The type and amount of dietary fibre that reaches the large intestine and the extent to which it is fermented by intestinal bacteria (i.e. its fermentability) are important factors for understanding its overall effects. In general, the bacterial fermentation of non-digestible carbohydrates affects the following: (1) the luminal environment, by influencing the composition and/or activity of the gut microbiota and the physico-chemical properties of the intestinal content( Reference Gibson and Roberfroid 1 , Reference Hara, Suzuki and Kobayashi 2 ), and (2) the intestinal epithelium, by regulating the gut hormone response and/or altering the intestinal barrier function, including the stimulation of mucus production and the regulation of cell proliferation, migration and survival, cellular permeability, and the mucosal immune system( Reference Kleessen, Hartmann and Blaut 3 – Reference Ishizuka, Tanaka and Xu 6 ). However, other factors could also modulate the fermentative process including the digestibility of food matrix components (such as resistant proteins, lipids and some micronutrients), intestinal motility and inter-individual differences in gut microbiota composition( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 5 , Reference Lobo, Mancini Filho and Alvares 7 – Reference Moran-Ramos, Tovar and Torres 9 ).
In recent years, several studies have also emphasised the effects of inulin-type fructans (ITF), such as fructo-oligosaccharides (FOS) and inulin, on the intestinal absorption of minerals. The acidification of intestinal contents from SCFA production by bacterial ITF fermentation increases mineral solubility and absorbability( Reference Levrat, Rémésy and Demigné 10 , Reference Rémésy, Levrat and Gamet 11 ). Furthermore, dietary ITF seem to affect mineral absorption in the large intestine by altering the permeability of tight junctions and the gene expression of mineral transporters such as calbindin-D9k (for Ca), divalent metal transporter (DMT-1) (for Fe) and zinc transporter 1 (ZnT1) (for Zn), supporting an effect on both paracellular and transcellular absorption( Reference Nzeusseu, Dienst and Haufroid 12 – Reference Tako, Yasuda and Glahn 14 ). In addition, changes in the mucosal architecture of the intestine as a result of increases in both the cellularity and number of crypts are factors that could contribute to an increase in the absorptive surface for mineral uptake( Reference Kleessen, Hartmann and Blaut 3 , Reference Goodlad, Lenton and Ghatei 4 , Reference Lobo, Mancini Filho and Alvares 7 , Reference Lobo, Colli and Alvares 15 ). These effects are reflected in the improved bioavailability of minerals, as demonstrated by the increased retention in their target tissues( Reference Lobo, Mancini Filho and Alvares 7 , Reference Nzeusseu, Dienst and Haufroid 12 – Reference Lobo, Cocato and Borelli 18 ).
We have previously demonstrated the positive effects of short-chain ITF (FOS), a rapidly fermentable fibre, on the bioavailability of Ca( Reference Lobo, Mancini Filho and Alvares 7 , Reference Lobo, Colli and Alvares 15 – Reference Lobo, Cocato and Jorgetti 17 ), Mg( Reference Lobo, Mancini Filho and Alvares 7 , Reference Lobo, Colli and Alvares 15 , Reference Lobo, Colli and Filisetti 16 ) and Fe( Reference Lobo, Cocato and Jorgetti 17 , Reference Lobo, Cocato and Borelli 18 ) in rats fed diets containing purified and non-purified ITF sources. In the present study, we used a Hb depletion–repletion method resembling the one proposed by the AOAC( 19 ) to investigate the effects of FOS on Fe bioavailability in anaemic rats. The effects of FOS as Fe bioavailability enhancers were confirmed and such effects were sustained by early changes in the caecal environment (decreased mucosal ferroportin (FPN)-1 expression and increased Fe absorbability and crypt bifurcation and cellularity).
Experimental methods
The experimental protocol was approved by the Commission on Ethics in Animal Experiments of the Faculty of Pharmaceutical Sciences at the University of São Paulo (FCF/USP) (CEEA 213/2009 FCF-USP) according to the guidelines of the Brazilian College on Animal Experimentation.
Iron deficiency protocol
Female Wistar rats (n 12) were obtained from the colonies for Animal Experimentation at FCF/USP, and each of them was breast-feeding six to eight male pups. These rats were housed in plastic cages with ripcurl and given an Fe-deficient powder diet( 19 ) (8 mg Fe/kg; n 10 female rats) or an AIN-93M diet( Reference Reeves, Nielsen and Fahey 20 ) (n 2 female rats) for 21 d.
On weaning day, a total of eighty-eight male rats initially weighing 54–58 g were transferred to individual stainless-steel wire-mesh metabolism cages (to limit coprophagy) under controlled temperature (22 ± 2°C) and relative humidity (55 ± 10 %) with a 12 h dark–12 h light cycle (lights on from 08.00 to 20.00 hours). Rats were given access to demineralised water ad libitum and an Fe-deficient powder diet (ID group; n 80) or an AIN-93G diet( Reference Reeves, Nielsen and Fahey 20 ) (control (CT) group; n 8) for 14 d (depletion period). During this period, ten ID rats were selected to determine their body weight and Hb concentration values. When the Hb concentration of these rats reached a mean value of 60 g/l, it was determined in all rats.
Hb repletion assay
For the Hb repletion assay, sixty-four rats were selected from the Fe-deficient group (n 80). These rats were distributed into four groups based on the product of body weight (g) and Hb concentration (g/l). Rats were fed modified AIN-93G diets containing 35 mg Fe/kg as microencapsulated ferrous sulphate (FS)( Reference Cocato, Ré and Trindade Neto 21 ) with alginate (Fermavi Eletroquímica Ltda) (FS group; n 16) or ferric pyrophosphate (FP; Fermavi Eletroquímica Ltda) in a mineral mix. FP was added to the diets of three different groups: one diet without fructan supplementation (FP group; n 16) and two other diets with fructan supplementation, with yacon (Smallanthus sonchifolius (Poepp & Endl.) H. Robinson, Asteraceae) flour (YF group; n 16) or Raftilose P95 (Clariant S/A) (RAF group; n 16) at 7·5 % FOS (75 g/kg diet) (repletion period; Table 1). Rats were killed after 1 (week 1; n 8 per group) and 2 (week 2; n 8 per group) weeks of repletion. The remaining eight healthy rats (CT group) continued to be fed the AIN-93G diet during the repletion period, and at the end of this period (week 2), the mean Hb concentration of this group was 140 (sd 22) g/l.
