Flavonoids are biologically active polyphenolic compounds ubiquitously found in fruits, vegetables, nuts and plant-derived beverages, such as tea or wine. The composition of flavonoids in different fruit species varies greatly. Quercetin, kaempferol, myricetin and isorhamnetin are common flavonols, with quercetin being the predominant one. A second flavonoid group in fruits is proanthocyanidins and their monomer units, catechins (procyanidin) or gallocatechins (prodelphinidins), which are the natural substrates of polyphenol oxidases and are, therefore, involved in the browning phenomenon of fruits. The main anthocyanins in fruits are glycosides of different anthocyanidins, mainly cyanidin, that are widespread and commonly contribute to the pigmentation of fruits. Citrus fruits differ in their flavonoid profiles from other fruit species, containing flavanones and flavones (hesperidin and naringenin) that are not common in other fruits(Reference Robards and Antolovich1). The major polyphenolic constituents present in green tea are epicatechin, epigallocatechin, epicatechin-3-gallate and epigallocatechin-3-gallate. In addition to small amount of catechins, black tea contains thearubigins and theaflavins, which are the polymerised forms of catechin monomers and are the major components formed during enzymatic oxidation and the fermentation process(Reference Katiyar2).
Flavonoids have been reported to possess a wide range of activities in the prevention of common diseases, including CHD, cancer, neurodegenerative diseases, gastrointestinal disorders and others(Reference González-Gallego, Sánchez-Campos and Tuñón3). These effects appear to be related to the various biological/pharmacological activities of flavonoids. A large number of publications suggest immunomodulatory and anti-inflammatory properties of these compounds. However, almost all studies are in vitro studies with limited research on animal models and scarce data from human studies. The majority of in vitro studies have been carried out with single flavonoids (generally aglycones) at rather supraphysiological concentrations, and few studies have investigated the anti-inflammatory effects of physiologically attainable flavonoid concentrations in healthy subjects(Reference Tuñón, García-Mediavilla and Sánchez-Campos4).
This review will summarise the evidence for the effects of fruits and tea flavonoids and their metabolites on inflammation and immunity. Mechanisms of the effects will be discussed, including those on enzyme function and regulation of gene and protein expression. Animal work will be included, and evidence from epidemiological studies and human intervention trials will be reviewed. Biological relevance and functional benefits of the reported effects, such as resistance to infection or exercise performance, will also be discussed.
Evidence from in vitro and animal studies
A large number of studies have shown inhibitory effects of fruit and tea flavonoids on the expression and activity of enzymes involved in the generation of inflammatory mediators such as nitric oxide (NO) or prostanoids and leukotrienes (Table 1). Thus, flavonols such as quercetin and kaempferol or flavones such as apigenin inhibit NO production and the expression of inducible NO synthase (iNOS) in the mouse macrophage-like cell line RAW 264·7(Reference Liang, Huang and Tsai5, Reference Shen, Lee and Lin6). Research using IL-1β-activated human chondrocytes or IL-1β-activated rat hepatocytes(Reference Martínez-Flórez, Gutiérrez-Fernández and Sánchez-Campos7, Reference Ahmed, Rahman and Hasnain8) also supports iNOS inhibition by different flavonoids. Down-regulation of cyclo-oxygenase (COX)-2 expression by apigenin and quercetin has been demonstrated in lipopolysaccharide (LPS)-stimulated J774A.1 cells(Reference Raso, Meli and Di Carlo9). Quercetin or kaempferol in mouse macrophages(Reference Jung and Sung10, Reference Kim, Park and Yun11) or the citrus polymethoxy flavone nobiletin in human synovial fibroblasts(Reference Lin, Sato and Takayama12) shows a similar effect. Different green tea polyphenols suppress mRNA and protein expression of COX-2 in RAW 264·7 cells(Reference Hou, Luo and Tanigawa13), and genistein down-regulates COX-2 promoter activity in colon cancer cells(Reference Mutoh, Takahashi and Fukuda14). iNOS and COX-2 protein levels are reduced by quercetin and kaempferol in Chang liver cells(Reference García-Mediavilla, Crespo and Collado15), and luteolin has a similar effect in LPS-stimulated macrophages(Reference Chen, Peng and Tsai16). Apigenin down-regulates COX-2 expression in lupus T cells, B cells and antigen-presenting cells, and causes their apoptosis(Reference Kang, Ecklund and Liu17). Although no clear structural/functional relationships have been established, it appears that the C-2,3-double bond and the hydroxyl substitutions on A- and B-rings are important contributors to this inhibitory activity(Reference Kim, Son and Chang18). Animal data confirm down-regulation of iNOS and COX-2 expression in different inflammatory diseases(Reference Moreira, Fraga and Alonso19, Reference Tieppo, Cuevas and Vercelino20).
