Vitamin D (vit-D) is a lipid-soluble vitamin well-documented for its role in the development and maintenance of skeletal health, as well as maintenance of Ca and P homeostasis(Reference Moreno, Furtner and Rivara1–Reference Mohr5). Advances in molecular and cellular biology now show that vit-D has pleiotropic effects and perhaps direct effects within the central nervous system (CNS)(Reference Anastasiou, Yannakoulia and Scarmeas6). Proposed mechanisms include maintaining neuronal Ca regulation and signalling, regulation of neurotrophic factors, enhanced neurotransmission, synaptogenesis, neurogenesis, neuroprotection and inhibition of degenerative processes, including apoptosis(Reference Groves, McGrath and Burne7–Reference Kalueff, Eremin and Tuohimaa10).
Presently, the recommended serum concentration range for vit-D is primarily based on historical studies in the context of skeletal health; however, it is unknown if this is appropriate when considering CNS integrity and cognitive function(Reference Manson, Brannon and Rosen11,Reference Peterlik12) . There is a substantive body of evidence that collectively demonstrates health risks associated with vit-D deficiency, including endocrine, autoimmune, metabolic, bone and cardiovascular disorders(Reference Afzal, Bojesen and Nordestgaard13–Reference Slinin, Paudel and Taylor23). However, the putative effects of vit-D deficiency and by extension vit-D supplementation on CNS function are contradictory. An emerging body of pre-clinical and clinical studies suggests an inverse U-shaped association with low and greater abundance of serum vit-D both correlated with neurocognitive deficits(Reference Annweiler, Dursun and Féron14,Reference Lam, Takechi and Pallabage-Gamarallage24,Reference Tsujikawa, Kurotaki and Fujimori26) . Whilst the mechanisms for the latter are presently not established, preclinical studies suggest that chronically heightened levels of serum vit-D may compromise cognitive performance through a cerebrovascular axis. In two rodent species, dietary-induced hypervitaminosis-D increased cerebral capillary permeability, resulting in inappropriate kinetics of plasma proteins from blood into brain parenchyme and neurovascular inflammation(Reference Lam, Takechi and Pallabage-Gamarallage24), whilst genetically engineered mice with hypervitaminosis-D show an accelerated ageing phenotype(Reference Tsujikawa, Kurotaki and Fujimori26,Reference Razzaque and Lanske27) . Whilst the physiological sequelae associated with vit-D deficiency are reasonably well established, the putative detrimental effects of exaggerated vit-D are noteworthy in the context of public health trends showing increased self-prescribed use of supplementary vit-D nutraceuticals and clinical trials with interventions of up to 7500 μg of vit-D3 per dose(Reference Wu, Staykova and Horne28–Reference Przybelski, Agrawal and Krueger32).
Collectively, at present, there is no substantiated serum reference range for vit-D that can reliably be indicated to support optimal CNS function(Reference Annweiler, Dursun and Féron14). Moreover, adding to the complexity of interpreting putative vit-D effects on cognitive performance is that clinically, blood vit-D status is ordinarily indicated by considering the concentration of the inactive metabolite of vit-D, serum 25-hydroxyvitamin-D (25(OH)D), a biomarker of storage rather than biological function per se (Reference Cavalier, Wallace and Knox33).
Serum vitamin D synthesis and homeostasis
Serum abundance of vit-D is partially indicative of the endogenous biosynthesis of cholecalciferol (vit-D3) which occurs within the epidermis of mammalian skin(Reference Groves, McGrath and Burne7,Reference Sintzel, Rametta and Reder34) during exposure to UV-B radiation (290–315 nm range). However, in addition, serum vit-D is influenced through exogenous sources provided through the ingestion of either plant-derived food commodities enriched in ergocalciferol (vit-D2); animal meats and dairy containing both cholecalciferol and ergocalciferol or through nutraceutical supplements (principally provided as cholecalciferol)(Reference Sintzel, Rametta and Reder34) (Fig. 1).
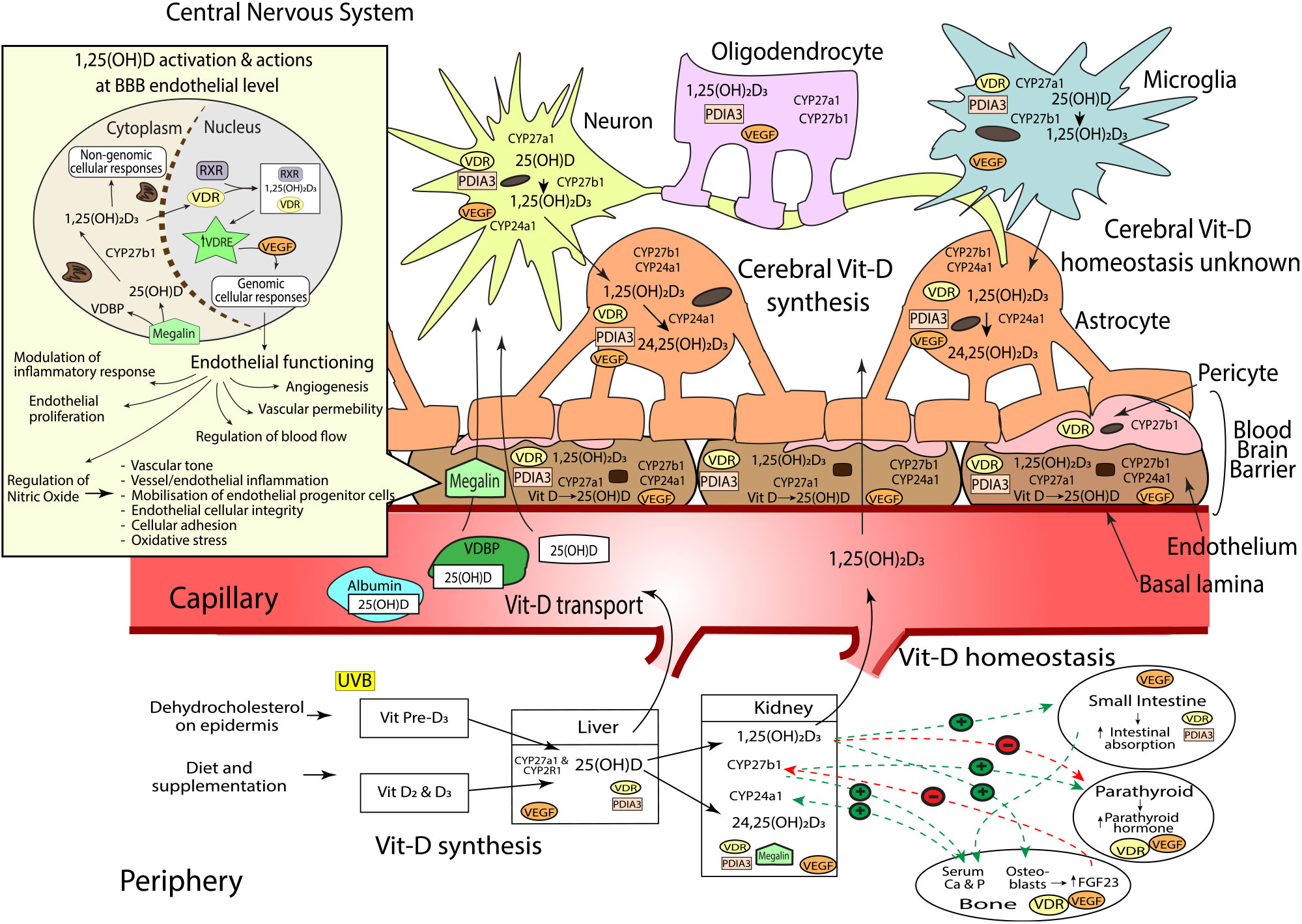
Fig. 1. Sources of vitamin D (vit-D), peripheral and cerebral synthesis and homeostasis, blood and blood–brain barrier (BBB) transport. Vit-D is obtained by the conversion of dehydocholesterol on the epidermis when exposed to UVB (vit-D3), diet and supplementation (vit-D2 and D3). Vit-D2 and D3 metabolites are converted to 25(OH)D (25-hydroxycholecalciferol) by CYP2R1 and CYP27A1 hydroxylase enzymes in the liver, before conversion to active 1,25(OH)2D3 (calcitriol, by CYP27B1, or inactive 24,25(OH)2D3 (24,25-Dihydroxycholecalciferol) by CYP24A1 in the kidneys. Hydroxylation enzymes CYP27A1, CYP27B1 and CYP24A1, and vit-D receptors, VDR and PDIA, have been shown in varying concentrations throughout the brain. Neurons, endothelial cells and microglial express CYP27A1, CYP27B1, CYP24A1, VDR and PDIA. Astrocytes express CYP27A1, CYP24A1, VDR and PDIA. Oligodendrocytes express CYP27A1 and PDIA3; and pericytes express CYP27B1 and VDR. Vit-D synthesis from vit-D to 25(OH)D takes place within endothelial cells and neurons, before further hydroxylation to 1,25(OH)2D3 in the neurons and microglia. 1,25(OH)2D3 activates either genomic actions via VDR or non-genomic actions via PDIA3. As VDR is expressed in lower quantities in brain compared with kidney, PDIA3 may act as the main cerebral vit-D receptor. Peripheral vit-D homeostasis is tightly regulated by hormones such as calcium, parathyroid hormone (PTH), fibroblast growth factors (FGF-23) and abundance of 1,25(OH)2D3; however, cerebral vitamin D homeostasis, along with the relationship between peripheral and cerebral homeostasis, remains unknown. Vit-D is predominantly transported in peripheral blood bound to VDBP, but can also be bound to albumin, or free (<1 %). Albumin can traverse the endothelium via transcytotic mechanisms, whilst free 25(OH)D and active 1,25(OH)2D3 enter the endothelial cell and move across the BBB into the central nervous system (CNS) via passive diffusion. The BBB consists of the basal lamina, endothelial cells, pericytes and astrocytes. 25(OH)D bound to VDBP is dependent on internalisation by megalin transport protein. Once internalised, 25(OH)D unbinds from VDBP before conversion to 1,25(OH)2D3 within the cytoplasm. 