain non-deficient levels of vitamin 25-hydroxyvitamin D3 in autumn in South-Western Norway through dietary intake alone.
Acknowledgements
The kind contributions of cod and salmon for the intervention trial by Lerøy Seafood Group ASA (Bergen, Norway) are highly appreciated. The authors thank all participants who contributed to the present study.
The present research was supported by funding from the Bergen Medical Research Foundation. The sponsor was not involved in the design of the study, data collection, analysis and interpretation of data, writing of the article or in the decision to submit the article for publication.
G. R., H. S., G. M. and O. A. G. designed the study. M. B., I. V. H., A. H. and O. A. G. conducted the study. M. B., I. V. H., A. H., Ø. M., A. U., P. M. U. and O. A. G. analysed the data. O. A. G. performed statistical analyses, drafted the paper and had primary responsibility for the final content. All authors contributed to the writing and approved the final version of the manuscript.
G. R. and H. S. are employed in Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA, respectively. Skretting Aquaculture Research Centre AS is a global leader in providing innovative and sustainable nutritional solutions for the aquaculture industry. Lerøy Seafood Group ASA is the leading exporter of seafood from Norway and the world’s second largest producer of Atlantic Salmon. Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA were not involved in on-site data collection. The other authors declare no conflicts of interest.