Table 1 Formulation of the experimental diets
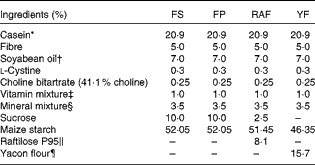
FS, ferrous sulphate; FP, ferric pyrophosphate; RAF, Raftilose P95; YF, yacon flour.
* Casein = 85 % protein (N × 6·25).
† Cargill Agrícola S/A.
‡ AIN-93-VX vitamin mixture( Reference Reeves, Nielsen and Fahey 20 ).
§ Modified from a mineral mixture (AIN-93G-MX( Reference Reeves, Nielsen and Fahey 20 )): Fe4(P2O7)3 at 4·12 g Fe/kg or FeSO4.H2O at 2·71 g Fe/kg mix was utilised in the mineral mix of the experimental diets.
∥ Orafti Active Food International (Clariant), 93 % inulin-type fructans (ITF).
¶ São Sebastião Farm (Ibiúna); 48 % ITF, 49 % fructose, 11 % total dietary fibre and 4 μg Fe/g.
Rats were anaesthetised through the intraperitoneal route with a 1:2 (v/v) mixture of ketamine (10 mg/kg body weight; Vetaset; Fort Dodge Animal Health) and xylazine (25 mg/kg body weight; Virbaxil 2 %, Virbac). After anaesthesia, blood was collected from each rat and the liver was perfused through the subhepatic vein with a NaCl solution (9 g/l) to drain blood out of the organ. The liver was then removed, rinsed with saline, weighed and stored at − 20°C until analysis. The caecum was cut adjacent to the ileocaecal valve and the caecocolonic junction, weighed (whole caecum) and placed in a Petri dish with ice( Reference Lu, Gibson and Muir 22 ). The pH of caecal content was measured in situ by inserting an electrode (UP-25; Denver Instrument, Denver) through the ileocaecal junction. The caecum was then cut open along the small curvature and aliquots of the caecal contents were collected, frozen in liquid N2 and adequately stored at − 20°C for Fe fractionation analysis. The caecal wall was flushed clean in ice-cold saline, blotted dry and weighed (to determine the caecal wall weight). The weight of the caecal contents was calculated by taking the difference between the weights of the whole caecum and those of the caecal wall. The caecal mucosa was scraped off with a glass slide before being snap-frozen in liquid N2 and stored at − 80°C until analysis. In addition, tissue samples from the caecal wall were fixed in formaldehyde solution and stored in 70 % ethanol.
Chemical composition of yacon flour and experimental diets
The diet fed to the CT group was formulated according to the AIN-93G diet( Reference Reeves, Nielsen and Fahey 20 ). In the RAF and YF diets (Table 1), maize starch, sucrose and dietary fibre were quantitatively substituted, taking into consideration the carbohydrate content of the FOS sources. The Raftilose P95 used in the present study was donated by Clariant S/A. According to the analysis certificate, this product contains approximately 93 % ITF-containing molecules (GFn) with an average polymerisation degree of 4 (FOS). The FOS in RAF were obtained by the partial hydrolysis of inulin, which was extracted from chicory roots (Cichorium intybus). Yacon tuberous roots were harvested 8 months after cultivation, and they were obtained from the Company of General Warehouses of São Paulo and processed properly as described in previous studies( Reference Lobo, Colli and Alvares 15 , Reference Lobo, Cocato and Borelli 18 ). The roots were autoclaved (121°C, 20 min), freeze-dried (Liotécnica Indústria e Comércio Ltda) and ground to obtain flour.
The total fructans and fructose in YF were analysed according to the method of Steegmans et al. ( Reference Steegmans, Iliaens and Hoebregs 23 ) using an enzymatic, spectrophotometric kit (R-Biopharm AG) for glucose and fructose determination with hexokinase, glucose-6-phosphate dehydrogenase and phosphoglucose isomerase. The total dietary fibre was determined by enzymatic–gravimetric method( Reference Prosky, Asp and Schweizer 24 ). Fe concentrations in the diets and in the YF were determined by atomic absorption spectrophotometry (AAnalyst 100; Perkin Elmer) using a hollow cathode lamp at 283·4 nm and a 0·2 nm slit after wet digestion (HNO3–H2O2, 5:1; v/v). The working standard solution was prepared with FeCl3 (Tritisol; Merck). Yacon flour analysis identified 48 % total fructans, 49 % fructose, 11 % total dietary fibre and 4 μg Fe/g. A certified reference material (lyophilised bovine liver, SRM1577c, National Institute of Standards and Technology) was used to check Fe recovery (Fe value = mean 188 (sd 14) μg/g, n 6) (certified value, 197 (sd 6) μg/g).