Table 1 Evidence of the effects of fruit and tea polyphenols from in vitro and animal studies
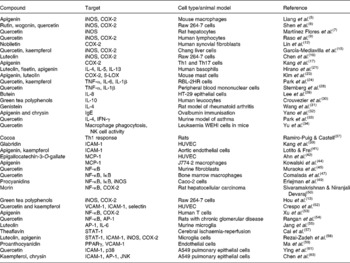
iNOS, inducible NO synthase; COX, cyclo-oxygenase; Th, helper T cell; LOX, lipoxygenase; IFN-γ, interferon-γ; NK, natural killer; ICAM, intercellular adhesion molecule; HUVEC, human umbilical vein endothelial cells; MCP, monocyte chemoattractant protein; VCAM, vascular cell adhesion molecule; AP-1, activator protein; STAT, signal transducer and activator of transcription; JNK, c-Jun NH2-terminal kinase.
The effects of flavonoids on cytokine expression have been studied in different cell types. Luteolin and apigenin have been shown to inhibit Th2-type cytokine production, including IL-4, IL-5 or IL-13 by activated human basophils(Reference Hirano, Higa and Arimitsu21). Quercetin inhibits TNF-α release by LPS-activated RAW 264·7 cells(Reference Wadsworth and Koop22). IL-8 production is inhibited in human nasal fibroblasts by green tea polyphenols(Reference Kim, Kim and Lee23). Quercetin and kaempferol inhibit gene expression and secretion of TNF-α, IL-1β or IL-6 in RBL-2H3 cells(Reference Park, Lee and Son24). Taxifolin glycoside has a significant inhibitory effect on the production of cytokines, formation of NO and change in intracellular Ca2+ levels in dendritic cells of bone marrow and spleen, suggesting that taxifolin glycoside may exert an inhibitory effect against dendritic cell-mediated immune responses(Reference Kim, Choi and Lee25). In human peripheral blood mononuclear cells, quercetin reduces cell proliferation in a dose-dependent manner and modulates the level of IL-1β and TNF-α released in the culture supernatants(Reference Sternberg, Chadka and Lieberman26). Morin decreases the IL-12 and TNF-α production in LPS-activated macrophages, suggesting that it may promote helper T type 2 (Th2) response in vivo and favours Th2 cell differentiation through modulating the maturation and function of bone marrow-derived dendritic cells(Reference Li, Suen and Chiang27). Silibinin, the primary active compound in silymarin, the Silybum marianum fruit extract, polarises Th1/Th2 immune response through the inhibition of immunostimulatory function of dendritic cells, with an impaired induction of Th1 response(Reference Lee, Kim and Kim28). Hydroxylations at positions 5,7,3′ and 4′, together with the double bond at C2–C3 and the position of the B-ring at 2, appear to be associated to the highest inhibition of pro-inflammatory cytokine expression(Reference Comalada, Ballester and Bailón29). Moreover, the inhibitory action on pro-inflammatory cytokines may be coupled to the enhancement of anti-inflammatory cytokines, and it has been reported that epigallocatechin-3-gallate, epicatechin-3-gallate or epigallocatechin enhance the production of IL-10 by human leucocytes(Reference Crouvezier, Powell and Keir30).
Data on the immunomodulatory effects of flavonoids obtained in vitro are supported by the results in experimental animals. Thus, genistein significantly suppresses the secretion of interferon (IFN-γ) and augments the IL-4 production by peripheral blood mononuclear cells, showing its immune modulation role of keeping the Th1/Th2 balance, in a rat model of rheumatoid arthritis(Reference Wang, Zhang and Jin31). A diet containing apigenin and chrysin suppresses the up-regulation of serum IgE induced by ovalbumin immunisation through the suppression of Th2-type immune response in animal models(Reference Yano, Umeda and Yamashita32). In a murine model of asthma, quercetin reduces the increased levels of IL-4 and augments IFN-γ production, regulating Th1/Th2 balance and playing a critical role in the amelioration of the pathogenetic process(Reference Park, Lee and Jung33). Quercetin protects from leukaemia WEHI-3 cells injected into BALB7c mice by modulating the immune response, with stimulation of macrophage phagocytosis and promotion of natural killer cell activity(Reference Yu, Lai and Yang34). Other studies have shown that rutin promotes immune response in vivo in a murine model of leukaemia(Reference Lin, Yang and Lu35), or silibinin dose dependently inhibits the production of Th1 cytokines in experimental autoimmune encephalomyelitis(Reference Min, Yoon and Kim36). In addition to exert regulatory activity on the secretion of inflammatory mediators from macrophages and other leucocytes in vitro, it has been shown that high dose intake of cocoa, rich in epicatechin, catechin and procyanidins, favours Th1 response in young rats and increases intestinal γδ-T lymphocyte count, whereas the antibody-secreting response decreases(Reference Ramiro-Puig and Castell37).
