A significant amount of vegetable oils produced across the world are used for deep-frying, and reuse of heated oil (deep-fried oil) is rampant in the street food joints of India and other Asian countries to save costs. Heating oil multiple times negatively alter the physicochemical and nutritional properties of oils. Reactive oxygen species (ROS), hydroperoxides, aldehydes, ketones and trans-isomers are some of the harmful derivatives produced during deep-frying(Reference Venkata and Subramanyam1). Destruction of endogenous antioxidants, particularly vitamin E, is another disadvantage of deep-frying(Reference Adam, Sulaiman and Top2). Therefore, long-term consumption of repeatedly heated oil not only causes fat-soluble vitamin deficiency but also may result in the cardio-hepatic oxidative stress (OS) due to their exposure to harmful derivatives produced during deep-frying. Though the incorporation of synthetic antioxidants to frying oils prevents their deterioration to some extent, they may not contribute to the rebuilding of weakened tissue antioxidant defence response. The quick re-establishment of weakened tissue response to the OS induced by the heated oils is critical for the normal functioning of the body(Reference Rangel-Zuñiga, Haro and Tormos3). Studies have shown that OS caused due to dampened antioxidant enzyme activity along with non-enzymatic antioxidants (glutathione, vitamin C and vitamin E) are predictors of CVD and hypertension(Reference Blankenberg, Rupprecht and Bickel4,Reference Talukder, Johnson and Varadharaj5) . Dysregulation of redox balance, particularly in the heart and liver, may derail their metabolic crosstalk necessary for normal physiological functions(Reference Baskin, Bookout and Olson6). Maintenance of systemic homoeostasis and the response to nutritional and environmental challenges require the coordination of multiple organs and tissues(Reference Priest and Tontonoz7). Though earlier studies have addressed the implications of repeatedly heated oil consumption on postprandial lipaemia and heart function(Reference Cohn8), no attempts have been made to evaluate the modulatory potentials of lipid-soluble bioactive components that may migrate from ginger (Zingiber officinale) and turmeric (Curcuma longa) to the oil during the heating process on cardio-hepatic response to OS. Gingerols (6-, 8- and 10-gingerols) are the major polyphenols present in ginger, and with heat treatment, they are transformed into corresponding shogaols and upon hydrogenation to paradols(Reference Mao, Xu and Cao9). Curcuminoids (curcumin demethoxycurcumin, 5’-methoxycurcumin and dihydrocurcumin) are colouring agents present in turmeric, with improved health benefits as a nanoparticle, and known to beneficially modulate inflammation under diabetic conditions(Reference Boarescu, Boarescu and Bocşan10,Reference Bulboacă, Boarescu and Bolboacă11) . Since ginger and turmeric are known to exhibit health benefits(Reference Bulboacă, Boarescu and Bolboacă11,Reference Anh, Kim and Long12) , we hypothesised that lipid-soluble molecules may migrate to the oil from these spices (ginger and turmeric) during the heating process which might attenuate the consequences of heated oil consumption. Besides, the inclusion of ginger or turmeric during deep-frying may need minimal changes in the industrial process technology and also acceptable to consumers. Hence, in this study, we aimed to assess the cardio-hepatic antioxidant response and blood pressure in experimental rats as a consequence of long-term consumption of repeatedly heated oil (both n-3- and n-6-rich oils) with or without ginger and turmeric. Wistar rats were used as a model system, as the dietary implications on the tissue antioxidant defence response and blood pressure parameters are well established.
Materials and methods
Materials
β-Mercaptoethanol, 1-chloro-2,4-dinitrobenzene (CDNB), cytochrome-C, 2′,7′-dichlorofluorescin diacetate (DCF-DA), thiobarbituric acid, xanthine and xanthine oxidase were obtained from Sigma Chemicals. Dinitrophenyl hydrazine, EDTA, oxidised glutathione, reduced glutathione, malonaldehyde, NADPH, t-butyl hydroperoxide and solvents were obtained from SRL Chemicals. Refined rapeseed oil (canola oil) and sunflower oil were purchased from the local market. Fresh ginger (Z. officinale) and turmeric (C. longa) were dried, powdered and kept in –20°C. AIN-93G-based mineral mix and vitamin mix were purchased from Hi-Media Laboratories.
