Intervention studies have demonstrated that maternal use of folic acid supplements before and early in pregnancy reduces the risk for neural tube defects in the fetus( Reference Smithells, Sheppard and Schorah 1 – Reference Botto, Moore and Khoury 6 ). There is increasing evidence that maternal intake of folic acid supplements in early pregnancy may also reduce the risk for oral clefts. However, the findings are divergent, and the effect of folic acid on oral clefts is debated( Reference Badovinac, Werler and Williams 7 – Reference Shaw, Lammer and Wasserman 11 ).
Facial structures that form the embryonic lip fuse during the 5th and 6th weeks of life, whereas the palatal shelves fuse during 7–10 weeks. Oral clefts occur when the lips and/or the roof of the mouth do not fuse properly( Reference Mossey, Little and Munger 12 ). On the basis of Global Burden of Disease regions, Mossey & Modell( Reference Mossey and Modell 13 ) have estimated an average world birth prevalence of non-chromosomal oral clefts at 1·25/1000 births. In Norway, the prevalence is about 2·2/1000 live births and stillbirths after 16 weeks of gestation, which is one of the highest prevalence levels in Europe( Reference Sivertsen, Wilcox and Johnson 14 ).
In general, oral clefts are classified into two primary categories: cleft palate only (CPO) and cleft lip with or without cleft palate (CL(P)). These two types may have distinct causal mechanisms. The majority of oral clefts are described as isolated, meaning that no accompanying birth defects are present. CPO, however, is more often combined with other congenital malformations than is CL(P). In non-isolated clefts, congenital heart defects and neural tube defects are more frequently combined with oral clefts, in addition to other severe anomalies( Reference Wyszynski 15 ).
The causes of oral clefts are largely unknown, but environmental factors such as maternal smoking, maternal use of drugs and maternal exposure to organic solvents are considered risk factors( Reference Watkins, Meyer and Strauss 16 ), and poor nutrition may also play a role( Reference Wyszynski 17 ). Genetic factors and the interplay between genes and the environment are also important when exploring the causes of oral clefts( Reference Watkins, Meyer and Strauss 16 ).
Norway introduced periconceptional folic acid recommendations to fertile women from 1998 onwards in an attempt to reduce the number of neural tube defects. There is no mandatory fortification of food supply with folic acid in Norway, unlike in many non-European countries. Since 1999, information on folic acid and multivitamin supplement use before and during pregnancy has been reported to the Medical Birth Registry of Norway (MBRN). In the present study, we investigated the association between women’s use of folic acid and/or multivitamin supplements before pregnancy and the risk for oral cleft in the newborn, with and without accompanying birth defects, in all live births and stillbirths in Norway during 1999–2013.
Methods
Data source
Since 1967, live births and stillbirths from 16 weeks of gestation onwards have been notified to the MBRN, and, since 2002, notifications are required from 12 weeks of gestation( Reference Irgens 18 , Reference Irgens 19 ). The population-based registry comprises demographic data on the parents and extensive medical information on maternal health before and during pregnancy, the delivery and on the condition of the newborn, including birth defects and other neonatal diseases. Data are collected by a standardised notification form for each birth, which is completed by attending health personnel at the time of birth and during the stay at the delivery unit. From 1967 to December 1998, the notification form remained unchanged. A revised version was then introduced to include information on maternal vitamin supplement use, smoking before and during pregnancy and ultrasound dating. At the same time, a separate notification from neonatal intensive care units was introduced for all infants transferred to such units after birth. In 1999, a separate registry for pregnancy terminations after 12 gestational weeks was established within the MBRN. All terminations carried out because of congenital anomalies or diseases in the fetus have since been routinely added to the birth registry to increase the data quality on congenital anomalies.
Vitamin supplementation
Information on vitamin use includes questions on regular folic acid and multivitamin supplement use before and during pregnancy. Data were collected from 1999 on the revised version of the notification form through the following check boxes: ‘Folic acid before pregnancy’, ‘Folic acid during pregnancy’, ‘Multivitamins before pregnancy’ and ‘Multivitamins during pregnancy’. Information on dose, frequency or exact duration of supplement use was not available. However, prenatal folic acid tablets used in Norway during the study period contained 0·4 mg, whereas most multivitamin supplements ranged from 0·0 to 0·2 mg of folic acid( 20 ).
