Wasting syndrome is common in cancer, and it affects about 50 % of all patients at the time of diagnosis(Reference Gomez Candela, Olivar Roldan and Garcia1, Reference Gomez-Candela, Luengo and Cos2) and >80 % of patients in advanced stages(Reference Agius, Nadulski and Kahl3, Reference Trabal, Leyes and Forga4). Weight loss and malnutrition in cancer patients increase the risk of infection and the cost of health care(Reference Bossola, Pacelli and Tortorelli5, Reference Murry, Riva and Poplack6), decrease the quality of life(Reference Laky, Janda and Kondalsamy-Chennakesavan7), and affect the response to anti-neoplastic treatment and overall survival(Reference Capra, Ferguson and Ried8). The early detection of malnutrition in cancer patients is important, and could increase the overall survival and quality of life(Reference Sosa-Sanchez, Sanchez-Lara and Motola-Kuba9). Nutritional screening includes anthropometric parameters (BMI and weight loss percentage) and biochemical parameters. However, it is not feasible to interpret some parameters(Reference Gupta, Lammersfeld and Vashi10) because they are influenced by other non-nutritional factors such as hydration status, tumour site and liver disease. Albumin may not be a good indicator of nutritional state because it is influenced by stress and illness(Reference Wong, Enriquez and Barrera11). Low serum albumin levels can also occur in patients receiving chemotherapy due to the effects of cytotoxic agents(Reference Arrieta, Michel Ortega and Villanueva-Rodriguez12).
Routine screening of malnutrition in cancer patients should be non-invasive and should include factors such as gastrointestinal (GI) symptoms that affect food intake and eating behaviour.
The aim of the present study was to evaluate weight-loss prevalence in cancer patients receiving chemotherapy based on the tumour site and the importance of GI symptoms in the presence of malnutrition.
Methods
The present retrospective study was conducted at the Medica Sur University Hospital's Oncology Centre, between January 2008 and July 2010. We reviewed the files of all patients treated with chemotherapy at this centre. Biochemical and clinical data were assessed after the second chemotherapy cycle.
The selection criteria were as follows: age between 19 and 75 years; complete diagnosis information (primary cancer site, type of tumour and stage at diagnosis); complete information regarding the main GI symptoms that occurred during chemotherapy, which were reported by the patient's clinical oncology doctor (e.g. nausea, vomiting, diarrhoea, constipation and anorexia); complete information for anthropometric variables (weight, height, habitual weight, and weight loss during the past 6 months). The exclusion criteria included incomplete files, patients who received radiotherapy treatment or a history of surgery as an oncological treatment. For descriptive purposes, patients were classified by their tumour sites as follows: colon; lung; breast; non-colorectal GI (gastric, oesophageal, gall bladder and pancreatic); gynaecological (cervico-uterine and ovarian); haematological (lymphoma, leukaemia and myeloma); others (prostate, thyroid and testicular). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Medica Sur ethics committee.
Statistical analysis
Descriptive statistics were used for qualitative and quantitative variables: frequency; percentage; mean and standard deviation. χ2 and Mann–Whitney U tests were applied to parametric and non-parametric data, respectively. Data analysis was performed using the SPSS program version 18 (SPSS, Inc.). P≤ 0·05 was considered significant.