Animal model, diet, feeding and growth parameters
The animal procedures were approved (CFT/IAEC/120/2018) and monitored by the Institutional Animal Care and Use Committee of CSIR-CFTRI (Council of Scientific and Industrial Research-Central Food Technological Research Institute). The authorised trainers gave comprehensive training to the animal handlers of this study, and extensive care was taken during the experimental procedures not to cause any distress to the animals. The animal experiment was conducted from 12 June 2019 to 16 September 2019. Sixty-four male Wistar rats (OUTB-Wistar, IND-cft (2C)) (Rattus norvegicus) weighing 50 ± 5 g were housed (four rats/cage) under a 12 h light–12 h dark cycle with water and diet available ad libitum. After acclimatisation, the rats (n 8) were randomly assigned to receive control (native rapeseed (N-CNO) and native sunflower (N-SFO)) and experimental diets (heated rapeseed (H-CNO) + ginger (GI), H-CNO + turmeric (TU), heated sunflower (H-SFO) + GI and H-SFO + TU) based on AIN-93G dietary formulations for 120 d(Reference Reeves, Nielsen and Fahey13). The dietary oils used were subjected to the heating process, as indicated in online Supplementary Table S1, and incorporated in the diet (10 g oil/100 g diet). Diets were stored in airtight pouches flushed with nitrogen and kept at the refrigerated condition. Following the completion of the experiment, rats were killed under CO2 anaesthesia and serum, heart and liver were harvested and stored at –80°C until further analysis. The fatty acid composition is given in online Supplementary Table S2. The growth and organ weights of rats are given in Table 1.
Table 1. Growth and organ weights (n 8)
(Mean values and standard deviations)

N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower.
a Mean values within a row with unlike superscript letters were significantly different (P < 0·05 and q < 0·1).
Oxidative stress markers, antioxidant enzymes and hepatic and cardiac enzymes
Tissue and serum lipid peroxides(Reference Miller and Aust14,Reference Wade and van Rij15) , nitric oxide(Reference Green, Wagner and Glogowski16), protein carbonyls(Reference Mesquita, Oliveira and Bento17), catalase(Reference Aebi18), superoxide dismutase(Reference Flohe19), glutathione peroxidase(Reference Tappel, Chaudiere and Tappel20) and glutathione transferase(Reference Habig, Pabst and Jakoby21) were measured using a microplate reader (Tecan). The liver and heart ROS was measured by the method, as described earlier(Reference Eruslanov and Kusmartsev22). Protein was measured by Lowry’s method(Reference Lowry, Rosebrough and Farr23). The activity of serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, alkaline phosphatase and creatinine kinase was analysed colourimetrically as per the commercially available kits (Agappe).
Western immunoblotting
Protein resolved on TRIS-glycine polyacrylamide gel (10 %) was transferred onto the polyvinylidene fluoride membrane, blocked using 5 % non-fat dry milk and probed using intercellular adhesion molecule-1 (ICAM-1) and β-tubulin (Cloud-Clone Corp.) and nitric oxide synthase-2 (NOS-2) (Santa Cruz Biotechnology) antibody. Membranes were washed and incubated with appropriate secondary antibody. Following further washes, the membranes were treated with chemiluminescent reagents and documented for densitometry.
Nuclear factor erythroid 2-related factor 2 in liver and heart
Heart and liver nuclear extracts were prepared using a nuclear extraction kit obtained from Abcam (ab113474), and nuclear factor erythroid-derived 2-like 2 (NRF-2) (ab207223) level in nuclear extracts was analysed as per kit instructions provided by Abcam.