Response to pro-inflammatory stimuli such as TNF-α and IL-1β and recruitment of leucocytes by endothelial cells are associated to the selective expression of adhesion molecules on their surface, which has been shown to be decreased by different dietary flavonoids. Hydroxyl flavones and flavonols inhibit cytokine-induced expression of vascular cell adhesion molecules-1, intercellular adhesion molecules (ICAM-1) and endothelial cell selectin (E-selectin) in human umbilical vein endothelial cells(Reference Gerritsen, Carley and Ranges38). Hydroxyl flavones, such as apigenin, and flavonols, such as galangin, kaempferol and quercetin, are able to inhibit endothelial adhesion molecule expression, whereas this effect is absent in the flavanone naringenin and the flavonol epicatechin(Reference Kang, Yoon and Han39, Reference Nowakowska40). This suggests that flavonoid effects on endothelial adhesion molecule expression depend on their molecular structure, with 5,7-dihydroxyl substitution of the A-ring and 2,3-double bond and 4-keto group of the C-ring being the main structural requirements. Metabolic transformation is also important, having been reported that exposure of apigenin and kaempferol to cultured hepatocytes, mimicking first pass metabolism, greatly diminishes the inhibitory effect of flavonoids on endothelial ICAM-1 expression(Reference Lotito and Frei41). Exacerbation of endothelial dysfunction is associated, in addition to the expression of adhesion molecules, to the IL-6-induced production of reactive-C protein (CRP) by hepatocytes. A dose-dependent reduction in CRP protein level has been demonstrated in Chang liver cells exposed to quercetin and kaempferol(Reference García-Mediavilla, Crespo and Collado15).
There is evidence that immunomodulatory properties of fruit and tea flavonoids are also related to inhibition of chemokines in different cell types. Thus, epigallocatechin-3-gallate down-regulates TNF-α receptor 1 and inhibits TNF-α-induced monocyte chemoattractant protein-1 production in bovine coronary artery endothelial cells(Reference Ahn, Xu and Davidge42). Quercetin inhibits TNF-induced IFN-γ-inducible protein 10 and macrophage inflammatory protein 2 gene expression in the murine small intestinal epithelial cell line Mode-K(Reference Ruiz, Braune and Hölzlwimmer43). Apigenin has been reported to inhibit monocyte chemoattractant protein-1 production in LPS-activated J774·2 macrophages(Reference Kowalski, Samojedny and Paul44).
Although the knowledge concerning the mechanisms of action of flavonoids responsible for their anti-inflammatory and immunomodulatory action is still limited, different regulatory processes affecting cell signalling have been investigated. The most widely researched has been the NF-κB pathway. It is known that quercetin inhibits the activation of NF-κB induced by IL-1β in murine fibroblasts(Reference Muraoka, Shimizu and Sun45) or H2O2-stimulated HepG2 cells(Reference Musonda and Chipman46), prevents LPS-induced IκB phosphorylation in bone marrow macrophages(Reference Comalada, Camuesco and Sierra47) and reduces IκB-α and IκB-β phosphorylation in human peripheral blood mononuclear cells(Reference Nair, Mahajan and Reynolds48). Quercetin and kaempferol diminish in parallel iNOS expression and the degradation of IκB in Chang liver cells(Reference García-Mediavilla, Crespo and Collado15). Quercetin abolishes iNOS overexpression and the activation of NF-κB in rat hepatocytes activated by IL-1β(Reference Martínez-Flórez, Gutiérrez-Fernández and Sánchez-Campos7). In Caco-2 cells, procyanidins inhibit NF-κB translocation and TNF-α-induced IκB phosphorylation and degradation(Reference Erlejman, Jaggers and Fraga49). Morin down-regulates the expression of both NF-κB and COX-2 in animal models of hepatocellular carcinoma(Reference Sivaramakrishnan and Niranjali Devaraj50). Nobiletin and tea prodelphinidin B-4 3′-O-gallate down-regulate COX-2 and iNOS by inhibiting NF-κB signalling pathways in LPS-activated RAW 264·7 cells(Reference Hou, Luo and Tanigawa13, Reference Choi, Hwang and Ko51). Research has also demonstrated that both quercetin and kaempferol down-regulate vascular cell adhesion molecules-1, ICAM-1 and E-selectin expression, and inhibit NF-κB binding activity in human umbilical vein endothelial cells stimulated by a cytokine mixture(Reference Crespo, García-Mediavilla and Gutiérrez52). In chronically activated human T cells, apigenin can suppress anti-apoptotic pathways involving NF-κB activation and COX-2 expression(Reference Xu, Zhang and Bertucci53). Modulation of the cascade of molecular events involved in inflammatory and immunological