We classified women as exposed before pregnancy (main exposure) if they used folic acid and/or multivitamins before pregnancy (21·5 %). The vast majority (20·4 %) of these women also used vitamins during pregnancy, whereas the minority used vitamins before pregnancy only (1·1 %) (Fig. 1). The exposure variable was included in the statistical regression models as categorical variables: no vitamin use; vitamin use before pregnancy (21·5 %); or vitamin use during pregnancy only. We also explored the use of folic acid alone (not concurrent with multivitamins) and the use of multivitamins alone (not concurrent with folic acid supplements). No use of vitamins (folic acid or multivitamin supplements) before or during pregnancy was used as the reference.
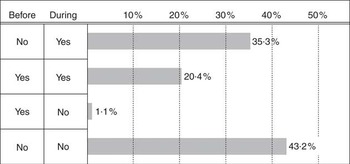
Fig. 1 Reported vitamin use, folic acid and/or multivitamins before and/or during pregnancy, to the Medical Birth Registry of Norway, 1999–2013. ‘No’ represents no vitamin supplement use; ‘yes’ represents use of vitamin supplements before and/or during pregnancy.
Unfortunately, data on terminated pregnancies do not include information on maternal folic acid or multivitamin supplement use. Consequently, our analyses focused on the relation between vitamin supplementation and oral clefts among live births and stillbirths from 1999 to 2013.
Classification of birth defects
Oral clefts were classified as follows: CPO (International Classification of Diseases (ICD)-10; Q 35), and CL(P) (ICD-10; Q 36–37). All oral clefts were also categorised as isolated or non-isolated. Isolated clefts were cleft cases with no major accompanying birth defects and less than three minor accompanying birth defects, defined according to the EUROCAT (European Surveillance of Congenital Anomalies) classification system( 21 , 22 ). Infants were classified as having registered major birth defects or not according to the same classification. Thus, infants classified with non-isolated oral clefts had at least one accompanying major birth defect or three or more minor accompanying birth defects. According to guidelines from EUROCAT, clefts with accompanying anencephaly or holoprosencephaly were not counted as clefts.
Potential confounders
Several known predictors of vitamin supplement use and predictors of oral clefts in the newborn were evaluated as potential confounders( Reference Nilsen, Vollset and Gjessing 23 ). These included parental age (<20 (reference), 20–24, 25–29, 30–34, ≥35 years), marital status (married/co-habiting (reference), single, other), parity (0 (reference), 1, ≥2 previous births) and maternal smoking habits at the beginning of pregnancy (non-smoker (reference), occasional smoker, daily smoker, missing data). In addition, maternal epilepsy and pregestational diabetes (type 1, type 2 and unspecified diabetes before pregnancy) were included as potential confounding factors.
Statistical analyses
Log-binomial regression was applied to investigate associations between maternal use of vitamins before pregnancy and the risk for oral clefts in infants, using Stata version 12.1 (Stata Corp.). Relative risks (RR) with 95 % confidence intervals were reported with and without adjustment for the above-mentioned potential confounders. Fig. 1 and 2 were made in R version 3.1.1( 24 ).

Fig. 2 Adjusted* relative risks (95 % confidence intervals) for oral clefts overall and in subgroups of non-isolated or isolated oral clefts associated with folic acid and/or multivitamin supplements†, Norway 1999–2013. Area of squares is proportional to the number of cases in each group. * Adjusted for year of childbirth, maternal and paternal age, marital status, parity, maternal smoking, maternal epilepsy and pregestational diabetes. † Reported use of folic acid and/or multivitamin supplements, including 20·4 % who reported use both before and during pregnancy and 1·1 % of women who reported use of supplements before pregnancy only. CPO, cleft palate only; CL(P), cleft lip with or without cleft palate.
Ethical approval
This study was approved by the Regional Medical Ethics Committee of Western Norway.