Blood pressure and haematology parameters
Blood pressure (systolic and diastolic) was measured by a non-invasive blood pressure system (IITC Life Sciences). Testing was carried out using rat tail-cuff by elevating ambient temperature in the chamber. For haematological examination, blood was collected in EDTA containing vials, and parameters like leucocytes, erythrocytes, Hb content, haematocrit value, mean corpuscular volume, mean corpuscular Hb content, mean corpuscular Hb concentration and platelets count were measured using automated blood analyzer (Sysmex, PocH-100i).
Statistical analysis
The sample size (n 8/group) was determined based on nutritional intervention studies reported earlier, with a statistical power of 0·8 and an α error of 0·05. The data were analysed by one-way ANOVA (non-parametric), followed by Tukey’s test (to compare the differences among the treatments) using GraphPad Prism version 7.04 (GraphPad Software; www.graphPad.com), and a P value < 0·05 was considered as statistically significant. The false discovery rate (q value of 0·1) was considered as the threshold for significance. Only the mean and standard deviation for each diet group are plotted in each figure.
Results
Oxidative stress markers in serum, liver and heart
OS markers (lipid peroxides, nitric oxide and protein carbonyls) in serum, liver and heart of heated oil (H-CNO and H-SFO) groups were significantly (P < 0·05) increased when compared with their respective control (N-CNO and N-SFO) groups (Table 2), whereas, when compared with the H-CNO group, the OS markers in the serum, liver and heart of the H-CNO + GI and H-CNO + TU groups decreased significantly (P < 0·05) (Table 2). Likewise, when compared with the H-SFO group, the OS markers in the serum, liver and heart of the H-SFO + GI and H-SFO + TU groups decreased significantly (P < 0·05) (Table 2).
Table 2. Oxidative stress markers (n 8)
(Mean values and standard deviations)
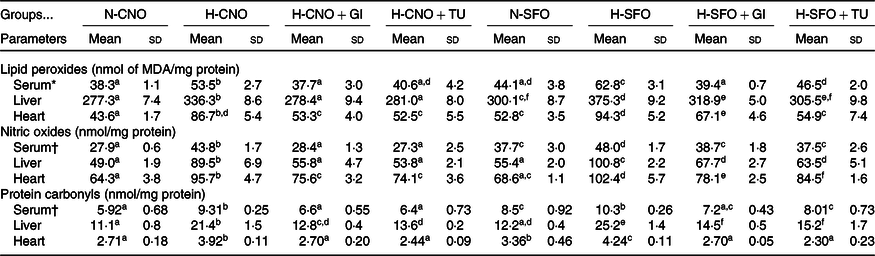
N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower; MDA, malondialdehyde.
a,b,c,d,e,f Mean values within a row with unlike superscript letters were significantly different (P < 0·05 and q < 0·1).
* For serum, units are nmol of MDA/ml serum.
† For serum, units are nmol/ml serum.
Reactive oxygen species and antioxidant enzyme activity in serum, liver and heart
ROS in the liver and heart of the H-CNO and H-SFO groups increased significantly (P < 0·05) compared with their respective control (N-CNO and N-SFO) groups (Table 3), whereas, when compared with the H-CNO group, ROS in the liver and heart of the H-CNO + GI and H-CNO + TU groups decreased significantly (P < 0·05) (Table 3). Likewise, when compared with the H-SFO group, ROS in the liver and heart of the H-SFO + GI and H-SFO + TU groups decreased significantly (P < 0·05) (Table 3). The activity of antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase and glutathione transferase) in serum, liver and heart of H-CNO and H-SFO groups decreased significantly (P < 0·05) compared with their respective control (N-CNO and N-SFO) groups (Table 4), whereas, when compared with H-CNO, the activity of antioxidant enzymes in the serum, liver and heart of the H-CNO + GI and H-CNO + TU groups increased significantly (P < 0·05) (Table 4). Likewise, when compared with H-SFO, the activity of antioxidant enzymes in the serum, liver and heart of the H-SFO + GI and H-SFO + TU groups increased significantly (P < 0·05) (Table 4).
Table 3. Reactive oxygen species (n 8)
(Mean values and standard deviations)

N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower; RFU, relative fluorescence units.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05 and q < 0·1).