processes may involve other transcription factors in addition to NF-κB. One of those factors is activator protein-1 (AP-1). It has been demonstrated that dietary quercetin inhibits AP-1 and does not reduce NF-κB in the renal cortex of rats with chronic glomerular disease(Reference Rangan, Wang and Harris54). Luteolin inhibits the LPS-induced DNA binding activity of AP-1 in LPS-activated mouse alveolar macrophages(Reference Chen, Peng and Tsai16), and effects on AP-1 are also responsible for luteolin-induced reduction in IL-6 in primary murine microglia and BV-2 microglial cells(Reference Jang, Kelley and Johnson55). The signal transducer and activator of transcription proteins are transcription factors contributing to the regulation of cellular responses to cytokines and growth factors, and it has been demonstrated that flavonoids inhibiting both NF-κB and signal transducer and activator of transcription-1 activation (i.e. quercetin, genistein and kaempferol) are the most potent inhibitors of iNOS expression and NO production(Reference Hamlinen, Nieminen and Vuorela56). It is also known that theaflavin significantly protects neurons from cerebral ischaemia reperfusion injury by limiting leucocyte infiltration and expression of ICAM-1, iNOS and COX-2, at least in part, reducing the phosphorylation of signal transducer and activator of transcription-1(Reference Cai, Li and Wu57). Luteolin and apigenin suppress IFN-γ-induced TNF-α and IL-6 production in parallel to IFN-γ-induced phosphorylation of signal transducer and activator of transcription-1 in microglia cells(Reference Rezai-Zadeh, Ehrhart and Bai58). PPAR are also involved in the inflammatory response, and it has been demonstrated that grape seed proanthocyanidin extracts induce an activation of PPARγ, which contributes to protect the function of endothelial cells through inhibition of vascular cell adhesion molecules-1(Reference Ma, Gao and Li59). Inhibition of mitogen-activated protein kinases (MAPK) may partly explain the effects of flavonoids on the binding capacity of different transcription factors. It has been shown that quercetin inhibits iNOS expression through inhibition of p38 MAPK and blocks AP-1 binding in LPS-induced RAW cells by inhibiting c-Jun N-terminal kinase(Reference Wadsworth and Koop60). In IL-1β-stimulated human A549 cells, quercetin inhibition of ICAM-1 is partially blocked by specific inhibitors of p38 MAPK(Reference Ying, Yang and Song61).
Modulation of MAPK by other flavonoids has also been reported. Thus, luteolin inhibits LPS-stimulated pathways through inhibition of some MAPK such as extracellular signal-regulated kinase and p38 in RAW 264·7 cells(Reference Xagorar, Roussos and Papapetropoulos62), and kaempferol or chrysin attenuates ICAM-1 expression in A549 cells through the attenuation of c-Jun N-terminal kinase and AP-1 activity(Reference Chen, Chow and Huang63). In summary, flavonoids express anti-inflammatory and immunomodulatory activity by modulation of gene expression and signal transduction pathways, but more in vitro studies are required to establish general rules concerning structural/activity relationships. Moreover, research on the intracellular effects of flavonoid metabolites in comparison to parent aglycones should be expanded.
Evidence from human studies
Most epidemiological and intervention studies on the beneficial effect of flavonoids have focussed on their antioxidant capacity. There is evidence that daily consumption of 10 ml of grape juice for 2 weeks results in an increased serum oxygen radical absorbance capacity in parallel to a decreased protein carbonyls concentration in a group of healthy volunteers(Reference O'Byrne, Devaraj and Grundy64). Acute intake of 400 ml of a phenolic juice, with grapes as a major ingredient, reduced lipid peroxidation, determined by plasma thiobarbituric acid-reduced substances in a group of six men and six women(Reference García-Alonso, Ros and Vidal-Guevara65). The consumption of an ellagitannin-enriched pomegranate dietary supplement (1 g) provides evidence of antioxidant activity through a significant reduction in thiobarbituric acid-reduced substances in a group of twenty-two overweight subjects(Reference Heber, Seeram and Wyatt66). In a pilot and randomised, double-blinded, placebo-controlled, cross-over study of twelve adults aged 19–52 years, an increase in serum antioxidants at 1 and 2 h following the intake of an antioxidant-rich fruit and berry juice blend has been reported, as well as an inhibition of lipid peroxidation (thiobarbituric acid-reduced substances) at 2 h post consumption(Reference Jensen, Wu and Patterson67).