Results
Study population
In the study period (1999–2013), a total of 528 220 women had 880 568 pregnancies resulting in 896 674 live births and stillbirths (≥22 weeks of gestation or birth weight ≥500 g), and 3378 pregnancy terminations (≥12 weeks). The total number of oral clefts was 1714; among them 1623 (94·7 %) were in live births or stillbirths, and ninety-one (5·3 %) were in terminated pregnancies. The birth prevalence of oral clefts was 1·81/1000 live births and stillbirths. The distributions of clefts among live births, stillbirths, neonatal deaths (during the first 28 d of life) and postneonatal deaths (from 28 to 364 d) as well as in terminated pregnancies are shown in Table 1. Among all clefts, the reported frequency of non-isolated clefts was 23·3 % (n 400), whereas 76·7 % were reported as isolated clefts (n 1314). Among infants with CPO, 30·0 % were classified as non-isolated clefts (n 183), whereas 19·7 % of those with CL(P) were non-isolated (n 217).
Table 1 Oral clefts in live births, stillbirths, neonatal deaths, postneonatal deaths and pregnancy terminations among 900 052 births and terminations, Norway, 1999–2013 (Numbers and percentages)

CPO, cleft palate only; CL(P), cleft lip with or without cleft palate.
* Live births excluding neonatal and postneonatal deaths.
† Neonatal deaths were deaths occurring during the first 28 d postpartum in live born infants.
‡ Postneonatal deaths occurred from 28 d postpartum to 1 year of age.
§ Isolated clefts were classified as cleft cases with no major accompanying birth defects and less than three minor accompanying birth defects defined according to a EUROCAT( Reference Milunsky, Jick and Jick 2 ) classification system.
|| Non-isolated clefts were classified as cleft cases with major accompanying birth defects and/or three or more minor accompanying birth defects. Clefts with accompanying anencephalies or holoprosencephalies on were not counted as oral clefts according to EUROCAT guidelines.
Parental characteristics are shown in Table 2. Folic acid and/or multivitamin use before and during pregnancy was reported by 20·4 % of the women, whereas 1·1 % of the women used vitamin supplements before pregnancy only; thus, 21·5 % of women were identified as having been exposed before pregnancy. Use of vitamin supplements during pregnancy only was reported by 35·3 %. In subgroups of women exposed to vitamins before pregnancy, we identified users of folic acid alone (not concurrent with multivitamins) in 8·1 % of the women and multivitamins alone (not concurrent with folic acid) in 2·1 %. Among women exposed before pregnancy, more women (55·6 %) combined the use of folic acid with the use of multivitamins, whereas 38·3 % of multivitamin users also took folic acid.
Table 2 Parental characteristics by maternal use of folic acid and multivitamin supplement before and/or during pregnancy among live births and stillbirths in Norway, 1999–2013 (Numbers and percentages)
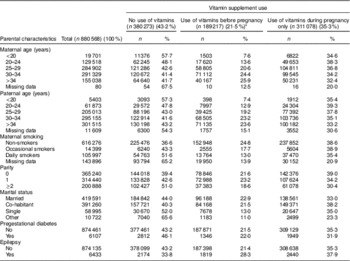
* Reported use of folic acid and/or multivitamin supplements, including 20·4 % who reported use both before and during pregnancy and 1·1 % of women who reported use of supplements before pregnancy only.
The overall proportion of women reporting no vitamin use before or during pregnancy was 43·2 % (Fig. 1).
Folic acid and multivitamin supplement use
Table 3 shows the association between maternal folic acid and/or multivitamins and infant oral clefts. For women exposed to vitamins before pregnancy, the adjusted RR was 0·90 (95 % CI 0·79, 1·04) for clefts overall, and 0·84 (95 % CI 0·66, 1·06) and 0·94 (95 % CI 0·79, 1·13) for CPO and CL(P), respectively.
Table 3 Crude and adjusted relative risks (RR) for oral clefts by folic acid and/or multivitamin supplement use, Norway 1999–2013 (Relative risks and 95 % confidence intervals)

Ref., referent values; CPO, cleft palate only; CL(P), cleft lip with or without cleft palate.
* Adjusted for year of childbirth, maternal and paternal age, marital status, parity, maternal smoking, maternal epilepsy and pregestational diabetes.