1,25(OH)2D3 then translocates into the nucleus where it binds to VDR and retinoid X receptor (RXR). The 1,25(OH)2D3, VDR, RXR complex then binds to target genes that contain a vitamin D response element (VDRE)(Reference Eyles, Liu and Josh62,Reference Eyles, Smith and Kinobe70,Reference Smolders, Schuurman and van Strien73,Reference Landel, Stephan and Cui75,Reference Beydoun, Tajuddin and Dore130) . Activation of VDRE promotes expression of vascular endothelial growth factor (VEGF)(Reference Sarkar, Chopra and Rohit234) which is located throughout the brain including astrocytes(Reference Mani, Khaibullina and Krum235), neurons(Reference Rosenstein, Krum and Ruhrberg236), oligodendrocytes(Reference Hayakawa, Pham and Som237), microglial cells(Reference Ding, Gu and Zhang238) and the endothelium at the BBB(Reference Shize, Rui and Yong239). VEGF binds to VEGF-specific receptors like those in the endothelium, controlling a wide range of endothelial cellular activities(Reference Sarkar, Chopra and Rohit234). This includes regulation of vascular permeability, endothelial proliferation, activation of angiogenesis, regulation of blood flow via vasodilation/vasoconstriction and modulation of inflammatory response including platelet activation, migration and cell survival(Reference Sarkar, Chopra and Rohit234,Reference Westerfeld, Miller, Levin and Albert240) . VEGF also induces the release of nitric oxide (NO) from endothelial cells(Reference Dulak, Jozkowicz and Dembinska-Kiec241). NO is an important biomolecule which mediates various metabolic pathways(Reference Al-Daghri, Bukhari and Yakout242) including vessel inflammation and integrity, regulation of vascular tone, cellular adhesion and oxidative stress. Additionally, NO is partly responsible for the mobilisation of endothelial progenitor cells essential for vessel maintenance and repair(Reference Dalan, Liew and Tan243). Vit-D and VEGF dysregulation can therefore substantially modulate endothelial cell function(Reference Sarkar, Chopra and Rohit234).
Peripheral synthesis and metabolism of vitamin D
Significant hepatic uptake of both skin and dietary-derived vit-D3 and vit-D2 occurs when they are hydroxylated by the microsomal cytochrome P450 25-hydroxylase enzyme, CYP2R1, to 25-hydroxyvit-D (calcidiol (25(OH)D)). In blood, the 25(OH)D is thereafter chaperoned associated with the vit-D binding protein (VDBP), which can subsequently be internalised by the proximal tubule within the kidney via megalin, a transmembrane receptor protein with high affinity for VDBP (Fig. 1)(Reference Veldurthy, Wei and Oz35). Principally within the kidney, CYP27B1 converts 25(OH)D to the biologically active metabolite 1,25-dihydroxyvit-D (calcitriol (1,25(OH)2D3)), where it is released into circulation as an endocrine modulator. In blood, the biologically active 1,25(OH)2D3 acts on target cells including cells of the parathyroid glands, osteoblasts, dendritic cells, T cells and keratinocytes. Ordinarily, homeostasis of the active metabolite is normally strictly controlled by highly regulated co-expression within kidney by CYP27A1, which deactivates 1,25(OH)2D3 as a consequence of conversion to 24,25-dihydroxyvitamin-D (24,25(OH)2D3)(Reference Lehmann and Meurer36–Reference Henry39).
The catalytic conversion of 25(OH)D to 1,25(OH)2D3 is thought to be principally regulated via ionised Ca homeostasis(Reference Jones, Prosser and Kaufmann40,Reference Sitara41) . When blood Ca is low, parathyroid hormone is released, stimulating expression of CYP27B1 and decreasing CYP24A1, resulting in an increase of 1,25(OH)2D3 synthesis. Increased levels of 1,25(OH)2D3 subsequently inhibit parathyroid hormone, thus avoiding potential risk for hypercalcaemia(Reference Veldurthy, Wei and Oz35,Reference Gil, Plaza-Diaz and Mesa42) . Moreover, a negative feedback loop occurs as a consequence of 1,25(OH)2D3 to the vit-D receptor (VDR), with down-regulation of transcription for CYP27B1(Reference Henry39) and an increased expression of inactivating enzyme, CYP24A1(Reference Veldurthy, Wei and Campbell43,Reference Bikle44) . Similarly, blood phosphate is a potent regulator of CYP-mediated conversion of vit-D metabolites. Dietary phosphates and heightened blood abundance stimulate fibroblast growth factor-23 (FGF-23) secretion(Reference Ferrari, Bonjour and Rizzoli45–Reference Urakawa, Yamazaki and Shimada47) by osteocytes in the bone matrix of skeletal tissue. The FGF-23 inhibits kidney CYP27B1 and increases CYP24A1 expression, collectively suppressing 1,25(OH)2D3(Reference Prié and Friedlander48). Conversely, circulating low levels of phosphate increases the production of CYP27B1 and thus increases 1,25(OH)2D3 concentrations in the blood; the latter would then stimulate FGF-23 production, which would complete the feedback loop and down-regulate CYP27B1 expression(Reference Sitara41,Reference Prié and Friedlander48) .
In 1987, Stumpf & O’Brien demonstrated that the bioactive metabolite of vit-D, 1,25(OH)2D3, could diffuse across the blood–brain barrier (BBB) into brain parenchyme and accumulate in nuclei of the amygdala, hippocampus, hypothalamus, thalamus, pallium, pons, midbrain and cerebellum(Reference Stumpf and O’Brien49). Stumpf and colleagues observed that the distribution of cerebral 1,25(OH)2D3 was non-random and in tight regulation with endocrine, autonomic, sensory and motor systems(Reference Stumpf and O’Brien49). Indeed, present clinical studies have substantiated that cerebrospinal fluid homeostasis of vit-D is associated with serum abundance of vit-D, supporting current dogma that modulating peripheral metabolism may have CNS modulatory effects(Reference Lee, Kim and Jung50).
The vitamin D receptor
The VDR is a member of the nuclear receptor superfamily and acts as a 1,25(OH)2D3-inducible transcription factor(Reference Ciesielski, Sato and Chebaro51–Reference Zittermann and Gummert53). In most cell types including neuronal cells, VDR are predominantly (58 %) found in the nucleus(Reference Klopot, Hance and Peleg54); however, observational studies have also shown VDR on the plasma membranes of cells(Reference Gezen-Ak55) and within the cytosol(Reference Klopot, Hance and Peleg54). Genomic effects mediated by VDR consist of the activation and repression of gene transcription. First, the internalisation of 1,25(OH)2D3 forms a complex with a retinoid X receptor (either RXRα, RXRβ or RXRγ) and VDR within the cell(Reference Kato52,Reference Litwack56) . The 1,25(OH)2D3-VDR-RXR complex then binds to specific enhancer elements, referred to as vit-D response elements (VDRE)(Reference Kato52), within the regulatory regions of target genes in the DNA. In a 1,25(OH)2D3-dependent manner, the complex recruits various chromatin-active coregulatory complexes, thereby facilitating gene-selective transcription(Reference Litwack56–Reference Mangelsdorf and Evans59) (Fig. 1).