Iron bioavailability, hepatic iron concentration and iron fractionation analyses in the caecal contents
To determine Hb concentration during the depletion period, blood was obtained by tail puncture. At the time of killing during the repletion period, blood was obtained from the abdominal aorta. Blood Hb concentration was determined with a commercial kit (reference no. 43, Labtest Diagnóstica) using the cyanide Hb method( Reference Drabkin and Austin 25 ) and a commercially available control material (Labtest Diagnóstica). The Hb Fe pool was calculated according to the following equation, assuming that 6·7 % of the body weight is blood and that Hb contains 0·335 % Fe( Reference Mahoney, Van Orden and Hendricks 26 ):
Values obtained in the Hb Fe pool and Fe intake calculations were used to estimate the Hb regeneration efficiency (HRE), according to the following equation( Reference Mahoney, Van Orden and Hendricks 26 ):
The bioavailability of Fe from FS (FS group) was considered as the reference and was therefore assigned a value of 100 %( Reference Lobo, Cocato and Borelli 18 , Reference Poltronieri, Arêas and Colli 27 ). Thus, the relative Fe bioavailability, or biological value (RBV) of each FP group (FP, RAF and YF groups), was determined at weeks 1 and 2 of the repletion period as follows:
The total Fe in the left lateral lobe of the liver and the caecal contents was determined as described previously for dietary Fe analysis. Caecal contents were subjected to a sequential solubilisation to analyse the intestinal Fe species distribution, as described by Simpson et al. ( Reference Simpson, Sidhar and Peters 28 ), which was modified from Tessier's method( Reference Tessier, Campbell and Bisson 29 ). In brief, approximately 1 g of the sample was subjected to extraction under stirring followed by centrifugation (10 000 g for 30 min). The supernatant was collected and combined with the washed pellet that was obtained by mixing the demineralised water after sample re-centrifugation. Each fraction of the extraction process corresponded to the supernatant added to its wash. The resulting pellet was subjected to the next extraction step. Fe concentrations were determined in the following five fractions: exchangeable (soluble in 1 m-magnesium chloride; for 1 h at room temperature); carbonate-bound (acid soluble; 1 m-sodium acetate, pH 5·0-adjusted with acetic acid, for 4 h at room temperature); oxide-bound (soluble in 0·04 m-hydroxylamine in acetic acid; for 16 h at 95°C); organic matter (soluble after treating with 0·02 m-nitric acid and H2O2 (pH 5·0-adjusted with nitric acid); for a total of 5 h at 85°C); residual (the remaining pellet)( Reference Simpson, Sidhar and Peters 28 , Reference Tessier, Campbell and Bisson 29 ). Fe concentrations were obtained directly from calibration curves that were prepared from the solution components used in different extraction steps. The analytical precision for each extraction step was evaluated by subjecting ten subsamples of a caecal content pool to the sequential procedure described above. The pool was obtained by homogenising the caecal contents of male Wistar rats (n 10; body weight approximately 200 g) fed commercial chow (664 mg Fe/kg). CV of 10·5 % (for the organic matter fraction) or lower (6·8, 9·4, 6·3 and 9·7 % for exchangeable, carbonate-bound, oxide-bound and residual fractions, respectively) were obtained. A comparison of the sum of Fe concentrations in each fraction was consistent with the total Fe concentrations (r 2 0·98; Fe value = mean 13·76 (sd 1·13) v. 13·95 (sd 1·14) μmol/g, respectively).
Immunoblotting
N-frozen samples of caecal mucosa were homogenised (T10 basic Ultra Turrax; IKA Works, Inc.) in T-PER Tissue Protein Extraction Reagent (Pierce Technology) containing protease and phosphatase inhibitors (Pierce Technology). The homogenates were centrifuged at 10 000 g for 5 min, and total protein concentrations in the supernatants were determined using the bicinchoninic acid assay (Pierce Technology). Equal amounts of total protein (50 μg) were denatured by boiling for 5 min in 4 × SDS Laemmli buffer (200 mm-Tris–HCl pH 6·8, 8 % (v/v) SDS, 40 % (v/v) glycerol, 20 % (v/v) β-mercaptoethanol and 0·4 % (v/v) bromophenol blue), separated by 12·5 % SDS–PAGE (Fisher Scientific) and then transferred onto nitrocellulose membranes (Whatman). The blots were pre-incubated in Blocker Casein Buffer (Pierce Technology) for 2 h at room temperature and then incubated with 1:500 (v/v) diluted rabbit polyclonal anti-FPN1 (Abcam Antibodies) for 14 h at 4°C. After being washed three times with 0·1 % PBS–Tween, the blots were incubated with 1:50 000 (v/v) diluted peroxidase-labelled anti-rabbit secondary antibodies (Sigma Chemical Company), and the immunoreactive band signals were visualised with an enhanced chemiluminescence kit (GE Healthcare). ImageQuant 400 equipment (GE Healthcare) and Quantity One Basic software (BioRad Laboratories) were used for scanning the photo-images and for densitometry analysis, respectively. Each membrane was re-probed with 1:50 000 (v/v) diluted peroxidase-labelled anti-β-actin monoclonal antibody (Sigma Chemical Company). FPN protein expression data were normalised to corresponding β-actin values and expressed as arbitrary units.
Caecal histology
To perform a histological examination, the fixed tissue fragments were embedded in paraffin and approximately 5 μm-thick sections were obtained and stained with haematoxylin and eosin. In each crypt, the cells in the left-hand column were counted (cells/hemicrypt) from the bottom to the top of the caecal crypt under an optical microscope. At least thirty hemicrypts per rat were assessed, and only crypts that were cut lengthwise were considered. To determine the percentage of bifurcating crypts, the crypts with an indentation at the base or those presenting longitudinal fission (one crypt mouth and two bases) were considered( Reference Maskens 30 ). The calculation was performed by determining the percentage of bifurcating crypts (only crypts cut lengthwise) per microscopic field. To estimate the total number of crypts per microscopic field, the obliquely sectioned crypts were also considered. At least twenty microscopic fields per rat were analysed. To evaluate the metaphase index, each rat was administered a single intraperitoneal injection of vincristine sulphate (Tecnocris, Zodiac Produtos Farmaceuticos S/A; 1 mg/kg body weight) 2 h before being killed. The metaphase index was determined by counting the number of cells arrested at metaphase under a light microscope (Nikon). Approximately 2000 cells per rat were counted in longitudinally sectioned crypts using an ocular grid (Zeiss Integration Eyepiece I Kpl 8; Carl Zeiss) at 100 × magnification. The metaphase index was determined as the ratio of the number of arrested metaphases:the total number of cells.
Statistical analysis
Statistical analysis was performed using Prism 5 for Windows (version 5.00, 2007; Graphpad Software, Inc.). All tests were performed by assuming the bilateral hypotheses and a 5 % significance level. Descriptive statistics were initially used to evaluate the means and standard deviations of the studied variable. During the depletion period, the mean values of the CT and ID groups were compared using an unpaired t test. During the repletion period, the variable means of the groups were compared using an ANOVA. Tukey's post hoc test was used to identify where significant differences occurred. A non-parametric Kolmogorov–Smirnov test was used to verify the normality of the observations, and when the normality hypothesis was rejected, ANOVA was substituted with non-parametric Kruskal–Wallis and Dunn's post hoc tests. The observed power was 85–95 % for most tests.
Results
Total food and iron intake, body weight gain, iron bioavailability parameters and hepatic iron concentrations
At the end of the depletion period, the blood Hb concentrations and body weights of the ID group were significantly reduced in comparison with those of the CT group (59 (sd 6) v. 122 (sd 19) g/l, P< 0·0001, and 82 (sd 18) v. 104 (sd 5) g, P= 0·005, respectively).