Results concerning inflammatory and immunoregulatory processes are less clear (Table 2). In various studies carried out in different countries, it has been found that the dietary pattern characterised by a higher portion of vegetables, fruits and legumes is inversely associated with blood inflammation markers such as CRP, IL-6 and adhesion factors(Reference Nanri, Yoshida and Yamaji68, Reference Salas-Salvadó, Garcia-Arellano and Estruch69). In a recent study of 285 adolescent boys aged 13–17 years, a diet rich in fruits and vegetables and, therefore, rich in antioxidants, folate and flavonoids was associated with lower levels of markers for inflammation such as CRP, IL-6 and TNF-α(Reference Holt, Steffen and Moran70). There is a report that intervention with an anthocyanin extract from blueberries (300 mg/d for 3 weeks) significantly reduced the plasma concentration of NF-κB-related pro-inflammatory cytokines and chemokines (IL-4, IL-13, IL-8 and IFN-α) in a group of 120 men and women aged 40–74 years(Reference Karlsen, Retterstol and Laake71). Results of a study with eighteen healthy men and women, which supplemented their diets with cherries (280 g/d) for 28 d, suggest a selective modulatory effect on CRP and NO(Reference Kelley, Rasooly and Jacob72). Epidemiological data from a cross-sectional study with 8335 subjects indicate that total flavonoid and also individual flavonol, anthocyanidin, and isoflavone intakes, estimated from the United States Department of Agriculture flavonoid databases, are inversely associated with plasma CRP concentrations(Reference Chum, Chung and Claycombe73). The analysis of dietary intake of 704 participants in the data from the Uppsala Longitudinal Study of Adult Men at age 70 years indicates that the intake of food rich in antioxidants was associated with reduced COX- and cytokine-mediated inflammation and oxidative stress at 7 years of follow-up(Reference Helmersson, Arnlöv and Larsson74). In a double-blind, randomised, placebo-controlled investigation of fifty-nine healthy law students who consumed either a commercially available encapsulated fruit and vegetable juice powder concentrate or placebo capsules for 77 d, the ingestion of the concentrate resulted in an increased plasma nutrients and antioxidant capacity, reduction in DNA strand breaks and an increase in circulating γδ-T cells(Reference Nantz, Rowe and Nieves75). In a epidemiological study conducted with 1031 healthy Belgian men, serum CRP concentrations were inversely associated with tea consumption(Reference De Bacquer, Clays and Delanghe76), and in another double-blind, placebo-controlled trial with thirty-seven healthy non-smoking men, regular tea consumption reduced platelet activation and plasma CRP concentrations(Reference Steptoe, Gibson and Vuononvirta77). A recent clinical trial study in forty-eight healthy men aged 20–48 years has demonstrated that a fermented food concentrate consisting of fruits, nuts and vegetables rich in polyphenols has promising immunoregulatory and anti-inflammatory potential, with significant reductions in ICAM-1 and vascular cell adhesion molecules-1 and changes in natural killer cell cytotoxicity in response to IL-2 stimulation(Reference Schoen, Schulz and Schweikart78).
Table 2 Evidence of the effects of fruit and tea polyphenols from epidemiological and intervention studies in healthy individuals

CRP, C-reactive protein; IFN-γ, interferon-γ; NO, nitric oxide; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; 8-OHdG, 8-hydroxydeoxyguanosine; ORAC, oxygen radical absorbance capacity.
However, short-term consumption of black tea (900 ml/d, 4 weeks) did not improve plasma antioxidant capacity; neither reduced urinary 8-hydroxydeoxyguanosine (8-OHdG) nor plasma CRP in a group of sixty-six patients with coronary artery disease(Reference Widlansky, Duffy and Hamburg79). No significant difference has been observed in plasma levels of CRP or ICAM-1 among healthy volunteers consuming diets rich or poor in berries and apple for 6 weeks(Reference Freese, Vaarala and Turpeinen80). In a randomised trial with sixty-four smokers with no previous medical history of chronic diseases, drinking black or green tea for 4 weeks did not affect levels of inflammation measured by plasma IL-6, TNF-α and CRP, or endothelial cardiovascular risk factors(Reference De Maat, Pijl and Kluft81). Moreover, after the acute consumption of 800 mg of pomegranate extract, the antioxidant capacity measured with the oxygen radical absorbance capacity assay increased in healthy human volunteers, with a maximum effect after 0·5 h, whereas the inflammation marker IL-6 was not significantly affected 4 h after the consumption of the extract(Reference Mertens-Talcott, Jilma-Stohlawetz and Rios82). Similarly, it has been shown that while quercetin dose dependently inhibited in vitro LPS-induced TNF-α production in the blood of healthy volunteers, 4-week administration of quercetin resulted in a significant increase in plasma quercetin concentration and increase in total plasma antioxidant capacity but did not alter ex vivo LPS-induced TNF-α levels(Reference Boots, Wilms and Swennen83).
Observational studies are limited in their conclusions because the protection afforded by the consumption of a particular nutrient may be multifactorial, with different components of the food exerting potential beneficial effects. Furthermore, in many studies, the daily intake of flavonoids has been estimated by questionnaires, and more precise analysis in quantity and quality is required. The disappointing outcome of various trials on the preventive effect of flavonoid supplementation in healthy subjects reinforces the necessity of more prospective randomised trials with larger sample sizes, longer follow-up and an extended duration of treatment, and gives some support to the suggestion that supplementation with antioxidants (including flavonoids) would probably be useful mainly in patients suffering from diseases associated with inflammation and oxidative stress(Reference Tuñón, García-Mediavilla and Sánchez-Campos4).