Results
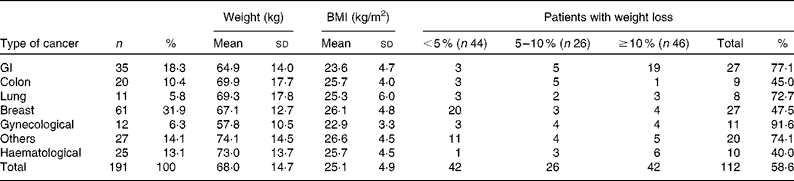
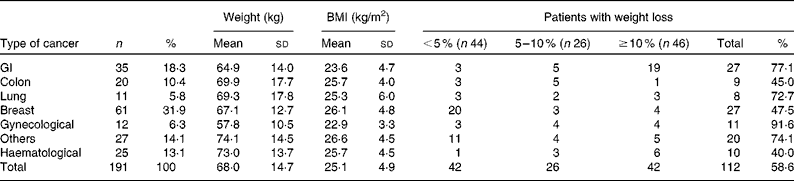
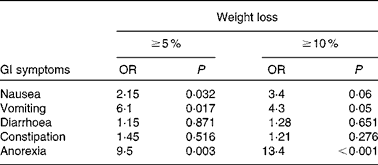
We reviewed 534 files of all patients who had undergone chemotherapy, of which a total of 191 files met the inclusion criteria, including 123 females and sixty-eight males. The most frequent primary tumour site was breast cancer (31·9 %). The mean age was 54·3 (sd 14·8) (range 28–72) years. The mean body weight was 68·0 (sd 14·6) kg and the mean BMI was 25·4 (sd 4·9) kg/m2. Over 63 % of the patients experienced unintentional weight loss, while 38·7 and 24·6 % of the patients had ≥ 5 % and ≥ 10 % weight loss, respectively, during the 6 months before the treatment (Table 1). Weight loss during the 6 months before the treatment had a higher prevalence in patients with gynaecological and GI cancers (91·6 and 77·1 %, respectively; Table 2). Patients with non-colorectal GI cancer had higher rates of weight loss after two cycles of chemotherapy ( ≥ 10 % of habitual weight; Table 2). The most common GI symptoms reported in all study patients were nausea (59·6 %) and anorexia (46 %). Patients with ≥ 5 % weight loss were significantly associated with nausea (OR 2·15, P= 0·03), vomiting (OR 6·1, P= 0·017) and anorexia (OR 9·5, P= 0·003). Patients with ≥ 10 % weight loss were significantly associated with vomiting (OR 4·3, P= 0·05) and anorexia (OR 13·4, P< 0·001) (Table 3).
Table 1 General characteristics (Mean values and standard deviations; number of patients and percentage values)
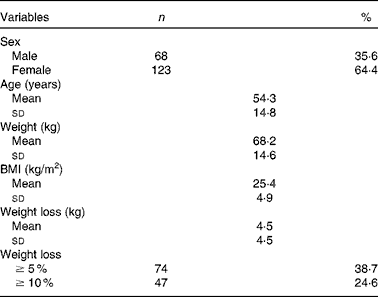
Table 2 Weight, BMI and weight loss by tumour location (Mean values and standard deviations; number of patients and percentages, n 191)
GI, gastrointestinal.
Table 3 Association between unintentional weight loss and gastrointestinal (GI) symptoms (Odds ratios and P values)
Discussion
The prevalence of unintentional weight loss in patients with cancer has been reported as 49–74 %, and is associated with the primary tumour location(Reference Dufau13–Reference Segura, Pardo and Jara15). The Eastern Cooperative Oncology Group(Reference Dewys, Begg and Lavin16) and others studies(Reference Zorlini, Akemi Abe Cairo and Salete Costa Gurgel17, Reference Laky, Janda and Bauer18) have reported that patients with gastric cancer have the highest rates of weight loss, whereas patients with breast cancer have the lowest rates. The present study found similar results, and more than 50 % of our patients experienced weight loss. The highest prevalence was found with GI (77 %), lung (72·7 %) and gynaecological tumours (91·6 %). The lowest prevalence was observed in colon and breast cancer patients. The mean BMI of Mexican patients with a cancer diagnosis has been reported as 25·5 kg/m2(Reference Fuchs and Gutierrez19). In the present study, the mean BMI of the patients was >25 kg/m2, with the exception of non-colorectal GI and gynaecological cancer patients. Therefore, the majority of patients would have been classified as overweight or obese without any malnutrition risk if we considered only the BMI as a nutritional parameter; however, the BMI classification ignores body composition. Recent research into the body composition of cancer patients, specifically the proportion of lean and fat tissue, has demonstrated that cancer patients may experience sarcopenic obesity (simultaneous obesity and low muscle mass)(Reference Prado, Lieffers and McCargar20). Therefore, it is essential to consider other tools to obtain a good nutritional evaluation, such as a recent history of weight change (i.e. proportional weight loss during the last 6 months, 2 weeks or compared with the habitual weight). Unintentional weight loss is a more powerful parameter than BMI for malnutrition detection in cancer patients(Reference Lipkin