Vitamin D receptors and the cytochrome P450 enzymes in brain
It was not until commercial antibodies became available that research groups such as Veenstra et al. (Reference Veenstra, Johnson and Tomlinson60) and Prufer et al. (Reference Prufer, Veenstra and Jirikowski61) were able to map VDR expression throughout the rodent brain. Many studies have demonstrated the presence of VDR in the brain via Western blot, immuno-fluorescent and quantitative RNA techniques(Reference Cui, Gooch and Groves9,Reference Gezen-Ak55,Reference Eyles, Liu and Josh62–Reference Cui, Pelekanos and Liu65) ; however, some researchers disputed these findings and suggested relatively low VDR abundance or exaggerated measures of VDR(Reference Wang, Becklund and DeLuca66,Reference Wang and DeLuca67) . The uncertainty with respect to CNS VDR abundance is partly based on the work by Wang et al., who did not detect VDR in the rat or human brain using ELISA and immunohistochemical methodologies(Reference Wang, Zhu and Deluca68). However, in 2014, Eyles and colleagues provided further evidence of cerebral abundance of VDR using proteomic techniques(Reference Eyles, Liu and Josh62).
Recently, another CNS-rich protein has been implicated in rapid intracellular signalling induced by 1,25(OH)2D3, namely, protein disulphide isomerase, family A member 3 (PDIA3), a membrane-bound VDR(Reference Boyan, Chen and Schwartz69). Notably, the PDIA3 expression in brain is orders of magnitude greater than hepatic and renal PDIA3 expression, particularly in the cerebral cortex and hippocampus, regional areas critical to cognitive function (Fig. 1)(Reference Boyan, Chen and Schwartz69). Consistent with an autocrine pathway, Eyles et al. (Reference Eyles, Smith and Kinobe70) also described the coexpression of CYP27B1 with VDR in human cerebral cortex, hippocampal formation and hypothalamus(Reference Cui, Gooch and Groves9). CYP27B1 and VDR were immunodetected in both neuronal and glial cells, with the expression of CYP27B1 restricted to the cytoplasm, and VDR within the nucleus, respectively (Fig. 1). VDR immunoreactivity was indicated within hippocampal formation regions, CA1 and CA2, with less prominence in CA3. Consistent with the regional distribution of VDR, CYP27B1 was clearly indicated throughout the entirety of the CA1, CA2 and CA3-hippocampal formation regions, brain regions pivotal for spatial learning and memory formation and considered one of the most vulnerable regions in Alzheimer’s disease pathology(Reference Eyles, Liu and Josh62,Reference Eyles, Smith and Kinobe70–Reference Landel, Annweiler and Millet72) (Fig. 1). The substantive findings by Eyles et al. demonstrating a de novo CNS pathway for 1,25(OH)2D3 biosynthesis and cellular activation in brain raises uncertainty as to the relative physiological significance of peripheral (serum) 1,25(OH)2D3 homeostasis in the context of CNS function.
Cellular distribution of vitamin D receptor and associated enzymes in the central nervous system
At a cellular level, Smolders et al. (Reference Smolders, Schuurman and van Strien73) reported immunostaining for VDR in microglia and astrocytes; however, the CYP24A1 enzyme was restricted primarily to astrocytes (Fig. 1). In alignment with these findings, El-Atifi et al. (Reference El-Atifi, Dreyfus and Berger74) demonstrated CYP2R1 and VDR expression in human pericytes. As major structural and functional cells comprise the neurovascular unit, the aforementioned evidence supports the autocrine/paracrine modes of action of vit-D on neurovascular function (Fig. 1).
Within the neurovascular unit, CYP27A1, CYP27B1 and CYP24A1 expressions have been confirmed in astrocytes, endothelial cells, microglia, oligodendrocytes and notably, with significant expression of CYP27B1 particularly indicated in neurons(Reference Landel, Stephan and Cui75). However, differential expression of the VDR binding proteins has been described. The VDR is expressed principally in astrocytes, whereas PDIA3 mRNA is ubiquitously indicated. For the latter, there was striking abundance found in endothelial cells consistent with a functional role of peripheral (blood) 1,25(OH)D on vascular function(Reference Landel, Stephan and Cui75). Figure 1 summarises all present evidence for vit-D associated metabolites and receptors that have been characterised in the brain.
Cerebral vitamin D homeostasis relative to peripheral vitamin D homeostasis
Vit-D metabolites are able to traverse the BBB from blood into the brain paranchyme through mechanism which have not been equivocally delineated(Reference Xue, He and Li76). Unfortunately, there is a paucity of literature considering putative associations between serum and cerebral vit-D homeostasis. Xue et al. (Reference Xue, He and Li76) showed that rats fed a vit-D3-deficient diet had markedly lower cerebral 25(OH)D3 metabolite concentration in comparison with rats maintained on a vit-D3 supplemented diet. Consistent with the possibility of diffusion or facilitated transport process across the BBB, Xue and colleagues showed that the serum vit-D3 metabolite concentrations correlated with brain vit-D3 metabolite levels. However, the relative significance of peripheral vit-D homeostasis in association with CNS vit-D homeostasis remains largely unexplored.
Vitamin D and cognitive performance
There is public popularity with the notion that higher vit-D status, indicated by increased serum vit-D (25(OH)D as the universal marker of vit-D status), is associated with better cognitive performance and reduced risk of neurodegenerative disorders. The public perception has resulted in the promotion of self-prescribed vit-D supplementation without clinical indication(Reference Razzaque77).
A number of randomised controlled trials (RCT) and meta-analyses suggest a positive association of serum vit-D with cognition(Reference Annweiler, Allali and Allain78), at a threshold serum concentration of 25(OH)D level >25 nmol/l. Benefits are indicated on global cognitive performance(Reference Wilkins, Sheline and Roe79) and in specific cognitive domains including visuospatial skills, language, working memory, memory recall, concentration and attention(Reference Przybelski and Binkley80). However, other studies indicate that exaggerated levels of vit-D are also associated with poorer cognitive function(Reference Granic, Hill and Kirkwood25,Reference McGrath, Scragg and Chant81–Reference Castle, Fiedler and Pop83) . Moreover, adding to the complexity of interpretating vit-D-associated CNS effects is the significant potential confounder of reverse causation and in lack of standardisation and heterogeneity of blood vit-D analytics(Reference Maddock, Geoffroy and Power82,Reference Maddock, Cavadino and Power84) .
Hypovitaminosis D and cognitive performance
Numerous studies report associations between vit-D deficiency and an increased risk of cognitive decline in older adults(Reference Annweiler, Dursun and Féron14,Reference Annweiler, Llewellyn and Beauchet19,Reference Littlejohns, Henley and Lang20,Reference Holick85) ; however, paradoxical findings suggest no such association(Reference McGrath, Scragg and Chant81) (See summary of studies to date in Table 1). An international task force considering vit-D and cognition in elderly individuals concluded that hypovitaminosis-D increases the risk of cognitive decline and dementia(Reference Annweiler, Dursun and Féron14). In Alzheimer’s disease, hypovitaminosis-D is associated with a 2·4-fold increased risk for cognitive impairment(Reference Annweiler, Dursun and Féron14). In the large community-based Framingham Heart Study, there was an association between lower vit-D status and reduced hippocampal volume, poorer measures of neuropsychological function and a greater risk of dementia(Reference Karakis, Pase and Beiser86). In agreement with these large cohort studies, Hooshmand et al. (Reference Hooshmand, Lökk and Solomon87) reported positive associations between vit-D (25(OH)D) status with cognitive function and a reduction in CSF amyloid-β and brain volume. Moreover, regional cerebral blood flow and brain function were found to be positively associated with serum vit-D concentration in Alzheimer’s disease patients. A lower concentration of serum vit-D was found to correlate with poorer executive functioning (heterogeneous set of complex processes that controls and regulates other abilities and behaviour); however, episodic memory was generally found to be unaffected(Reference Hooshmand, Lökk and Solomon87). Furthermore, Chaves et al. (Reference Chaves, Feitosa and da S Araújo88) recently concluded vit-D insufficiency as an independent risk factor for Alzheimer’s disease.