Table 4. Antioxidant enzyme activity (n 8)
(Mean values and standard deviations)

N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower; SOD, superoxide dismutase; GPx, glutathione peroxidase; GST, glutathione transferase.
a,b,c,d,e Mean values within a row with unlike superscript letters were significantly different (P < 0·05 and q < 0·1).
Nuclear translocation of nuclear factor erythroid 2-related factor 2 and nitric oxide synthase-2 and intercellular adhesion molecule-1 expression in liver and heart
NRF-2 level in the nuclear extract of the liver and heart of the H-CNO and H-SFO groups decreased significantly (P < 0·05) compared with their respective control (N-CNO and H-SFO) groups (Fig. 1), whereas, when compared with H-CNO, the NRF-2 level in H-CNO + GI and H-CNO + TU groups increased significantly (P < 0·05). Likewise, when compared with H-SFO, the NRF-2 level in H-SFO + GI and H-SFO + TU groups increased significantly (P < 0·05). The NOS-2 and ICAM-1 expression in the liver and heart of the H-CNO and H-SFO groups increased significantly (P < 0·05) compared with their respective control (N-CNO and N-SFO) groups (Figs. 2 and 3), whereas, when compared with H-CNO, the NOS-2 and ICAM-1 expression in H-CNO + GI and H-CNO + TU groups decreased significantly (P < 0·05). Likewise, when compared with H-SFO, the NOS-2 and ICAM-1 expression in H-SFO + GI and H-SFO + TU groups decreased significantly (P < 0·05) (Fig. 4).

Fig. 1. Nuclear factor erythroid 2-related factor 2 (NRF-2) level in the nuclear extracts of the liver (a and b) and heart (c and d) in rats fed native and repeatedly heated oil with or without ginger (GI) and turmeric (TU). Values are means and standard deviations of four rats. (a and c) ![]() , native rapeseed (N-CNO);
, native rapeseed (N-CNO); ![]() , heated rapeseed (H-CNO);
, heated rapeseed (H-CNO); ![]() , heated rapeseed and GI (H-CNO+GI);
, heated rapeseed and GI (H-CNO+GI); ![]() , heated rapeseed and TU (H-CNO+TU). (b and d)
, heated rapeseed and TU (H-CNO+TU). (b and d) ![]() , native sunflower (N-SFO);
, native sunflower (N-SFO); ![]() , heated sunflower (H-SFO);
, heated sunflower (H-SFO); ![]() , heated sunflower and GI (H-SFO + GI);
, heated sunflower and GI (H-SFO + GI); ![]() , heated sunflower and TU (H-SFO + TU).
, heated sunflower and TU (H-SFO + TU).

Fig. 2. Nitric oxide synthase-2 (NOS-2) (a and b) and intercellular adhesion molecule-1 (ICAM-1) (c and d) expression in the liver of rats fed native and repeatedly heated oil with or without ginger (GI) and turmeric (TU). Values are means and standard deviations of four rats. (a and c) ![]() , native rapeseed (N-CNO);
, native rapeseed (N-CNO); ![]() , heated rapeseed (H-CNO);
, heated rapeseed (H-CNO); ![]() , heated rapeseed and GI (H-CNO+GI);
, heated rapeseed and GI (H-CNO+GI); ![]() , heated rapeseed and TU (H-CNO+TU). (b and d)
, heated rapeseed and TU (H-CNO+TU). (b and d) ![]() , native sunflower (N-SFO);
, native sunflower (N-SFO); ![]() , heated sunflower (H-SFO);
, heated sunflower (H-SFO); ![]() , heated sunflower and GI (H-SFO + GI);
, heated sunflower and GI (H-SFO + GI); ![]() , heated sunflower and TU (H-SFO + TU).
, heated sunflower and TU (H-SFO + TU).