During the repletion period, no significant differences were observed in the total food intake among the groups (112 and 260 g at weeks 1 and 2, respectively, in the FS group). Moreover, there were no differences in total Fe intake (5·5 and 13 mg at weeks 1 and 2, respectively, in the FS group), considering that the different diets had similar Fe concentrations, as measured by atomic absorption spectrophotometry (49·4, 44·9, 45·3 and 47·0 mg Fe/kg diet in the FS, FP, RAF and YF diets, respectively). There were no differences in body weights among the groups at the end of week 1, but at week 2, YF supplementation resulted in a higher weight gain in comparison with that in the other groups (P< 0·05).
Blood Hb concentrations, Hb Fe pool, HRE values, RBV and hepatic Fe concentrations during the repletion period are shown in Fig. 1. In general, the effects of FOS in anaemic rats were observed within the 1st week of the repletion period. Hence, considering that blood Hb concentrations were similar among the groups at the start of this period (P= 0·78), changes in Hb concentrations in FOS-fed rats at week 1 were greater than those in the FP group (P< 0·01) and similar to those in the FS group. At week 2, there were no differences in blood Hb concentrations among the groups (Fig. 1(A)). Moreover, no changes were observed in the Hb Fe pool among the groups throughout the repletion period (Fig. 1(B)).
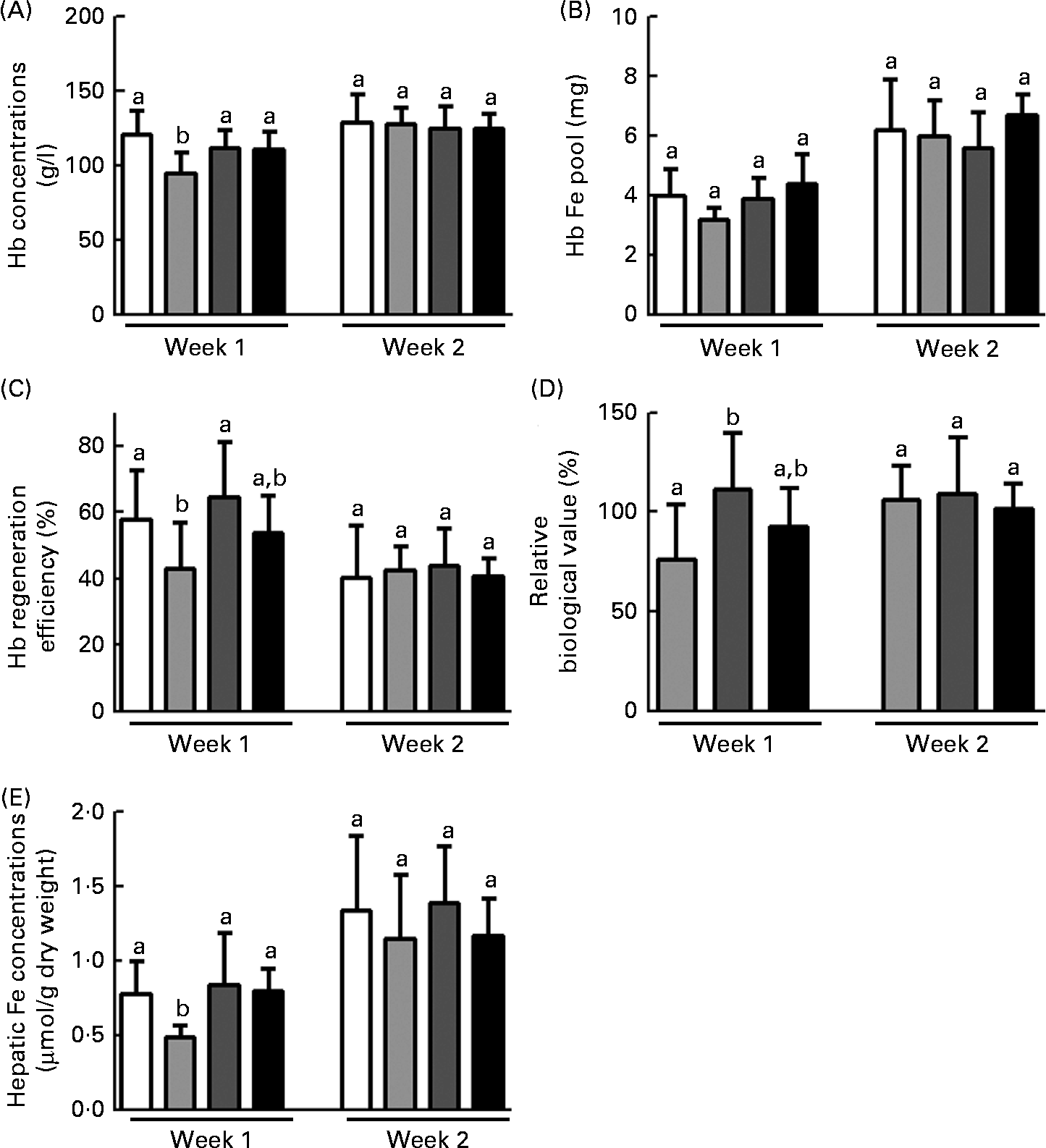
Fig. 1 Effects of fructo-oligosaccharides on the bioavailability of iron from ferric pyrophosphate (FP) during the 1st week of recovery of Hb in iron-deficient rats. The iron bioavailability of iron-deficient rats fed diets containing ferrous sulphate (□), FP (![]() ) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (
) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (![]() ) or yacon flour (■) for 1 and 2 weeks (weeks 1 and 2, respectively). (A) Blood Hb concentrations; (B) Hb iron pool; (C) Hb regeneration efficiency; (D) relative biological value; and (E) hepatic iron concentrations. Values are means (n 8), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA and Tukey's post hoc tests).
) or yacon flour (■) for 1 and 2 weeks (weeks 1 and 2, respectively). (A) Blood Hb concentrations; (B) Hb iron pool; (C) Hb regeneration efficiency; (D) relative biological value; and (E) hepatic iron concentrations. Values are means (n 8), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA and Tukey's post hoc tests).