Table 1. Summary of randomised controlled trials and observational studies exploring vitamin D (vit-D) and cognitive outcomes
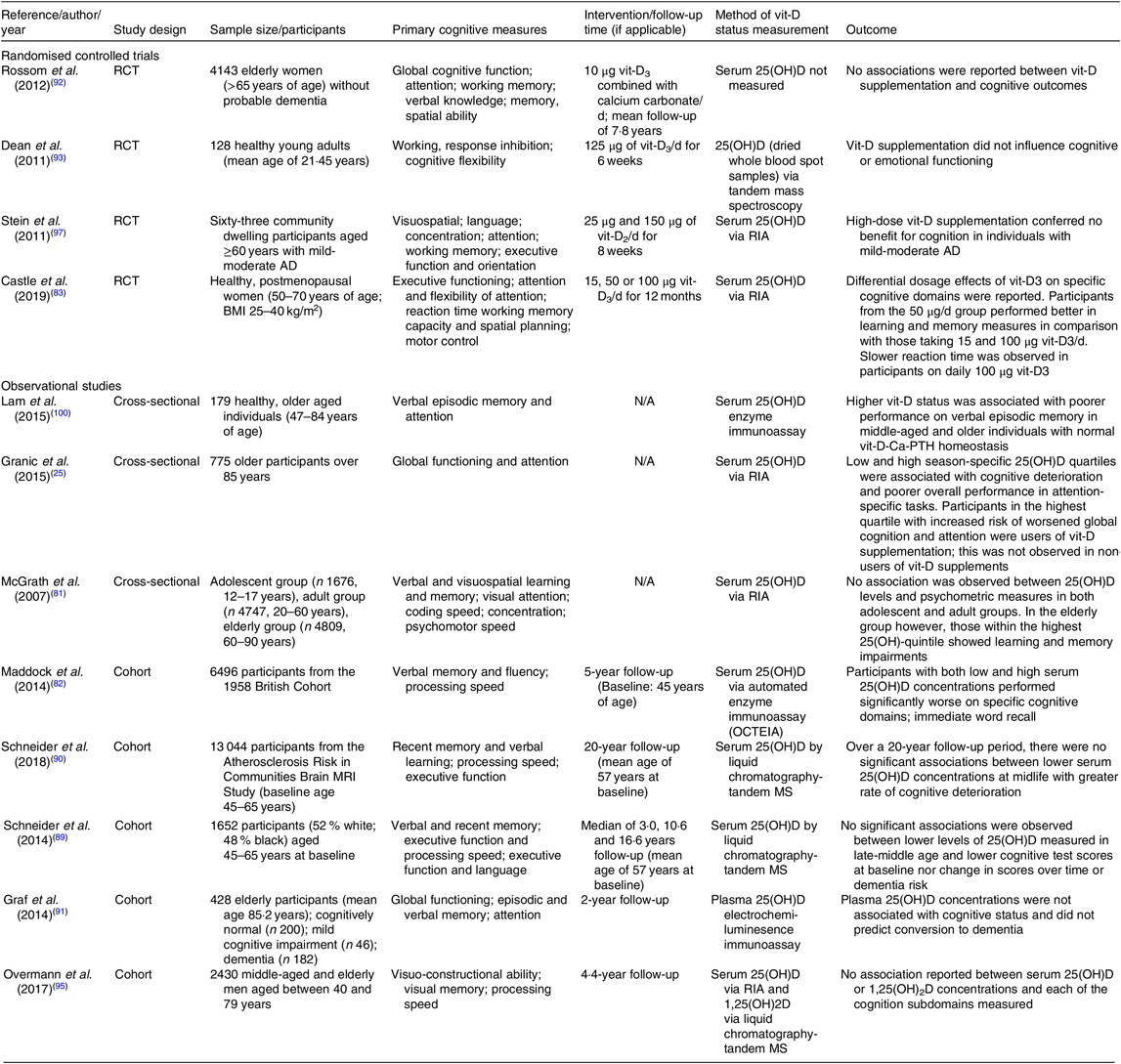
RCT, randomised controlled trial; 25(OH)D, 25-hydroxyvitamin D; AD, Alzheimer’s disease; N/A, not applicable.
In contrast to studies which indicate a positive association between serum vit-D and cognitive performance, an increasing number of prospective cohort studies, including in early childhood to individuals of advanced age, have failed to show a correlation between serum vit-D status and cognitive outcomes. Schneider et al. (Reference Schneider, Lutsey and Alonso89,Reference Schneider, Zhao and Lutsey90) found no association between vit-D deficiency with lower cognitive test scores in a 20 year (median) longitudinal study in late to middle aged adults. Graf et al. (Reference Graf, Rossi and Giannelli91) reported no association between vit-D and increased risk for mild cognitive impairment, nor did serum vit-D predict the conversion from cognitively normal or mild cognitive impairment to dementia. Other studies have not found any association between low 25(OH)D and cognitive performance(Reference Rossom, Espeland and Manson92,Reference Dean, Bellgrove and Hall93) . Using a Mendelian randomisation study design in a recent study, Maddock et al. (Reference Maddock, Zhou and Cavadino94) explored the relationship between serum 25(OH)D concentration and cognitive function. Data collected from seventeen observational cohort studies, including over 170 000 participants, concluded no evidence of an association between serum 25(OH)D concentration as a causal factor for cognitive performance in later life. Similarly, a 4·5-year longitudinal male ageing study by Overman et al. (Reference Overman, Pendleton and O’Neill95) found no association between serum 25(OH)D and 1,25(OH)2D3 concentrations and multiple cognitive subdomains in 3369 men aged between 40 and 79 years of age.
Vitamin D supplementation and cognitive function
Results from RCT linking vit-D deficiency and cognitive decline have not been promising(Reference Schneider, Lutsey and Alonso89,Reference Manders, Groot and Hoefnagels96,Reference Stein, Scherer and Ladd97) (Table 1). There was no benefit on cognition or memory realised in vit-D-deficient older participants supplemented with vit-D(Reference Rossom, Espeland and Manson92,Reference Stein, Scherer and Ladd97,Reference Bartali, Devore and Grodstein98) . Results from the Women’s Health Initiative study found that vit-D and Ca supplementation over a period of 8 years had no beneficial effects on cognition(Reference Jackson, Lacroix and Cauley99). Studies by Stein et al. (Reference Stein, Scherer and Ladd97) show that supraphysiological levels of vit-D did not confer beneficial effects in cognitive performance in individuals with mild-moderate Alzheimer’s disease. A study by Rossom et al. (Reference Rossom, Espeland and Manson92) failed to demonstrate positive effects of vit-D supplementation in attenuating cognitive dysfunction, or on lowering the risk of dementia when compared with placebo. Moreover, Castle and colleagues recently reported on the differential dosage effects of vit-D on specific cognitive domains in healthy, post-menopausal women whereby higher doses were found associated with poorer reaction time and measures of learning and memory(Reference Castle, Fiedler and Pop83). Furthermore, a recent literature review by Landel et al. (Reference Landel, Annweiler and Millet72) determined that there was no solid evidence to suggest that exogenous supplementation of vit-D improves cognition in those who already have sufficient serum levels of vit-D. Collectively, results of RCT do not consistently suggest postive effects of exogenous vit-D on cognitive performance.
Hypervitaminosis-D and cognitive performance
Recent studies suggest cognitive impairment may indeed also be indicated in individuals with greater levels of serum vit-D, especially those taking vit-D supplements. Granic et al. (Reference Granic, Hill and Kirkwood25) reported that both the lowest and highest season-specific serum 25(OH)D quartiles had increased risk of cognitive impairment compared with those in the middle quartiles adjusted for socio-demographic, health and lifestyle confounders. An increased risk of poorer global cognition and attention amongst those in the highest quartile was observed, specifically in users of vit-D supplements.
Earlier reports by McGrath et al. (Reference McGrath, Scragg and Chant81) did not find significant associations between lower vit-D status and neurocognitive performance in adolescent and adult groups. However, there was a significant association between vit-D levels and cognitive performance in the elderly group, demonstrating unexpected results that those with increased levels of vit-D, particularly those taking vit-D supplements performing worse in learning and memory measures. Consistent with these findings, Lam et al. (Reference Lam, Albrecht and Takechi100) found an association between higher serum vit-D status and poorer verbal episodic memory performance in those with normal Ca-parathyroid hormone homeostasis in middle-aged and older individuals.