Fig. 3. Nitric oxide synthase-2 (NOS-2) (a and b) and intercellular adhesion molecule-1 (ICAM-1) (c and d) expression in the heart of rats fed native and repeatedly heated oil with or without ginger (GI) and turmeric (TU). Values are means and standard deviations of four rats. (a and c) ![]() , native rapeseed (N-CNO);
, native rapeseed (N-CNO); ![]() , heated rapeseed (H-CNO);
, heated rapeseed (H-CNO); ![]() , heated rapeseed and GI (H-CNO+GI);
, heated rapeseed and GI (H-CNO+GI); ![]() , heated rapeseed and TU (H-CNO+TU). (b and d)
, heated rapeseed and TU (H-CNO+TU). (b and d) ![]() , native sunflower (N-SFO);
, native sunflower (N-SFO); ![]() , heated sunflower (H-SFO);
, heated sunflower (H-SFO); ![]() , heated sunflower and GI (H-SFO + GI);
, heated sunflower and GI (H-SFO + GI); ![]() , heated sunflower and TU (H-SFO + TU)
, heated sunflower and TU (H-SFO + TU)

Fig. 4. Modulation of cardio-hepatic antioxidant defence response by ginger and turmeric lipid-solubles in heated oil-fed rats. ROS, reactive oxygen species; NOS-2, nitric oxide synthase-2; ICAM-1, intercellular adhesion molecule-1; NRF-2, nuclear factor erythroid 2-related factor 2.
Enzyme indicators of hepatic and cardiac function
The serum level of SGOT, SGPT, alkaline phosphatase and creatine kinase in the H-CNO and H-SFO groups increased significantly (P < 0·05) compared with their respective control (N-CNO and N-SFO) groups (Table 5), whereas, compared with the H-CNO group, the serum level of SGOT, SGPT, alkaline phosphatase and creatine kinase in H-CNO + GI and H-CNO + TU groups decreased significantly (P < 0·05). Likewise, when compared with the H-SFO group, the serum level of SGOT, SGPT, alkaline phosphatase and creatine kinase in H-SFO + G, I and H-SFO + TU groups decreased significantly (P < 0·05) (Table 5).
Table 5. Hepatic and cardiac function enzymes (n 8)
(Mean values and standard deviations)

N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; ALP, alkaline phosphatase; CK-MB, creatine kinase.
a,b,c,d,e Mean values within a row with unlike superscript letters were significantly different (P < 0·05 and q < 0·1).
Haematology, blood pressure and heart rate
No significant change was observed in the haematological parameters (leucocytes, erythrocytes, Hb, haematocrit, mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration and platelets count) between any of the groups studied (Table 6). The systolic blood pressure, diastolic blood pressure and heart rate increased significantly (P < 0·05) in H-CNO and H-SFO groups compared with their respective control (N-CNO and H-SFO) groups (Table 6), whereas, when compared with the H-CNO group, the systolic blood pressure and diastolic blood pressure, as well as the heart rate in H-CNO + GI and H-CNO + TU groups, decreased significantly (P < 0·05). Likewise, when compared with the H-SFO group, the systolic blood pressure and diastolic blood pressure, as well as the heart rate in H-SFO + GI and H-SFO + TU groups, decreased significantly (P < 0·05) (Table 6).