The efficiency of Hb recovery reflects the ratio of dietary Fe conversion into Hb:the amount of ingested Fe over the course of the repletion period. In the present study, only the RAF group exhibited higher HRE values than the FP group and had HRE values similar to those of the FS group at week 1 of the repletion period (P <0·05; Fig. 1(C)). The Hb concentration observed at the end of each week during the repletion period (weeks 1 and 2) in the FS group was considered as the reference. Hence, the FS group was used as a reference to express the bioavailability of Fe from FP (FS is assigned a 100 % value) (RBV). The RBV of the FP in the RAF group were higher than those of the FP in the FS group (111 % after the 1st week and 109 % after the 2nd week). These values were significantly higher than those in the FP group at week 1 (P <0·05; Fig. 1(D)). Moreover, there was no significant difference in the RBV between rats provided with either dietary FOS source at week 1.
As expected, hepatic Fe concentrations were lower in the FP group than in the FS group at week 1 of the repletion period, whereas FOS supplementation led to the recovery of hepatic Fe stores to levels comparable to those observed in the FS group (P =0·01; Fig. 1(E)). In addition, there were no differences in hepatic Fe concentrations between the two FOS groups.
Effects of fructo-oligosaccharides on caecal parameters during recovery from anaemia: early changes in iron species distribution, mucosal ferroportin-1 expression, morphometry and crypt cell proliferation
Because the effects of FOS on Fe bioavailability had already been observed at week 1 of the repletion period, the intestinal changes in response to the supplementation of these fermentable fibres were evaluated. The effects of FOS supplementation on caecal parameters (caecum weight, caecal content pH values and Fe species distribution in the caecal contents) are summarised in Table 2. FOS supplementation resulted in heavier caeca (total, wall and contents), irrespective of the dietary FOS source supplemented, when compared with those of the FP group (P< 0·05). Moreover, the caecal content pH was decreased by FOS supplementation, but only the values of the RAF group were significantly different from those of the CT rats (P< 0·05). Accordingly, although the total Fe concentrations in the caecal contents were not significantly different among the groups, RAF supplementation redistributed Fe from the carbonate-bound fraction (which is sensitive to pH changes) to the exchangeable (more bioavailable) and oxide-bound (susceptible to reduction) fractions (Fig. 2). Thus, the concentrations identified for exchangeable Fe were higher in rats fed the RAF diet (P= 0·01) than in those fed the YF and CT diets (an increase from 2 to 5 % relative to the total Fe concentrations in the CT and RAF rats, respectively (Fig. 2)). Similarly, the levels of Fe bound to oxides were significantly increased by RAF supplementation (P< 0·01). Fe concentrations in the oxide-bound fraction represented 6–7 % of those in the CT rats and increased to 17 % in RAF rats (Fig. 2). The levels of Fe bound to organic matter were lower than their detection limits, representing a negligible fraction of the total amount of Fe in the caecal contents (which are therefore not shown in Fig. 2). Fe concentrations in the residual fraction were higher than those observed in any of the preceding extractions (Fig. 2) and were not different among the groups (Table 2).
Table 2 Caecal parameters (organ weight, pH values, total iron concentrations and iron species distribution in the caecal contents) of iron-deficient rats fed diets containing ferrous sulphate (FS) or ferric pyrophosphate (FP) supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF) or yacon flour (YF) during the 1st week of recovery of Hb (Mean values with their standard deviations, n 8)
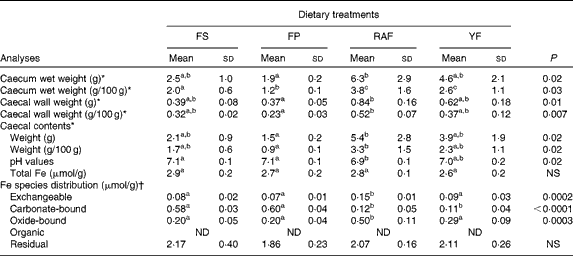
ND, not detected.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Data were analysed using ANOVA and Tukey's post hoc tests.
† Data were analysed using non-parametric Kruskal–Wallis and Dunn's post hoc tests.
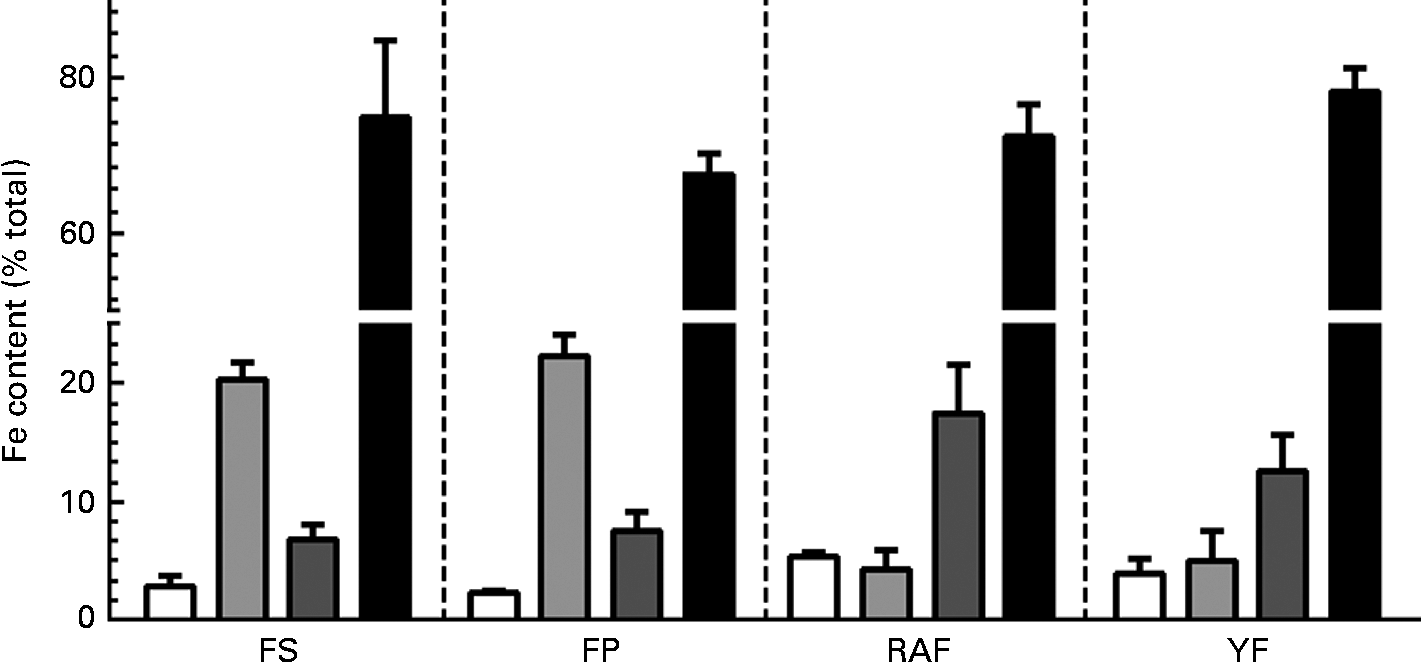
Fig. 2 Effects of fructo-oligosaccharides on the distribution of iron species in caecal contents during the 1st week of recovery of Hb in anaemic rats. Iron species distribution in the caecal contents of iron-deficient rats fed diets containing ferrous sulphate (FS), ferric pyrophosphate (FP) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF) or yacon flour (YF). The fractions are as follows: exchangeable (□); carbonate-bound (![]() ); oxide-bound (
); oxide-bound (![]() ); residual (■). Values are means (n 8), with standard deviations represented by vertical bars.