Whilst a significant number of cross-sectional, epidemiological and population studies have explored the association of serum vit-D status and cognition, mechanistic studies are lacking. Cell studies by Brown et al. (Reference Brown, Bianco and McGrath101) demonstrated that vit-D treatment of cultured hippocampal cells can initiate cellular apoptosis and similar findings have been indicated in cancerous cell lines(Reference Flanagan, Packman and Juba102–Reference Chen, Hsieh and Wu104). Excessive activation of the VDR causes gene transcription associated with mitochondrial export of cytochrome C and subsequent cleavage of caspase-9, which consequently promotes DNA fragmentation and thereby apoptosis(Reference Huebbe, Nebel and Siegert105).
Vitamin D homeostasis and ageing
Population studies exploring the association between vit-D homeostasis and cognitive capacity in late-aged individuals may be confounded by ‘reverse causation’ due to lifestyle changes and immobility(Reference Clipp, Burke and Hoffman-Bolton106–Reference Youl, Janda and Kimlin109). Moreover, the ability to synthesise endogenous vit-D within the epidermis or to activate 25(OH)D may be age-dependent(Reference Gallagher110). Paradoxical reports have been published with some studies suggesting increased, decreased or unchanged vit-D metabolite concentrations with ageing in both preclinical and clinical studies(Reference Veldurthy, Wei and Oz35,Reference Gallagher110,Reference Eastell, Yergey and Vieira111) . In addition to putative age-associated changes in peripheral biosynthetic and conversion of vit-D metabolites, pre-clinical and clinical data indicate that there is a marked reduction in intestinal, kidney and skeletal responsiveness to 1,25(OH)2D with age through mechanisms which are presently unclear(Reference Veldurthy, Wei and Oz35). Several studies have demonstrated a reduction in intestinal VDR expression(Reference Ebeling, Yergey and Vieira112–Reference Horst, Goff and Reinhardt114) with ageing in both animal and human studies, although others have reported no change(Reference Kinyamu, Gallagher and Prahl115,Reference Wood, Fleet and Cashman116) . A putative age-related diminished intestinal responsiveness to 1,25(OH)2D3 may be associated with modified recruitment of VDR, VDR co-activators or epigenetic changes(Reference Veldurthy, Wei and Oz35). Presently, no studies have investigated cerebral vit-D homeostasis, receptors or ‘responsiveness’ in the context of ageing, an important consideration in the context of CNS function and cognitive performance.
Hypervitaminosis-D and accelerated ageing
The global prevalence of hypovitaminosis-D during ageing is well documented(Reference Huang, Huang and Lai117). However, possible effects of hypervitaminosis-D on the ageing process are not yet clear(Reference Razzaque and Lanske27). Based on the strict regulation of vit-D activation, hypervitaminosis-D is rare in humans based on dietary sources. Nonetheless, vit-D supplementation has been associated with early ageing, hypercalcaemia, cardiovascular complications (vascular-related) and early death, supporting the association between hypervitaminosis-D and accelerated ageing(Reference Rajakumar, Greenspan and Thomas118). Two genes of interest in this area of study are FGF-23, involved in suppression of renal expression of 1,25(OH)2D3, resulting in decreased production of calcitriol, and KLOTHO; a membrane protein involved with cellular functions and cell–matrix interactions(Reference Razzaque119). Recent in vivo genetic-manipulation studies have shown increased serum levels of vit-D and altered mineral-ion homeostasis in mice that lack either FGF-23 or KLOTHO genes(Reference Razzaque and Lanske27). Furthermore, hypervitaminosis-D in these mutant mice exhibits an accelerated ageing phenotype(Reference Tsujikawa, Kurotaki and Fujimori26). Genetic ablation of FGF-23 or KLOTHO genes in other rodent models results in hypervitaminosis-D, hypercalcaemia and hyperphosphataemia, corresponding with a phenotype consistent with premature ageing(Reference Tsujikawa, Kurotaki and Fujimori26). Remarkably, Tsujikawa et al. (Reference Chen, Kuro-O and Chen120) reported that dietary restriction of vit-D reverses the premature-ageing phenotypes and indeed prolongs life in the KLOTHO knockout mouse model, an observation now reported in other studies. Polymorphisms in the human KLOTHO gene have also been associated with the occurrence of a number of age-related pathologies such as CVD(Reference Arking, Atzmon and Arking121). These findings provide strong evidence that hypervitaminosis-D may be causally associated with the ageing process.
Vitamin D receptor genetic polymorphisms and cognitive function
Genetic variability for vit-D metabolism may be associated for an individual’s susceptibility to cognitive decline. Polymorphisms of the VDR gene have been shown to influence the susceptibility to age-related changes in cognitive functioning and progression of neurodegenerative diseases such as Alzheimer’s disease, mild cognitive impairment, Parkinson’s disease and implicated with cognitive function(Reference Pettersen, Fok and Robin Hsiung122,Reference Kuningas, Mooijaart and Jolles123) . However, literature contributing to the delineation of the putative metabolic pathways that result in cognitive dysfunction is varied and inconclusive.
The VDR is synthesised by the VDR gene, located on chromosome 12 and composed of nine exons(Reference Monticielo, Brenol and Chies124), of which several genetic variations have been recognised. Approximately 1 % of the population has a DNA gene variation of the VDR(Reference Vilarino, Bianco and Lerner125). These genetic alterations can lead to significant defects on gene activation, affecting cell proliferation, mineral and hormonal metabolism and immune function. The most investigated polymorphisms of the VDR gene include Cdx-2, FokI, BsmI, ApaI and Taq1 and are often associated with phenotypes involving bone mineral density, osteoporotic fracture risk and cancer(Reference Pettersen, Fok and Robin Hsiung122,Reference Kuningas, Mooijaart and Jolles123) .
Kuningas et al. (Reference Kuningas, Mooijaart and Jolles123) observed a significant decline in cognitive performance in those carrying the BsmI and Taq1 polymorphisms; however, no cognitive deficit was observed in participants carrying the Cdx-2 or FokI polymorphisms (Table 2 for summary of studies to date). Interestingly, individuals with the ApaI polymorphisms performed better on tests measuring processing speed, attention and memory. These findings complemented those reported by Uitterlinden and colleagues in 2004(Reference Uitterlinden, Fang and van Meurs126). In contrast, Leymann et al. (Reference Lehmann, Refsum and Warden127) found the ApaI VDR polymorphisms to be significantly associated with cognitive decline and increased risk of AD, particularly in people under 75 years. Similarly, Keyimu et al. (Reference Keyimu, Zhou and Miao128) found both BsmI and ApaI polymorphisms significantly associated with an increased risk of mild cognitive impairment in a cohort of elderly Uygur people. A 2015 meta-analysis, totalling seven studies with 2034 Parkinson’s disease cases and 2432 healthy controls, found polymorphisms of ApaI, Bsm1 and Taq1 were not associated with the susceptibility to Parkinson’s disease, while the FokI (C and T allele) polymorphisms were associated with an increased risk in Parkinson’s disease(Reference Niu, Wang and Xie129).
Table 2. Summary of cross-sectional and cohort studies exploring the link between vitamin D receptor (VDR) gene polymorphisms and cognitive osutcomes
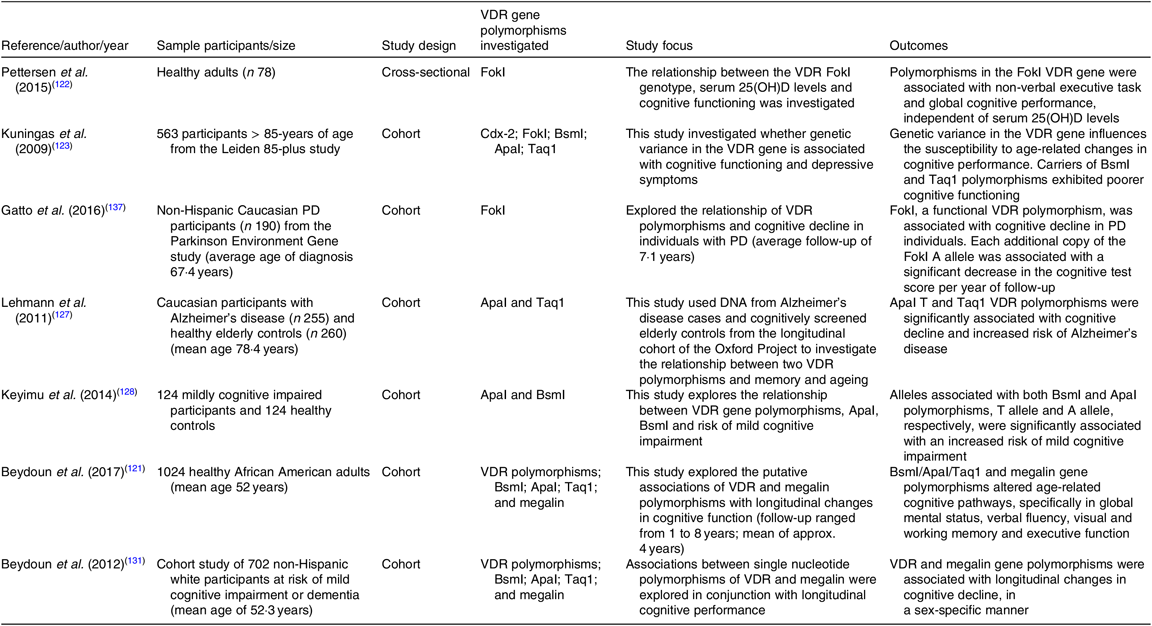
25(OH)D, 25-hydroxyvitamin D; PD, Parkinson’s disease.