† Reported use of folic acid and/or multivitamin supplements, including 20·4 % who reported use both before and during pregnancy and 1·1 % of women who reported use of supplements before pregnancy only.
‡ No use of vitamin supplements before pregnancy.
§ Reported use of folic acid supplements (not concurrent with multivitamins).
|| Reported use of multivitamin supplements (not concurrent with folic acid supplements, but most multivitamins contain 0·0–0·2 mg of folic acid).
Fig. 2 shows the adjusted RR for oral clefts in the newborn to women exposed to vitamins before pregnancy in relation to non-users. Results are shown for clefts overall, CPO and CL(P), as well as for the subgroups non-isolated and isolated clefts. Among non-isolated clefts, vitamin use was significantly associated with a lower risk for total clefts with the adjusted RR being 0·63 (95 % CI 0·45, 0·88). To explore this finding we performed sub-analyses after excluding infants with oral clefts and accompanying chromosomal abnormalities (n 52) and genetic syndromes (n 21): in total seventy-one infants. The adjusted RR of overall clefts remained nearly unchanged (adjusted RR 0·94; 95 % CI 0·81, 1·08), but changed slightly for vitamin use and non-isolated clefts (adjusted RR 0·54; 95 % CI 0·34, 0·85). After excluding chromosomal abnormalities and genetic syndromes, the associated anomalies found in combination with oral clefts were mainly heart defects (n 69), limb defects (not including hip dysplasia/dislocation) (n 50), urinary tract defects (n 33), nervous system defects (n 29, of which six were neural tube defects), digestive defects (n 27), ear, nose and throat defects (n 25) and genital defects (n 20). When we excluded cleft cases with heart defects (n 69), the relations remained nearly unchanged for both clefts overall and non-isolated oral clefts. The same was true when excluding cleft cases with accompanying nervous system defects (n 29). When excluding limb defects (n 50), the association was almost unchanged for clefts overall, but slightly weaker for non-isolated clefts (RR 0·67; 95 % CI 0·40, 1·11), and was no longer statistically significant.
We found no significant association between women exposed to vitamins and isolated oral clefts. The adjusted RR for all isolated clefts was 0·98 (95 % CI 0·84, 1·15); for CPO the adjusted RR was 0·92 (95 % CI 0·70, 1·21) and for CL(P) the RR was 1·01 (0·84, 1·23). We also divided our study period into two different time intervals (1999–2005 and 2006–2013). We did find a significantly reduced risk for oral clefts overall by vitamin use in the first period (the adjusted RR 0·76; 95 % CI 0·59, 0·97), but not in the second (the adjusted RR 1·07; 95 % CI 0·88, 1·29).
Adjustment for potential confounding factors did not change the estimates substantially (Table 3). Maternal smoking was strongly associated with both vitamin use and oral clefts. Occasional smokers and daily smokers were less likely to use vitamin supplements than were non-smokers, and daily smoking during pregnancy was associated with increased infant risk for oral cleft (RR 1·36; 95 % CI 1·19, 1·56). Information on smoking habits was missing for nearly 17 % of the women. The final model was analysed with and without missing data on smoking habits. In analyses restricted to women with complete smoking information, no major changes in the risk estimates were seen; the adjusted RR for oral clefts overall was 0·93 (95 % CI 0·79, 1·08) and that for non-isolated oral clefts was 0·53 (95 % CI 0·32, 0·87). All analyses have been performed for various combinations of folic acid and/or multivitamin supplement use before and/or during pregnancy and risk for oral clefts in the newborn, with essentially similar results (data not shown).
Discussion
In this large population-based study of 528 220 women and 880 568 pregnancies, 1623 newborns or stillborns were affected by oral clefts in Norway in the time period 1999–2013. Use of folic acid and/or multivitamins before pregnancy was reported by 21·5 % of the women. We found no association between maternal supplement use and risk for oral clefts in the newborn overall. Women who used vitamin supplements did, however, have a significantly lower risk for non-isolated oral clefts in the newborn.