); residual (■). Values are means (n 8), with standard deviations represented by vertical bars.
The expression of FPN-1, the transporter responsible for cellular Fe efflux, was increased in the caecal mucosa of the FP group (P= 0·04; Fig. 3), which accounted for the highest expected absorption efficiency in rats from the FP group that still remained anaemic at week 1 of the repletion period. Moreover, there were no differences in FPN-1 expression between FOS-fed rats and FS rats (Fig. 3).
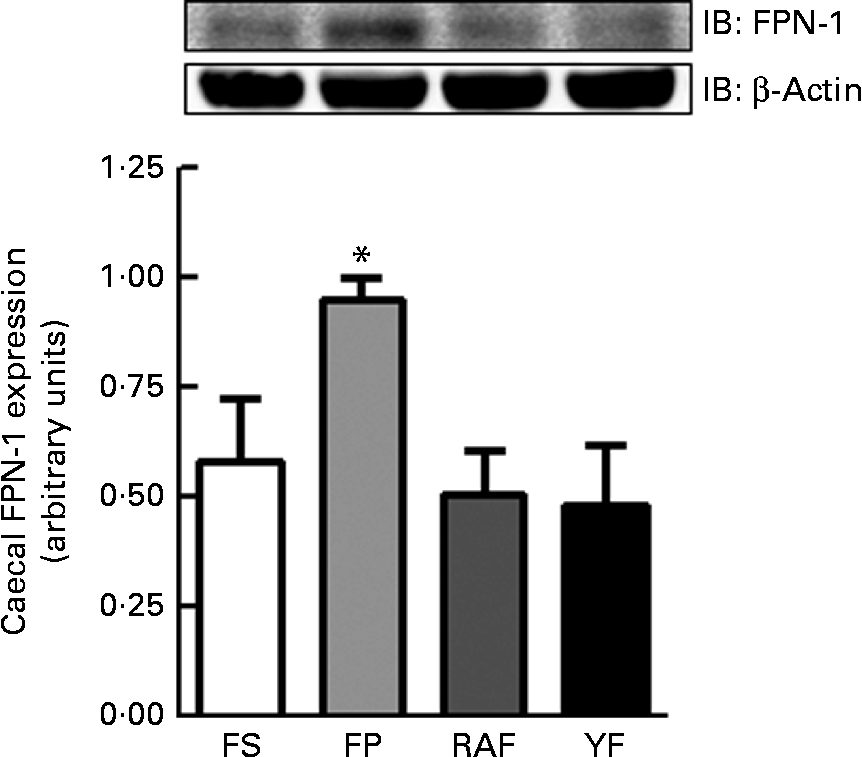
Fig. 3 Effects of fructo-oligosaccharides on ferroportin-1 (FPN-1) expression in the caecal mucosa of anaemic rats during the 1st week of recovery of Hb. FPN-1 expression in the caecal mucosa of iron-deficient rats fed diets containing ferrous sulphate (FS, □), ferric pyrophosphate (FP, ![]() ) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF,
) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF, ![]() ) or yacon flour (YF, ■). Values are means (n 4), with standard deviations represented by vertical bars. *Mean value was significantly different from that of the YF group (P< 0·05; non-parametric Kruskal–Wallis and Dunn's post hoc tests). IB, immunoblotting.
) or yacon flour (YF, ■). Values are means (n 4), with standard deviations represented by vertical bars. *Mean value was significantly different from that of the YF group (P< 0·05; non-parametric Kruskal–Wallis and Dunn's post hoc tests). IB, immunoblotting.
Caecal enlargement was in agreement with the histological changes observed in FOS-fed rats; there was a noticeable increase (P< 0·05) in the number of cells per crypt (Fig. 4(A)), the number of crypts per field (Fig. 4(B)) and the percentage of bifurcating crypts per field (Fig. 4(C)) when compared with those in the CT rats. Consistent with these findings, cell proliferation as assessed by the 2 h accumulation of vincristine-arrested metaphases per crypt was increased by FOS supplementation (P< 0·05), and this effect was more pronounced in the RAF group (Fig. 4(D)).

Fig. 4 Changes in the caecal mucosa architecture of iron-deficient rats fed fructo-oligosaccharides during the 1st week of recovery of Hb. The number of cells (A), number of crypts (B), percentage of bifurcating crypts (C) and metaphase index in the crypts (D) in the caecum of iron-deficient rats fed diets containing ferrous sulphate (FS, □), ferric pyrophosphate (FP, ![]() ) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF,
) or FP supplemented with 7·5 % inulin-type fructans in the form of Raftilose P95 (RAF, ![]() ) or yacon flour (YF, ■). Values are means (n 5), with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; non-parametric Kruskal–Wallis and Dunn's post hoc tests).
) or yacon flour (YF, ■). Values are means (n 5), with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; non-parametric Kruskal–Wallis and Dunn's post hoc tests).