In a recent study, Beydoun et al. (Reference Beydoun, Tajuddin and Dore130) evaluated associations of VDR polymorphisms (Cdx, BsmI, ApaI and Taq1) and LDL receptor, megalin, with a decline in longitudinal cognitive performance in 1024 healthy African American adults. Megalin is expressed in endothelial cells of the BBB and potentially mediates vit-D transport from the blood to brain parenchyma (Fig. 1). Beydoun and others have reported that VDR (BsmI/ApaI/Taq1) and megalin gene polymorphisms to correlate with age-related cognitive decline, specifically in performance tasks assessing global mental status, verbal fluency, visual/working memory and executive function(Reference Kuningas, Mooijaart and Jolles123,Reference Beydoun, Ding and Beydoun131–Reference Gatto, Sinsheimer and Cockburn134) . Studies investigating VDR polymorphisms and cognitive indices are heterogeneic, inconclusive and exacerbated by the confounder of uncertainy in plasma and cerebral homeostasis of vit-D(Reference Bornman135).
Genetic and environmental interactions regulating vitamin-D homeostasis, vitamin D receptor polymorphisms and cognitive performance
The relationship between VDR polymorphisms, vit-D metabolite concentrations and CNS function remains unclear(Reference McGrath, Saha and Burne136). It has been demonstrated by multiple researchers that expression and functionality of VDR polymorphisms to transactivate specific DNA gene sequences are regulated by both genetics, environment and abundance of bioactive vit-D(Reference Keyimu, Zhou and Miao128,Reference Gatto, Sinsheimer and Cockburn134,Reference Gatto, Paul and Sinsheimer137) . For example, Wilkinson et al. (Reference Wilkinson, Llewelyn and Toossi138) observed the TT/Tt VDR genotype of Taq1 polymorphism was associated with tuberculosis in a UK Indian population, but only in a vit-D-deficient state. Wong et al. (Reference Wong, Seow and Arakawa139) found an individual’s susceptibility to colon cancer doubles when genotyped with ff FokI polymorphisms when consuming a low Ca (increased 1,25(OH)2D3 synthesis) in comparison with FF genotypes. Collectively, it is apparent the impact of VDR polymorphisms on the function of the VDR may be strongly influenced by an individual’s vit-D status and thereby associated disease risk(Reference Bornman135). By extension, similar effects may be indicated with respect to CNS function and cognition.
Whilst plasma insufficiency of vit-D concomitant with expression of selected VDR isoforms has been associated with Alzheimer’s disease and cognitive decline(Reference Pettersen, Fok and Robin Hsiung122), the possibility of strong associations between vit-D concentrations above normal physiological levels and VDR polymorphisms remains to be investigated. Interestingly, some studies have shown that FokI polymorphisms change the VDR translation initiation site and alter its functional properties, producing multiple isoforms of the receptor which influence transcription factor expression(Reference Bornman135,Reference Abrams, Griffin and Hawthorne140) . The f allele (T nucleotide) on the FokI gene produces a longer VDR protein which is proposed to be less active in influencing transcription factor and thus affecting downstream effects(Reference Bornman135). Conversely, the F allele (C nucleotide) on the FokI gene results in a VDR protein truncated by three amino acids, which is more effective in activating transcription factor(Reference Bornman135,Reference Miyamoto, Kesterson and Yamamoto141,Reference Arai, Miyamoto and Taketani142) . Orton et al. (Reference Orton, Morris and Herrera143) found lower concentrations of 25(OH)D (25·8 ± 2·2 ng/ml) when coding for homozygous genotypes for the shorter VDR isoform, compared with greater 25(OH)D concentrations (33·3 ± 1·6 ng/ml) when coding for the longer VDR isoform in heterozygous and homozygous genotypes.
Vitamin D and neurovascular inflammation
The BBB is a semipermeable membrane comprising the cerebrovascular wall which separates the blood serum from the brain parenchyma(Reference van de Haar, Burg-Mans and Jansen144). The unique endothelial junctions of the BBB ensure the tight regulation of substances entering the CNS(Reference Hawkins and Davis145–Reference Sandoval and Witt147). BBB dysfunction is characterised by compromised cerebrovascular integrity leading to unregulated extravasation of serum constituents into the brain parenchyma. An increasing number of studies suggest that impairment of cerebral capillaries at the endothelial cell may be a major risk factor prior to the progression of clinical cognitive dysfunction(Reference Lam, Takechi and Pallabage-Gamarallage24,Reference van de Haar, Burg-Mans and Jansen144,Reference Janelidze, Hertze and Nägga148) . Recent experimental and clinical studies have shown therapeutic benefit in attenuating progression of neurodegeneration/cognitive decline if cerebrovascular disturbances are attenuated(Reference Gorelick, Scuteri and Black149,Reference Takechi, Pallebage-Gamarallage and Lam150) .
Lam et al. (Reference Lam, Takechi and Pallabage-Gamarallage24) demonstrated that vit-D-enriched diets resulted in increased brain capillary permeability and neuroinflammation in a dose-dependent manner and independent of serum Ca homeostasis, or suppression of parathyroid hormone. Lam’s study is the first to demonstrate that provision of exogenous vit-D supplementation above ordinary physiological levels has significant cerebrovascular-regulating properties. Furthermore, Durk et al. (Reference Durk, Chan and Campos151) recently investigated VDR expression on cerebral endothelial cells, which when activated by its bioactive ligand, 1,25(OH)2D3 was found to alter the kinetics of endothelial p-glycoprotein expression and its substrates. As p-glycoprotein is an ATP-driven efflux pump and a major blockade in the prevention of small-molecule delivery across the BBB and into the brain(Reference Cannon, Peart and Hawkins152), Durk’s findings, whilst not directly implicating vit-D and the regulation of cerebral capillary function, certainly support this notion that vit-D can influence BBB permeability.
High-dose vit-D supplementation has been associated with an increased systemic inflammatory phenotype in concert with increased colitis susceptibility in animal studies(Reference Ghaly, Kaakoush and Lloyd153). In alignment with the pro-inflammatory phenotype exacerbated by high-dose vit-D intake, a recent study by Krementsov et al. (Reference Krementsov, Asarian and Fang154) reported intriguing results in a pre-clinical model of autoimmune neuroinflammation (multiple sclerosis) whereby high-dose vit-D supplementation unexpectedly exacerbated disease susceptibility, in a sex- and genotype-specific manner. It is apparent that present evidence suggests restoration of vit-D homeostasis to sufficient levels from a vit-D-deficient state has been shown to ameliorate CNS oxidative stress, mitochondrial dysfunction, neuroinflammation and apoptosis that culminate in neurodegeneration(Reference Briones and Darwish155–Reference Taniura, Ito and Sanada159). However to date, no studies have reported the effects of exaggerated vit-D metabolism on the aforementioned mechanisms on the CNS, and indeed, warrants further investigation.
Clinical considerations: reference serum vitamin D concentration
The definition for appropriate serum vit-D ranges remains controversial(Reference Binkley, Dawson-Hughes and Durazo-Arvizu160). The U.S. Institute of Medicine states insufficient vit-D levels are defined by 25(OH)D levels below 50 nmol/l(Reference Ross, Taylor, Yaktine and Del Valle161), the Endocrine Society whom reports insufficiency of 25(OH)D below 72·5 nmol/l(Reference Holick, Binkley and Bischoff-Ferrari162), whilst the Vitamin D Council refers to insufficiency at a concentration below 97·5 nmol/l of circulating 25(OH)D(29). There are also meta-analyses, RCT and observational studies that suggest sufficient vit-D levels are approximately 100 nmol/l(Reference Pludowski, Holick and Grant163–Reference Wimalawansa166), a concentration that some organisations would dispute and suggest may be potentially reaching toxic levels(Reference Holick, Binkley and Bischoff-Ferrari162).