and Bell21, Reference Gudny Geirsdottir and Thorsdottir22). Subjective Global Assessment and body composition measurements using bioelectrical impedance or computerised tomography are very useful(Reference Prado, Lieffers and McCargar20, Reference Prado, Baracos and McCargar23, Reference Prado, Baracos and McCargar24). The present study considered the percentage of weight loss, and most patients experienced a 5 % reduction in their habitual weight. Based on these results, we conclude that weight loss may be a better malnutrition parameter than BMI in patients with cancer who are receiving chemotherapy. Marin et al. (Reference Marin Caro, Gomez Candela and Castillo Rabaneda25) evaluated 226 oncology patients, and 10 and 64 % of patients presented malnutrition based on a consideration of BMI and Subjective Global Assessment, respectively. Similar studies have reported a malnutrition prevalence of 6–15 % based on BMI(Reference Collins, Wight and Partridge26), whereas the prevalence was higher when Subjective Global Assessment was applied(Reference Laky, Janda and Cleghorn27–Reference Edington, Winter and Coles29). These variations could be explained by changes in water distribution due to tumours (oedema, ascites, dehydration, diarrhoea and vomiting), or accelerated tumour growth(Reference Sánchez-Lara, Turcott and Sosa-Sánchez30). Other mechanical and metabolic complications can occur in addition to the primary tumour site. GI symptoms are important components of malnutrition in cancer patients. In the present study, the most frequent GI symptoms were nausea (59·6 %), anorexia (46 %) and constipation (31·9 %). A similar prevalence of GI symptoms was found in studies conducted by Tchekmedyian et al. (Reference Tchekmedyian14), Segura et al. (Reference Segura, Pardo and Jara15), Walsh et al. (Reference Walsh, Donnelly and Rybicki31) and others(Reference Grosvenor, Bulcavage and Chlebowski32–Reference Sarhill, Mahmoud and Walsh34). In the present study, nausea, anorexia and vomiting were significantly associated with ≥ 5 % weight loss, whereas diarrhoea and constipation were not associated. These symptoms could result in weight loss by changing the body water distribution. The use of a retrospective series and the under-reporting of toxicity symptoms were limitations of the present study. Minor GI symptoms such as dysphagia, taste disorders and early satiety were reported inconsistently in the patients' files. The poor reporting of toxicity or its symptoms is common following cancer treatments. The association of GI symptoms with nutritional status could be relevant, and should be considered in further studies. It was also important that only about one-third of patients could be included because missing basic clinical data, such as weight, were not measured or recorded. This data deficiency must be addressed in clinical practice because of the implications of malnutrition for cancer patients' outcomes.
Conclusion
In our population, non-colorectal GI and lung cancer patients had a higher prevalence of weight loss compared with patients with other primary cancer sites. GI symptoms such as anorexia, nausea and vomiting were significantly correlated with weight loss. The present study confirmed that the primary tumour site, weight-loss history and GI symptoms were determinant factors of nutritional status in oncology patients, and they must be included in the screening, evaluation and treatment of cancer patients.
Acknowledgements
This study received no financial support. The authors declare that there are no conflicts of interest. The authors' contributions are as follows: U.-M. E. and M.-K. D. participated in the data collection; S.-L. K. was involved in writing and analysis; G. D. contributed to the writing and editing assistance.