Comparisons with previous studies
Our study showed no risk reduction for oral clefts overall among live born or stillborn infants to women using vitamins before pregnancy, a finding supported by other observational studies( Reference Little, Gilmour and Mossey 25 , Reference Shaw, Carmichael and Laurent 26 ). However, yet other studies have shown associations between vitamin use and oral clefts( Reference Botto, Olney and Erickson 27 , Reference van Rooij, Ocke and Straatman 28 ). Consequently, the evidence is mixed and is likely caused by differences in design, sample selection and study size, different measures of vitamins and classifications of oral clefts. It may also be that the underlying nutritional status of the general population of the place where the study is conducted is of importance.
A population-based case–control study from 2007 by Wilcox et al. explored the role of folate in the prevention of oral clefts in the time period 1996–2001 in Norway. They found a 39 % decrease in CL(P) risk associated with using folic acid supplements and a 64 % decrease when women used folic acid supplements in addition to a folate-rich diet( Reference Wilcox, Lie and Solvoll 8 ). This study partly overlapped with our study population, outcome data and time period (3-year overlap), but differed in terms of collection of exposure information. Our study was registry based, where information about maternal vitamin use was reported to the population-based MBRN for all live births and stillbirths during 1999–2013, whereas Wilcox’s study was a case–control study in which information on vitamin use was collected months after delivery. There were contradictory findings for the subtypes of oral clefts in the two studies. Differences in the study design, time periods, cleft populations, sample size and exposure information may have played a role. When we divided our study period into two (1999–2005 and 2006–2013), we found a significantly reduced risk for oral clefts overall by vitamin use in the first time period, closer in time to Wilcox’s study, but not in the second.
A Hungarian study reported 20–30 % risk reduction for oral clefts associated with the use of high-dose folic acid (3–6 mg/d)( Reference Czeizel, Toth and Rockenbauer 29 ). Other studies have also shown a dose-dependent effect of vitamins on oral clefts( Reference Czeizel, Timar and Sarkozi 30 , Reference Czeizel 31 ). Some studies have shown a risk reduction for CL(P), but not for CPO( Reference Wilcox, Lie and Solvoll 8 , Reference van Rooij, Ocke and Straatman 28 ). In a meta-analysis by Johnson & Little( Reference Johnson and Little 32 ), use of multivitamin supplements was inversely associated with CL(P), but to a lesser extent with CPO. There was not enough evidence to prove an association between folate or folic acid and oral clefts, but there was substantial heterogeneity between studies( Reference Johnson and Little 32 ). In a meta-analysis by Badovinac et al. ( Reference Badovinac, Werler and Williams 7 ), estimates from case–control studies showed that use of folic acid-containing supplements during pregnancy decreased the risk for any oral cleft by 33 %, that for cleft lip and palate by 29 % and that for CPO by 20 %. Estimates among prospective studies were in the same protective direction for any oral cleft (45 %) and for cleft lip and palate (49 %). However, the prospective studies indicated that supplement use increased the risk for CPO by 19 %( Reference Badovinac, Werler and Williams 7 ).
Strengths and limitations
The strength of our study includes a large study population with information on maternal vitamin use for all live births and stillbirths in Norway during 1999–2013. Together with comprehensive information on maternal determinants of health, this gave us the opportunity to study women’s use of folic acid and multivitamins and its association with oral clefts in the newborn. However, our study had some limitations. Misclassification of exposure may have biased the association measures due to possible incorrect reporting of vitamin supplement use by the hospitals. This could include misclassification regarding time and type of vitamin use, and also missing data on vitamin use. We were not able to identify missing data on vitamin use in this study. Therefore, some women may have been misclassified as non-users although being vitamin users.
The prevalence of folic acid and/or multivitamin supplement use is somewhat lower in the MBRN compared with what has been found in two Norwegian studies based on the Norwegian Mother and Child Cohort Study (MoBa)( Reference Nilsen, Vollset and Gjessing 23 , Reference Suren, Roth and Bresnahan 33 ). In the MoBa population (giving birth 1999–2009), use of folic acid in the period 1–4 weeks before the start of pregnancy was reported by 33 % of participating women, whereas 50 % reported use of folic acid during the early weeks of pregnancy. However, the MoBa study, although nationwide, consists of a quite selected population, with strong under-representation of younger women (<25 years), single mothers, multiparous mothers, smokers and mothers with low education( Reference Nilsen, Vollset and Gjessing 34 ). All these factors may be associated with maternal periconceptional use of folic acid. The MBRN, on the other hand, includes all women giving birth in Norway and thus a non-selected population, and this may partly explain the difference in the registered use of folic acid between the two populations.