Ross et al. (Reference Ross, Manson and Abrams167) and a more recent study by Manson et al. (Reference Manson, Brannon and Rosen11) state vit-D recommendations are based on bone health, while benefits for other non-skeletal systems, such as the brain, remain unclear. A diagnosis of vit-D deficiency is generally based on the findings that supplementing with 15–20 μg of vit-D/d (Institute of Medicine RDA for adults) fails to increase vit-D serum levels above 50 nmol/l in a population of North America. Manson et al. suggests, however, that a vit-D level of 40 nmol/l would serve the requirement of half of the North American population, whilst vit-D levels of 50 nmol/l are considered adequate to the majority of the indicated population. Presently, it is difficult to robustly define a physiological reference range, particularly in context of optimal CNS function, given the numerous factors that may influence vit-D status and response. Moreover, there is a paucity of studies which consider potential adverse effects associated with persistently higher blood or tissue concentration of bioactive vit-D metabolites. The importance of defining a suitable range and appropriate biomarker to accurately reflect vit-D levels is urgently needed.
Measurement of vitamin D homeostasis
Blood vit-D homestasis is indicative of 25(OH)D bound to VDBP (85 %), 25(OH)D bound to albumin (15 %) and unbound (free) 25(OH)D (Fig. 1)(Reference Lai, Bikle and Lizaola168).
Serum 25(OH)D is ordinarily used as the surrogate biomarker of homeostasis based on its long half-life, a critical intermediary in the utilisation of 1,25(OH)D by the body, and because tissue level hydroxylase enzymes, such as CYP27B1, function below their Km values and are below detectable limits. However, recent studies have challenged the validity of utilising 25(OH)D as a surrogate marker of active biological effects illicited as a consequence of 1,25OHD binding to high-affinity receptors(Reference Hilger, Friedel and Herr169,Reference Reid, Toole and Knox170) . The recent updated international recommendations by a panel of 12 vit-D experts concluded that 25(OH)D was not an appropriate marker of vit-D physiological homeostasis(Reference Annweiler, Dursun and Féron14).
Hilger et al. (Reference Hilger, Friedel and Herr169) recently conducted a systematic review of vit-D status in global populations in which 195 studies were analysed from forty-four countries, involving over 168 000 participants. The study reported substantial variability in mean serum 25(OH)D concentrations (range 4·9–136·2 nmol/l) within the same geographical regions around the world. Clearly on a global scale, there are huge discrepancies in using 25(OH)D metabolite alone as a reflection of total vit-D status(Reference Holick, Binkley and Bischoff-Ferrari162,Reference Ross, Manson and Abrams167) .
Common methods to assess vit-D metabolites and homeostasis include MS and high-pressure liquid chromatography (HPLC-MS), enzyme immunoassays, competitive protein binding assays, RIA, chemiluminescent immunoassays and automated chemiluminescence protein-binding assays(Reference Snellman, Melhus and Gedeborg171,Reference Zahedi Rad, Neyestani and Nikooyeh172) . Clinical measures are not harmonised internationally making appropriate reference ranges difficult to compare (Table 1). Due to the significant assay variation in 25(OH)D measurement and substantial inter-assay and laboratory variability(Reference Binkley, Krueger and Cowgill173–Reference Lai, Lucas and Banks175), unsubstantiated assumptions for 25(OH)D as a robust marker of vit-D status have been realised(Reference Carter, Berry and Durazo-Arvizu176).
The Vitamin D External Quality Assessment Scheme is the world’s largest specialist scheme for assessing and evaluating the reliability of 25(OH)D assays and has been in operation since 1989. As of January 2017, the data collected by Vitamin D External Quality Assessment Scheme was a contribution of fifty-six countries and covered analysis from approximately thirty different assay methods(Reference Carter, Berry and Durazo-Arvizu176). Indeed, multiple studies, including reports from Vitamin D External Quality Assessment Scheme, have indicated a great deal of variability between different 25(OH)D metabolite assays as well as inter-laboratory disagreement(Reference Binkley, Krueger and Cowgill173,Reference Binkley, Krueger and Gemar177–Reference Carter182) .
Snellman et al. (Reference Snellman, Melhus and Gedeborg171) investigated the precision and accuracy of three common commercially available assays (HPLC-MS, RIA and chemiluminescent immunoassays) with diverse results. Researchers accounted for a multitude of confounders such as age, sex, ethnicity, season, altitude, geography, as well as limiting genetic variability, in their cohort of Swedish twins (n 204). Mean 25(OH)D concentrations between assays showed up to 30 % (25 nmol/l) variability (HPLC-MS 85 nmol/l; RIA 70 nmol/l; chemiluminescent immunoassays 60 nmol/l). Moreover, Black et al. (Reference Black, Anderson and Clarke183) investigated inter-laboratory 25(OH)D concentrations using Australian participants (n 840) from three different laboratories. The three laboratories used DiaSorin Liaison and HPLC-MS-based 25(OH)D detection assays, which were analysed against a certified laboratory using a standardised HPLC-MS-based assay. Results from all four laboratories were wide-ranging. Researchers are now engaged in an international effort to suspend meta-analyses publications on whose methodologies are based on unstandardised 25(OH)D data(Reference Binkley, Dawson-Hughes and Durazo-Arvizu160). According to the National Institute of Health, Office of Dietary Supplements USA (2017), an international effort to standardise the measurement of 25(OH)D and its metabolites is currently being led by the Vitamin D Standardization Program(Reference Sempos, Heijboer and Bikle184).
Contemporary liquid chromatography-MS (LC-MS/MS) analytical methods have been developed and validated to simultaneously quantify a comprehensive panel of vit-D compounds in human serum. Historically, the extraction and chromatographing of vit-D compounds have been particulary challenging due to the range of polarities and different molecular moieties. However, in the recent years, optimised precipitation and separation techniques have produced high-sensitivity, recovery and resolution results which can quantitate up to fifteen vit-D compounds (bioactive, inactive, catabolites) and indeed, an appropriate alternative to standardise analysis of vit-D status(Reference Abu Kassim, Shaw and Hewavitharana185,Reference Hewavitharana, Abu Kassim and Shaw186) .
In cases of suspected clinical vit-D deficiency, physicians generally recommend either ergocalciferol (vit-D2) or cholecalciferol (vit-D3) supplementation(Reference Swanson, Nielson and Shrestha187). Measurement of total vit-D status includes circulating serum calcifediol (25(OH)D) and metabolites 25(OH)D2 and 25(OH)D3; however, not all the immunoassays are able to detect 25(OH)D2 in clinical practice(Reference Cavalier, Wallace and Knox33). Nonetheless, Tripkovic et al. (Reference Tripkovic, Lambert and Hart188) conducted a meta-analysis and systematic review of RCT from 1966 to 2011 that directly compared the effect both vit-D2 and vit-D3 on raising circulating serum 25(OH)D2 or 25(OH)D3 levels, respectively. Significantly higher 25(OH)D3 levels than 25(OH)D2 were achieved when participants were given 20 μg of vit-D3, compared with those given 20 μg of vit-D2, findings replicated in subsequent studies(Reference Romagnoli, Mascia and Cipriani189,Reference Nguyen190) . Swanson et al. (Reference Cashman, Dowling and Škrabáková191) sought to quantify and examine the associations between 25(OH)D2, and 25(OH)D3, with their bioactive forms 1,25(OH)2D2, and 1,25(OH)2D3, respectively, in a large cohort of older men (n 679) to better understand how 25(OH)D2 relates to the other vit-D metabolites. Interestingly, greater levels of 25(OH)D2 were associated with lower levels of both 25(OH)D3 and biologically active 1,25(OH)2D3. Furthermore, 25(OH)D2 was not found to be associated with higher total levels of 25(OH)D or physiologically relevant 1,25(OH)2D. Collectively, the findings suggest that differences between 25(OH)D2 and 25(OH)D3 are due to dissimilar affinities for the VDR, which appears to be linked to an extra step of 24-hydroxylation that inactivates 1,25(OH)2D(Reference Houghton and Vieth192). Additionally, it is thought that 25(OH)D3 is the preferred substrate for hepatic 25-hydroxylase enzyme, CY2R1, which in combination may alter the rate of 24-hydroxylation(Reference Tripkovic, Lambert and Hart188).