Effects in chronic diseases
Most studies on the beneficial effects of flavonoids on diseases that are associated with inflammation/oxidation have focussed on CVD, which is reviewed in another article from this series of reviews, and thus we will discuss the effects on other chronic diseases in the following section, considering both epidemiological data and those from clinical studies (Table 3).
Table 3 Clinical effects of fruit and tea polyphenols

PFP, purple passion fruit peel; RGJ, red grape juice; MCP, monocyte chemoattractant protein; CRP, C-reactive protein; GSE, grape seed extract; LBR, lyophilised black raspberries; 8-iso-PG, 8-epimer of PG; 8-OHdG, 8-hydroxydeoxyguanosin; COX, cyclo-oxygenase; iNOS, inducible nitric oxide synthase; PSA, prostate-specific antigen.
Different researchers have analysed the potential benefits of flavonoids as anti-allergic substances. In a cohort of approximately 10 000 male and female participants, a significant inverse association between the intake of flavonols, flavones and flavanones and the incidence of asthma has been reported(Reference Knekt, Kumpulainen and Järvinen84). In a cross-sectional study of 174 asthmatics, it was observed that a high adherence to traditional Mediterranean diet (intake of fresh fruits) increased the likelihood of asthma to be under control in adults(Reference Barros, Moreira and Fonseca85). However, results of a population-based, case–control study of 1471 adults in London suggest that dietary intake of catechins, flavonols and flavones is not associated with asthma(Reference Garcia, Arts and Sterne86). Patients with asthma have been studied in a 4-week randomised, placebo-controlled, double-blind trial with oral administration of purple passion fruit peel extract, a novel mixture of bioflavonoids or placebo pills, and it was concluded that the prevalence of wheeze, cough, as well as shortness of breath, was reduced significantly in the group treated with purple passion fruit peel extract(Reference Watson, Zibadi and Rafatpanah87). In another randomised, double-blind, placebo-controlled study, positive effects of apple polyphenols have been reported in thirty-three patients aged 15–65 years with moderate or severe persistent allergic rhinitis(Reference Enomoto, Nagasako-Akazome and Kanda88).
In a group of twenty-seven haemodialysis patients, regular ingestion of concentrated red grape juice (100 ml) reduced neutrophil NADPH oxidase activity and plasma concentrations of oxidised LDL and the inflammatory biomarker monocyte chemoattractant protein-1 to a greater extent than vitamin E(Reference Castilla, Dávalos and Teruel89). In a study in which forty relatively healthy, institutionalised HIV-infected individuals were recruited for assessment before or 3 months after fresh fruit and vegetable supply, it was found that the increase in dietary fruits and vegetables intake had some beneficial effects on total antioxidant status and immune parameters (CD38+/CD8+ count), although no change in hydroperoxides, malondialdehyde or DNA damage was noted(Reference Gil, Lewis and Martínez90). A prospective randomised, controlled trial including thirty men has shown that quercetin (500 mg twice daily for 1 month) is well tolerated and provides significant symptomatic improvement in a group of thirty men with chronic prostatitis(Reference Shoskes, Zeitlin and Shahed91). Quercetin treatment (1 g/d) over 4 weeks has also been found to provide significant symptomatic improvement in twenty-two patients with interstitial cystitis(Reference Katske, Shoskes and Sender92). On the contrary, results from a recent randomised, double-blind, placebo-controlled study indicate that a 4-week treatment with quercetin+vitamin C (166+133 mg) has no effects on disease severity or serum concentration of CRP and pro-inflammatory cytokines in a group of twenty-two patients with rheumatoid arthritis(Reference Bae, Jung and Lee93).
Association of dietary flavonol and flavone intake with type 2 diabetes and markers of insulin resistance and systemic inflammation has been investigated in a group of 38 018 women aged ≥ 45 years. Although there was a modest inverse association of diabetes risk with intake of apple or tea, no relationship was observed between the intake of flavonols and flavones and plasma concentration of insulin, CRP or IL-6(Reference Song, Manson and Buring94). A similar absence of effects on inflammation (CRP and IL-6) and insulin resistance has been reported in a group of fifty-five type 2 diabetic patients after green tea consumption (9 g/d, 4 weeks)(Reference Ryu, Lee and Lee95). Results of a randomised, controlled trial on the effects of green tea extracts/powder (544 mg polyphenols, 456 mg catechins) in sixty-six patients with borderline diabetes or diabetes further appear to support the absence of effects of flavonoids on insulin resistance or inflammatory markers(Reference Fukino, Shimbo and Aoki96). However, it has been very recently reported that following administration of a grape seed extract (600 mg/d) for 4 weeks to a group of thirty-two type 2 diabetic patients, there is a significant improvement in markers of insulin resistance and plasma CRP(Reference Kar, Laight and Rooprai97).