Discussion
The primary strategies for correcting Fe deficiency in populations are dietary modification or diversification to improve Fe intake and bioavailability; Fe supplementation and Fe fortification of foods; and biofortification by plant breeding( Reference Zimmermman and Hurrel 31 ). Although the efficiency of Fe absorption increases as Fe stores become depleted, the amount absorbed from foods, especially when diets are low in meat, fish, fruit and vegetables, is not enough to prevent Fe deficiency in many women and children, especially in developing countries( Reference Zimmermman and Hurrel 31 , Reference Benito, House and Miller 32 ). For instance, the primary cause of increasing Fe deficiency in Brazil is that the consumption of food items considered to be Fe sources has continually decreased( Reference Szarfarc, Braga, Amancio and Vitalle 33 ). Indeed, the search for new food standards, proposals for food distribution and knowledge about the dietary composition must be the researcher's target.
The effects of dietary fibre or fibre-rich diets on mineral absorption have been discussed for many years and appear to fundamentally depend on whether they are observed in in vitro or in vivo studies( Reference Kelsay, Behall and Prather 34 , Reference Fernandez and Phillips 35 ). On the one hand, some fibres (e.g. cellulose, hemicelluloses and pectin) or fibre-associated substances (e.g. phytates and phenolics) negatively affect the absorption of minerals by binding to or entrapping them in the lumen of the small intestine( Reference Fernandez and Phillips 35 ). On the other hand, these negative effects seem to be reduced if the fibres are fermented in the large intestine. In this context, the positive effects of a group of fermentable fibres (namely ITF (FOS and inulin)) on the intestinal absorption and bioavailability of some minerals have been repeatedly demonstrated in animal( Reference Lobo, Mancini Filho and Alvares 7 , Reference Nzeusseu, Dienst and Haufroid 12 – Reference Lobo, Cocato and Borelli 18 ) and human( Reference Van Der Heuvel, Muys and Van Dokkum 36 , Reference Abrams, Griffin and Hawthorne 37 ) studies. Most of these studies focused on the bioavailability of Ca and Mg. Nevertheless, the number of studies concerning the bioavailability of trace elements (Fe, Cu and Zn) is proportionally lower, and these studies have thus far yielded contradictory results. In particular, there is some evidence in rats( Reference Lobo, Cocato and Borelli 18 , Reference Weber, Freitas and Amancio 38 , Reference Freitas, Amancio and Morais 39 ) and pigs( Reference Tako, Glahn and Welch 13 , Reference Tako, Yasuda and Glahn 14 , Reference Yasuda, Urban and Dawson 40 , Reference Patterson, Yasuda and Welch 41 ) that Fe bioavailability is positively affected. However, these effects have not been consistently demonstrated in human studies( Reference Petry, Egli and Chassard 42 ). To our knowledge, there are no studies that have compared the effects of purified and non-purified sources of ITF on Fe bioavailability and on histological changes in the large intestine of rats subjected to an experimental model of Fe-deficiency anaemia. We had previously demonstrated the improved bioavailability of Fe from FP (as measured by Hb repletion efficiency) by supplementing diets with 7·5 % ITF from either RAF (a purified source) or yacon tuberous roots (a non-purified source) in anaemic rats( Reference Lobo, Cocato and Borelli 18 ). In the present study, we confirmed these findings and demonstrated that early changes in the caecal environment (luminal Fe species redistribution, decreased mucosal FPN-1 expression, and morphological alterations) could be related to Fe bioavailability improvement by FOS supplementation.
Fe in foods is released into the lumen as haem or as non-haem ferric complexes( Reference Cremonesi, Acebron and Raja 43 , Reference Colli, Braga, Amancio and Vitalle 44 ). However, non-haem Fe is most affected by the food matrix composition and the physico-chemical conditions in the intestinal lumen( Reference Colli, Braga, Amancio and Vitalle 44 ). Among the various oxidation states of Fe, divalent Fe (Fe2+) and trivalent Fe (Fe3+) are more stable in aqueous media, and they occur naturally in foods( Reference Colli, Braga, Amancio and Vitalle 44 ). The two forms are soluble at the pH of the stomach; nonetheless, Fe3+ precipitates more rapidly than Fe2+ because of the alkalinisation of the alimentary bolus in the duodenum during the digestive process. However, Fe precipitation can be inhibited by complexing agents in foods, and these agents include amino acids, carboxylic acids, carbohydrates and phosphates( Reference Cremonesi, Acebron and Raja 43 , Reference Colli, Braga, Amancio and Vitalle 44 ). Thus, factors that affect the luminal pH or chelating properties of certain dietary components can also affect their redox potential and interfere with their solubility( Reference Cremonesi, Acebron and Raja 43 , Reference Colli, Braga, Amancio and Vitalle 44 ). In this context, physico-chemical changes in the caecal environment resulting from the bacterial fermentation of non-digestible carbohydrates could contribute to alterations in mineral speciation and thus mineral absorbability( Reference Levrat, Rémésy and Demigné 10 , Reference Rémésy, Levrat and Gamet 11 , Reference Lobo, Cocato and Borelli 18 , Reference Yasuda, Roneker and Miller 45 ). In the present study, the lower luminal pH observed in the caecum of FOS-fed rats could have contributed to Fe mobilisation from the carbonate-bound to the exchangeable and oxide-bound fractions. Only 10 % of dietary Fe is absorbed in the small intestine (primarily in the duodenum), which indicates that significant amounts of Fe are recovered in the luminal content of the large intestine( Reference Lund, Wharf and Fairweather-Tait 46 ). Thus, our findings indicate that FOS fermentation could have increased the fraction of Fe available for uptake in the caecum by approximately 15 %.