Regarding unplanned pregnancies, we only know one study in Norway addressing this issue. In one of the mentioned studies based on the MoBa population( Reference Suren, Roth and Bresnahan 33 ), about 20 % of the women did not plan their pregnancy. As the MoBa population is a selected population, the actual numbers of unplanned pregnancies among Norwegian women are most likely higher, but this is not known. However, the lower prevalence of vitamin supplement use before conception in the whole population of Norwegian women most likely reflects a higher rate of unplanned pregnancies.
We expected a lower ascertainment for CPO than for CL(P), as a previous study showed that reporting of oral clefts to the MBRN, 1967–1998, was dependent on the severity of the cleft. Of all cleft cases recorded in the MBRN, 57 % of CPO and 94 % of CL(P) live birth cases had been recorded( Reference Kubon, Sivertsen and Vindenes 35 ). CPO is more frequently associated with other birth defects, which likely increases the ascertainment of non-isolated CPO. We found the prevalence of oral clefts overall to be 1·81/1000 live and stillbirths in our study (0·65/1000 for CPO and 1·16/1000 for CL(P)). This is a lower prevalence compared with the findings of another Norwegian study from the period 1967 to 1998( Reference Sivertsen, Wilcox and Johnson 14 ) and indicates that a few oral clefts may have been under-reported after 1998, or that termination of pregnancy for oral clefts has been increasing since 1998. However, the percentage of terminated pregnancies with cases of oral clefts among the total number of oral clefts only increased from 1·4 % in 1999 to 4·0 % in 2013 (http://mfr-nesstar.uib.no/mfr/) and should not have affected the association of vitamin use with non-isolated clefts. Further, it seems that heart defects and neural tube defects cannot explain the strong association of vitamin use with non-isolated oral clefts.
Associations in observational studies may be subject to unknown or residual confounding( Reference Lawlor, Davey Smith and Kundu 36 ). Several factors were not available in our study, like information on maternal dietary intake, alcohol consumption and maternal BMI. It is possible that other healthy behaviours – for instance, healthy diets – are also more common in vitamin supplement users. However, we did adjust for maternal age, smoking and marital status, which could partly explain the patterns in use of folic acid and multivitamins and could also affect the prevalence of oral clefts.
We found a stronger effect on oral clefts with multivitamins than with folic acid. However, because most multivitamin supplements in Norway contain some folic acid, ranging from 0·0 to 0·2 mg, it is not possible to create a multivitamin group without folic acid within our data. As the association is somewhat stronger for this group than for the folic acid-only group, it seems that other micronutrients than folic acid may also play a role, or that the association is confounded by lifestyle factors associated with the use of multivitamins only.
Maternal smoking has been associated with increased risk for oral clefts( Reference Honein, Rasmussen and Reefhuis 37 , Reference Little, Cardy and Munger 38 ), also present in our study. However, adjusting for smoking only slightly attenuated the associations between vitamin use and oral clefts. We also restricted analyses to women with complete smoking information. As this did not affect the association of vitamin use with risk for oral cleft, it is not likely that results would be different among women with missing smoking information.
Conclusion
We found no statistically significant association between use of vitamins before pregnancy and isolated oral clefts in the newborn. We did, however, find lower risk for oral clefts that occurred in combination with other malformations.
Acknowledgements
This study was funded by The Western Norway Regional Health Authority, project numbers 911647 and 911629 (N. Ø.).
T. G., T. B., K. K., S. E. V., R. M. N., Ø. A. H. and N. Ø. all substantially contributed to conception, design and interpretation of data. T. G. drafted the article and analysed the data. All the authors revised it critically for important intellectual content and gave their approval of the final version.
There are no conflicts of interest to declare.