Flavonoids have shown many biological properties that may account for cancer chemoprevention, and multiple mechanisms have been identified for the anti-neoplastic effects(Reference Yao, Jiang and Shi98). However, some studies have failed to find a positive association between intake of flavonoids and reduced risk for different types of cancer. Thus, no significant association between dietary flavonoids intake and total cancer risk was observed in a cohort study in which black tea provided 61 % of total dietary flavonoid intake(Reference Hertog, Feskens and Hollman99). Results of the Netherlands Cohort Study on Diet and Cancer among 58 279 men and 62 573 women aged 55–69 years did not support the hypothesis that consumption of black tea protected against the subsequent risk of stomach, colorectal, lung and breast cancers(Reference Goldbohm, Hertog and Brants100). In a cohort study in Japan involving more than 25 000 stomach cancer patients, no association was observed between gastric cancer risk and consumption of green tea(Reference Tsubono, Nishino and Komatsu101).
Nevertheless, there is some epidemiological evidence that intake of flavonoids is associated with reduced cancer risk. For example, one epidemiological cohort study conducted years ago among 384 cancer patients showed that cancer onset was delayed by 8·7 and 3·0 years in women and men, respectively, who increased the consumption of green tea from less than three to over ten cups per day(Reference Imai, Suga and Nakachi102). There is some evidence that green tea at high levels of intake may provide some benefit in preventing cancers of the digestive tract, especially gastric cancer(Reference Higdon and Frei103). It has also been reported that flavonols may be protective against lung cancer(Reference Knekt, Järvinen and Seppänen104) and oesophageal cancer(Reference Rossi, Garavello and Talamini105), flavones and flavonols may protect against renal cell carcinoma(Reference Bosetti, Rossi and McLaughlin106), and increased intakes of anthocyanidins, flavones and flavonols may lower the risk of colorectal cancer(Reference Rossi, Negri and Talamini107).
Some of the polyphenolic compounds with cancer-preventive effects are the anthocyanins in berries(Reference Rietveld and Wiseman108). Daily consumption of lyophilised black raspberries (32 and 45 g, female and male, respectively, for 6 months) promoted reductions in the urinary excretion of 8-iso-PGF2, and to a lesser more variable extent, 8-OHdG, among patients with Barrett's oesophagus(Reference Kresty, Frankel and Hammond109). The topical application of a mucoadhesive gel of lyophilised black raspberries to oral intraepithelial neoplastic lesions in seventeen patients with human premalignant oral lesions results in a reduction in the expression of COX-2 in dysplastic lesions and a suppression of genes associated with inhibition of apoptosis(Reference Mallery, Zwick and Pei110).
Beneficial effects of other fruit and tea flavonoids have also been reported. Thus, topical epigallocatechin-3-gallate treatment 20 min before UV exposure significantly protects epithelial cells and reduces DNA damage in human skin(Reference Elmets, Singh and Tubesing111). A phase II trial has found no change in prostate-specific antigen (PSA) levels in forty-two patients with prostate carcinoma(Reference Jatoi, Ellison and Burch112). However, in another clinical trial in patients with prostate cancer receiving pomegranate juice (221 ml/d), significant prolongation of PSA doubling time, coupled with positive effects of patients' serum on cell proliferation, apoptosis and oxidative stress in LNCaP cells, was observed(Reference Pantuck, Leppert and Zomorodian113). Results from a phase II trial in 124 individuals with in high risk of liver cancer receiving 500–1000 mg green tea polyphenols for 3 months decreased urinary 8-OHdG(Reference Luo, Tang and Tang114).
In addition to the limitations indicated in the previous section of this review, another important aspect that requires consideration when exploring the beneficial effects of flavonoids on diseases is the fact that increased intake of flavonoids with higher in vitro activity should not simply be recommended because low absorption and rapid elimination cause a limited bioavailability, and metabolisation originates derivatives that do not necessarily share the biological activity of the parent compounds(Reference Williams, Spencer and Rice-Evans115). Therefore, research regarding bioavailability will be essential for the establishment of dietary management of diseases.
Effects in exercise-induced immune and inflammatory changes
A new interesting effect of flavonoids has been proposed in the area of sport sciences, as a consequence of reports indicating that exercise causes oxidative stress, which has led to the use of antioxidant supplements by athletes(Reference Vassilakopoulos, Karatza and Katsaounou116). Different authors have suggested that the intake of a diet rich in antioxidants would be a prudent recommendation to minimise the deleterious actions of free radicals resulting from exercise(Reference Williams, Strobel and Lexis117) because physical training associated with a low intake of antioxidant nutrients may represent a period of greater vulnerability to oxidative stress(Reference Watson, Callister and Taylor118) and may, if given at the appropriate amount and time, complement the ability of exercise to enhance immune responsiveness to potential pathogens(Reference Lyall, Hurst and Cooney119). However, although studies investigating supplement effects have used exercise performance and/or changes in oxidative stress or markers of inflammation as outcome measures, contradictory results have been obtained, and it is difficult to draw definitive conclusions due to the differences in amount or type of the supplement, the length of supplementation and the various outcome measures used (Table 4).