Interestingly, Jones et al. (Reference Jones, Assar and Harnpanich193) compared the serum half-lives of 25(OH)D2 and 25(OH)D3 in two separate populations from the UK and Gambia, Africa (n 36), with differing 25(OH)D status. Results showed that not only was the half-life of 25(OH)D2 shorter than 25(OH)D3 but also the half-lives were affected by VDBP concentration and genotype.
Vitamin D binding protein measurement and polymorphisms
Numerous tissues express the VDBP with liver as the major source for plasma abundance. However, VDBP expression has also been demonstrated in the brain, spinal cord, kidney, skeletal muscle, heart, lung, intestine and bone(Reference Yang, Qin and Zhu194,Reference Jirikowski, Kaunzner and Dief Ael195) . The VDBP can be determined in blood serum, cerebrospinal fluid, saliva, seminal fluid and breast milk(Reference Speeckaert, Speeckaert and van Geel196). VDBP is the primary chaperone protein for 25(OH)D due to higher affinity compared with albumin(Reference Kamboh and Ferrell197). The free hormone hypothesis postulates that protein-bound hormones are biologically inactive, while unbound hormones are biologically free to exert their physiological activity(Reference Jassil, Sharma and Bikle198).
Notionally, only 1 % of total 25(OH)D concentration is available for conversion to the bioactive 1,25(OH)D(Reference Lai, Bikle and Lizaola168). VDBP serves as a sink for 25(OHD) and may be critical in the context of conversion to 1,25(OH)D(Reference Kamboh and Ferrell197). There are three forms of VDBP polymorphisms that exist, originally referred to as GC1F, GC1S and GC2. These allow for six allelic phenotypes (1s/1s, 1s/ 1f, 1s/2, 1f/1f, 1f/2 and 2/2)(Reference Yousefzadeh, Shapses and Wang199). These phenotypes can be identified by genotyping for the two SNP, rs7041 and rs4588, in the GC gene(Reference Jorde, Mathiesen and Rogne200). These polymorphisms occur at a diverse range of frequencies among different races and ethnicities(Reference Kamboh and Ferrell197). Allelic variants of VDBP are at varied concentrations within blood(Reference Lauridsen, Vestergaard and Nexo201) and different binding affinities for 25(OH)D and 1,25(OH)D(Reference Arnaud and Constans202,Reference Chun, Peercy and Adams203) . Engelman et al. (Reference Engelman, Fingerlin and Langefeld204) showed that homozygosity for the CG1F allele occurred in 53 % of African Americans but only 6 % of Caucasians and 13 % of Hispanics. Interestingly, over 50 % of African Americans has a vit-D deficiency diagnosis, yet their low 25(OH)D concentrations do not appear to be linked with a higher risk of bone fractures, as they do in white Americans(Reference Hoofnagle, Eckfeldt and Lutsey205). Consistent with the latter, Powe et al. (Reference Powe, Evans and Wenger206) found racial, ethnic variation in VDBP polymorphism concentrations in black and white Americans and showed difference in total 25(OH)D, but comparable abundance of bioavailable 25(OH)D. Harris(Reference Harris207) previously compared vit-D metabolites in American Caucasians and African Americans, indicating African Americans have higher circulating concentrations of the bioactive vit-D at a given level of 25(OH)D compared with Caucasians. In studies by Lutsey et al. (Reference Lutsey, Michos and Misialek208), they reported lower VDBP levels in African Americans in comparison with Caucasians were associated with a higher concentration of 25(OH)D. Furthermore, Sinottee et al. (Reference Sinotte, Diorio and Bérubé209) evaluated the association between VDBP polymorphisms and 25(OH)D concentrations in white premenopausal Caucasian French women (n 741) and concluded that circulating 25(OH)D is highly correlated with VDBP polymorphisms. Other studies investigating VDBP polymorphisms have also found varied vit-D metabolite concentrations amongst race and ethnic groups(Reference Engelman, Fingerlin and Langefeld204,Reference Wang, Zhang and Richards210–Reference Santos, Mascarenhas and Boguszewski212) .
In Tromsø, Northern Norway, longitudinal population-based health surveys covering general, medical and pathological information have been conducted at 6–7 years intervals since 1974(Reference Jorde, Mathiesen and Rogne200). Thus far, blood samples have totalled 27 000 participants and genotyping for VDBP polymorphisms has been undertaken in 11 704 participants. The prevalence in VDBP polymorphisms ranged between 4·4 and 30·9 % within population. Findings clearly demonstrate that 25(OH)D levels were not only dependent on VDBP and albumin concentrations but also significantly associated with VDBP polymorphisms. Additionally, factors influencing VDBP concentration and ultimately, VDBP polymorphisms and vit-D status, include age and sex(Reference Pop, Shapses and Chang213,Reference Carpenter, Zhang and Parra214) , diurnal rhythms(Reference Jones, Redmond and Fulford215) and obesity(Reference Karlsson, Osmancevic and Jansson216,Reference Ashraf, Huisingh and Alvarez217) . Recent studies have further demonstrated that serum VDBP concentration is decreased in pathological states including, type 1 diabetes mellitus(Reference Blanton, Han and Bierschenk218), chronic liver disease(Reference Lai, Bikle and Lizaola168,Reference Stokes, Volmer and Grunhage219) and renal disease(Reference Denburg, Kalkwarf and de Boer220).
Clinical indications and health promotion
Vit-D insufficiency as currently determined is suggested in approximately 14 % of the world’s population(Reference Lerner, Sharony and Miodownik221). This has resulted to public vit-D recommendations from Government institutions and agencies between 25 and 250 μg/d(29–31). In response to public health promotion of vit-D deficiency to resolve what has now been classed as a ‘major global epidemic’(Reference Soliman222), a significant number of middle-aged and elderly individuals are supplementing their vit-D intake in an attempt to reduce the putative health risks associated with vit-D deficiency, including cognitive decline and osteoporosis(223).
It is estimated that 86 % of the global population is reporting as having sufficient vit-D levels(Reference Lerner, Sharony and Miodownik221). By extension, it is possible that recommendation for exogenous intake through fortified foods/supplements at 15 to 20 μg may be potentially harmful in some individuals, particularly if endogenous levels of 1,25(OH)2D3 are already heightened(223). Researchers in the USA analysed data from a national survey over 15 years (1999–2014) and found an 18 % increase in the people taking vit-D over 25 μg/d and a 2·8 % increase in the amount of people taking of over 100 μg of vit-D/d(223). Unfortunately, this trend is not isolated and can be seen on a global scale. In Australia, the majority (77 %) of Australian citizens have supposedly sufficient vit-D levels, yet remarkably, one in twenty adults were reported to be taking vit-D supplements. Among those with high serum vit-D ((25(OH)D) >100 nmol/l), one in ten reported regular intake of exogenous vit-D(224). Similarly, despite more than two-thirds (68 %) of Canadians reported having sufficient vit-D serum levels ((25(OH)D); >50 nmol/l), 34 % still report taking a regular vit-D supplements(Reference Janz and Pearson225). Furthermore, several large national and international clinical trials have confirmed participant numbers between 5000 and 30 000 per trial for a variety of indications. These trials are currently administering from 50 μg vit-D/d(Reference Manson, Bassuk and Lee226) or 1500–2500 μg vit-D/month(Reference Scragg, Waayer and Stewart227,Reference Neale, Armstrong and Baxter228) , exploring therapeutic rates of 250–7500 μg vit-D/dose(Reference Wu, Staykova and Horne28,Reference Bacon, Gamble and Horne229–Reference Rizzoli, Boonen and Brandi233) without adequate evidence such levels are safe in the context of cognitive function. Clearly, serum measures of vit-D must be considered carefully as a putative surrogate marker of CNS vit-D homeostasis.
Conclusion
There is substantial scientific, clinical and public health interest in how vit-D modulates CNS function. On the basis of purported benefits in cognitive performance, supplementary use of vit-D has increased markedly in developed countries, often without clinical indication. The latter may be of some concern given an emerging body of evidence which suggests either no benefit or possibly even harm in subjects taking exogenous vit-D supplementation who have otherwise adequate levels of vit-D as currently assessed. Significant limitations in our contemporary understanding of vit-D effects on the CNS include the relevance of serum measures to CNS homeostasis, regulation of conversion and deactivation of bioactive metabolites with studies suggesting significant subject variability. Other challenges include understanding longitudinal/life-long and possibly epigenetic effects when considering causal association realised over decades of life. Greater insight into fundamental physiological processes realised through robust pre-clinical models would be informative in supporting clinical conisiderations of vit-D homeostasis in the context of CNS health.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia.
All authors contributed to the literature search, manuscript design, manuscript writing and revisions of the manuscript. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.