Complementarily, mucosal FPN-1 expression in the caecum was decreased in FOS-fed rats at a magnitude similar to that in the FS group. Some studies have also demonstrated that, under specific circumstances, the proximal colon may significantly contribute to Fe absorption( Reference Tako, Glahn and Welch 13 , Reference Yasuda, Urban and Dawson 40 , Reference Frazer, Wilkins and Anderson 47 , Reference Takeuchi, Bjarnason and Laftah 48 ). In fact, feeding rats with an Fe-deficient diet induces an increase in the gene expression of Fe transporters (DCYTB, FPN-1 and DMT-1) both in the duodenum and in the large intestine( Reference Takeuchi, Bjarnason and Laftah 48 ). Accordingly, the highest caecal FPN-1 expression in FP animals is consistent with the fact that these animals were still anaemic after 1 week of repletion. Some authors have suggested that the expansion of the caecal compartment by fibre fermentation could contribute to Fe absorption via enhanced DMT-1 expression( Reference Hara, Onoshima and Nakagawa 49 ). Caecal enlargement has been observed frequently and tends to be proportional to the fermentability of dietary fibre( Reference Lobo, Mancini Filho and Alvares 7 , Reference Lobo, Cocato and Borelli 18 , Reference Lu, Gibson and Muir 22 , Reference Campbell, Fahey and Wolf 50 ). In this context, this trophic effect was believed to be caused by the SCFA (particularly butyrate) that are produced as a result of the increased metabolic activity of the microbiota( Reference Gibson and Roberfroid 1 , Reference Walker, Duncan and Leitch 8 , Reference Campbell, Fahey and Wolf 50 – Reference Weaver, Tangel and Krause 52 ). Butyrate is recognised as the primary source of energy for colonocytes, playing an important role in the stimulus of cell division in the intestinal mucosa( Reference Sakata 51 , Reference Weaver, Tangel and Krause 52 ). We had previously demonstrated increased caecal SCFA production by FOS supplementation and found YF to be more butyrogenic than RAF( Reference Lobo, Cocato and Borelli 18 ). However, the yacon roots used in that study had lower fructan contents than those used in the present study (18 %( Reference Lobo, Cocato and Borelli 18 ) v. 48 %), most probably because of the increased fructan depolymerisation catalysed by fructan 1-exohydrolase( Reference Itaya, Carvalho and Figueiredo-Figueiredo 53 ), which led us to use a larger amount of YF in the formulation of experimental diets (41·7 %( Reference Lobo, Cocato and Borelli 18 ) v. 15·7 %). Thus, although the fructan content was the same (7·5 %) in the different diets (RAF and YF), the dietary fibre (soluble and insoluble) concentrations (as measured by the enzymatic–gravimetric method( Reference Prosky, Asp and Schweizer 24 )) in the additional YF used for diet formulation may have contributed to the higher fermentability observed in the YF group in that study( Reference Lobo, Cocato and Borelli 18 ). In fact, in the present study, besides exhibiting a lower caecal content pH, RAF rats also had heavier caeca compared with YF rats. Factors other than dietary fibre fermentability may also directly influence the mucosal growth of the caecum, such as increased intestinal content viscosity( Reference Hara, Suzuki and Kobayashi 2 ). In addition, caecum weight can be influenced by stool water content because FOS remain in solution in the chyme and contribute to increased osmotic pressure, resulting in increased water flow to the intestinal lumen( Reference Lobo, Colli and Filisetti 16 , Reference Lobo, Cocato and Jorgetti 17 , Reference Leegwater, De Groot and Van Kalmthout-Kuyper 54 ).
Bacterial fermentation has also been implicated in morphological changes in the intestinal epithelium of FOS-fed animals. In this context, germ-free rats were found to exhibit an increase in intestinal weights, but exhibited no proliferative effects in the intestinal mucosa in response to fibre consumption, confirming that the fermentation products (SCFA) are responsible for trophic effects on the intestinal mucosa( Reference Kleessen, Hartmann and Blaut 3 , Reference McCullough, Ratcliffe and Mandir 55 ). Thus, that FOS-containing diets indirectly affect mucosal morphometry (with increases in the number and depth of crypts and crypt bifurcation) in the rat large intestine has previously been demonstrated by us( Reference Lobo, Mancini Filho and Alvares 7 , Reference Lobo, Colli and Alvares 15 ) and other authors( Reference Kleessen, Hartmann and Blaut 3 , Reference McCullough, Ratcliffe and Mandir 55 ). Moreover, the increased number of caecal crypts was supported by the present data on crypt cell proliferation and bifurcation, which are complementary mechanisms to increase tissue mass and were stimulated upon FOS supplementation. The production of new crypts by fission is a process that is thought to be related to the expansion of the crypt stem cell population and is increased in postnatal development, during intestinal recovery from injury and in crypts isolated from adenomas and hyperplastic polyps( Reference Maskens 30 , Reference Cheng, Araki and Furuya 56 , Reference Goodlad and Englyst 57 ). Thus, FOS fermentability must be addressed with some caution, because the observed effects may, on the one hand, be interesting for promoting mineral absorption and bioavailability by increasing the absorptive surface and contributing to the improvement of mineral solubility in the caecum. On the other hand, the intestinal changes caused by this type of carbohydrate (rapidly fermentable) have been linked to the promotion of intestinal tumour development( Reference Goodlad and Englyst 57 , Reference Mandir, Englyst and Goodlad 58 ). Furthermore, some studies in rats have demonstrated detrimental effects on the intestinal barrier function after FOS supplementation in Ca-restricted diets( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 5 , Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 59 ). Thus, from a nutritionist's point of view, a dietary prescription for fermentable fibres should consider not only the type and amount of fibre but also whether the consumed fibre is given in a purified or non-purified form. In addition, the composition of the food matrix, in which the fibre is present, is another important factor to be taken into consideration.
In conclusion, dietary FOS supplementation improves Fe bioavailability in anaemic rats by changing luminal Fe solubility, most probably as a result of bacterial fermentation in the caecum. These effects were more pronounced on using a purified source (RAF) than on using a non-purified source (YF) of fructans. These effects, if confirmed in humans, might contribute to the formulation of specific diets for individuals with Fe deficiency. The changes observed in the caecal tissue of FOS-fed animals must be evaluated with caution because increased cell proliferation and crypt fission have been implicated in the carcinogenic process within the large intestine.
Acknowledgements
The authors thank Cruz Alberto Mendoza Rigonatti and Natália Nadur de Souza for their technical assistance, the Fundação de Amparo à Pesquisa do Estado de São Paulo (2009/01760-8) for supporting this research and the Conselho Nacional de Desenvolvimento Científico e Tecnológico for the fellowships awarded to A. R. L. (505758/2008-3) and C. C. (309026/2009-1). The present study was also supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
The authors’ contributions are as follows: A. R. L. was responsible for the study concept and design and for the acquisition, analysis and interpretation of the data; E. H. S. G. and E. D. C. were responsible for the acquisition and analysis of the data; E. P. A. was responsible for the acquisition, analysis and interpretation of the data; C. C. was responsible for the study concept and design, acquired, analysed and interpreted the data, and obtained funding.
The authors declare no financial, professional or personal conflicts of interest.