Table 4 Effects of fruit and tea polyphenols on exercise-induced immune and inflammatory changes

8-OHdG, 8-hydroxydeoxyguanosine; TBARS, thiobarbituric acid-reduced substances; FRAP, ferric reducing anti-oxidating power; CRP, C-reactive protein; CK, creatine kinase; LDH, lactate dehydrogenase; COX, cyclo-oxygenase.
In a study with cyclists, competing at a national or regional level, who had been engaged for at least 1 year in a controlled physical training program, antioxidant ingestion prior and during 90 min test on a bicycle ergometer at 70 % VO2max significantly attenuated the increase in the plasma levels of the markers of carbonyl proteins and 8-OHdG induced by exercise(Reference Morillas-Ruiz, Zafrilla and Almar120). In another study in which twenty-five men and twenty-three women ran for 30 min at 80 % VO2max, once before and once after 2 weeks of supplementation with a fruit and vegetable powder, treatment attenuated the glutathione (GSH) decrease and the oxidised glutathione (GSSG) and protein carbonyls increase compared with placebo group, with no sex differences, but no clear effects were observed on malondialdehyde or OHdG plasma levels(Reference Bloomer, Goldfarb and McKenzie121, Reference Goldfarb, McKenzie and Bloomer122). In young men undergoing resistance exercise, green tea consumption has been reported to reduce the post-exercise concentration of lipid hydroperoxide and to increase the plasma values of total polyphenols, to reduce GSH and ferric reducing antioxidanting power(Reference Panza, Wazlawik and Ricardo Schütz123). In a recent study, it has been observed that short-term blackcurrant extract consumption reduces transient increases in plasma oxidative generating capability and protein carbonyls generated by a 30 min row in parallel to a reduction in creatine kinase activity(Reference Lyall, Hurst and Cooney119). However, it has been reported that supplementation with oral quercetin (250 mg, four times per day) 3 weeks before and during a 160 km run did not affect run performance, trolox equivalent antioxidant capacity, F2 isoprostanes or protein carbonyls in a group of sixty-three athletes(Reference Quindry, McAnulty and Hudson124).
Concerning the markers of inflammation, when forty untrained men were supplemented with flavonoids (quercetin 200 mg/d+hesperidin 100 mg/d) in conjunction with 300 mg/d of mixed tocopherols and 800 mg/d of docosahexaenoate for 7 d before eccentric exercise and during 7 d of recovery, although no group differences were noted for creatine kinase, lactate dehydrogenase, delayed onset muscle soreness or range of motion, the results indicated a significant reduction in CRP and IL-6(Reference Phillips, Childs and Dreon125). However, in a study where sixty-three ultramarathon athletes were randomised to quercetin and placebo groups and under double-blinded methods ingested 1 g/d quercetin for 3 weeks before a run, the flavonoid failed to attenuate muscle damage, inflammation, increases in plasma cytokine levels and alterations in leucocyte cytokine mRNA expression(Reference Nieman, Henson and Davis126). In the same line, subjects consuming 1 g/d quercetin for 6 weeks before and during 3 d of cycling at 57 % work maximum for 3 h did not show significant effects on plasma trolox equivalent antioxidant capacity, F2-isoprostanes, nitrite or CRP(Reference McAnulty, McAnulty and Nieman127). In a group of forty trained male cyclists who ingested quercetin (1 g/d) for 3 weeks before and during a 3 d period in which subjects cycled for 3 h/d at approximately 57 % maximal work rate, leucocyte IL-8 and IL-10 mRNA were significantly reduced; however, quercetin did not influence any muscle measure, including NF-κB content, IL-8 and TNF-α mRNA or COX-2 mRNA expression(Reference Nieman, Henson and Davis128). In another study from the same group with a similar protocol, it has been found that although natural killer cell activity, PHA-stimulated lymphocyte proliferation or polymorphonuclear oxidative burst activity are not modified by quercetin, the incidence of upper respiratory tract infections is significantly reduced(Reference Nieman, Henson and Gross129). However, later research has shown that quercetin supplementation in a group of sixty-three athletes for 3 weeks before, during and 2 weeks after a 160 km race did not modify granulocyte respiratory burst, natural killer cells, neutrophil and monocyte counts, with no significant change in the incidence rates of respiratory infections(Reference Henson, Nieman and Davis130).
In summary, despite the well-established in vitro antioxidant and anti-inflammatory potential of flavonoids, inconsistent or null effects of human interventions do not tend to support a role of flavonoid supplementation as a countermeasure to exercise-induced immune and inflammatory changes.
This work was supported by GlaxoSmithKline. CIBERehd is supported by Instituto de Salud Carlos III, Spain. All the authors contributed equally for intellectual input and writing of the manuscript. All the authors declare no conflict of interest.