Table 6. Haematology, blood pressure and heart rate (n 8)
(Mean values and standard deviations)

N-CNO, native rapeseed; H-CNO, heated rapeseed; GI, ginger; TU, turmeric; N-SFO, native sunflower; H-SFO, heated sunflower; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; PLT, platelets; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate in beats/min.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
In this study, we showed that the long-term consumption of repeatedly heated oil (both n-6 and n-3) adversely affects cardio-hepatic antioxidant defence response and blood pressure in experimental rats. We evaluated cardio-hepatic responses to the heated oil-induced OS as the mutual interaction between heart and liver is crucial for the normal functioning of the body(Reference Fouad and Yehia24). Both heart and liver showed a similar response to heated oils, wherein the activity of critical antioxidant enzymes like catalase, superoxide dismutase, glutathione peroxidase and glutathione transferase was found to be reduced. However, when spices like ginger or turmeric were included during the heating process, the dampened cardio-hepatic antioxidant defence response was ameliorated. To decipher the mechanism for cardio-hepatic protection against OS by ginger and turmeric lipid-solubles, we analysed the NRF-2 transcription factor. NRF-2 is an emerging regulator of cellular resistance to OS and induces the expression of antioxidant response element-dependent genes(Reference Ma25). Nuclear translocation of NRF-2 following its dissociation from Kelch-like ECH-associated protein-1 complex and its subsequent binding to antioxidant response element triggers antioxidant enzyme expression needed to overcome the OS(Reference Kobayashi, Kang and Okawa26). As evident in this study, the drop in the nuclear translocation of NRF-2 in the heart and liver of heated oil-fed rats, but not in rats fed oil heated with ginger or turmeric, possibly resulted in the decrease of antioxidant defence response. The electrophile reaction with cysteines of Kelch-like ECH-associated protein-1 leads to the formation of adducts that prevent the ubiquitination of NRF2, resulting in its stabilisation, nuclear translocation and transcriptional induction of NRF2-target genes(Reference Cuadrado, Rojo and Wells27), and from this perspective, the modulation of NRF2 by lipid-solubles of ginger and turmeric needs to be further elucidated.
NOS-2 catalyses the production of NO, a powerful pro-oxidant, that causes tissue damage under various conditions(Reference McAdam, Haboubi and Forrester28). While relatively small amounts of NO produced by eNOS are essential to cardiovascular homoeostasis, high NO levels produced due to increased NOS-2 activity may have detrimental consequences to the cardiovascular system and contribute to hypertension(Reference Oliveira-Paula, Lacchini and Tanus-Santos29). ICAM-1 plays a critical role in the docking of the neutrophils to the endothelium, thus allowing the activated neutrophils to release its arsenals, mainly ROS and pro-inflammatory lipid mediators to trigger the tissue injury(Reference Mittal, Siddiqui and Tran30). As evident in this study, both NOS-2 and ICAM-1 were up-regulated in the heart and liver of rats fed heated oils, whereas their expressions were diminished in rats fed oils heated with ginger or turmeric. Thus, the protective effects of ginger and turmeric lipid-solubles through down-regulation of ICAM-1 and NOS-2 may beneficially alter the vascular tone. Our results support that the consumption of repeatedly heated oil (whether n-3 or n-6 rich) not only increases heart rate but also adversely affects the blood pressure(Reference Jaarin, Mustafa and Leong31), possibly through the up-regulation of vascular adhesion molecules, including ICAM-1(Reference Ng, Kamisah and Faizah32). Even though n-3 fatty acids are known to exhibit beneficial effects, both n-3 (present in CNO) and n-6 fatty acid (present in SFO) rich oils were equally detrimental to the cardio-hepatic response to OS when subjected to the repeated heating process.
In conclusion, our study established that chronic intake of repeatedly heated oils (whether n-3 or n-6 rich) induces loss of cardio-hepatic antioxidant defence response, thereby affecting their functional crosstalk. Lipid-solubles from ginger and turmeric that migrate to the oil during heating prevent the cardio-hepatic OS and blood pressure triggered by heated oils in rats. The amount of ginger (7·5 g dry powder/100 g oil) and turmeric (7·5 g dry powder/100 g oil) as used in this investigation may be reasonably adapted to human applications. Further characterisation of lipid-solubles from ginger and turmeric migrating to oil during heating and also their inclusion during the deep-frying process is warranted.
Acknowledgements
M. Z. acknowledges ICCR, New Delhi, for the award of a Research Fellowship and CSIR-CFTRI, Mysore, for providing the facilities.
This work was financially assisted by the 12th five-year plan project (Nutri-ARM, BSC-0404) from the Council of Scientific and Industrial Research.
M. Z. was responsible for the execution of the experiment; P. A. was responsible for data assessment with M. Z.; R. R. T. was responsible for the research question, design, data assessment and manuscript writing. All the authors that contributed to this manuscript approved the final version.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003967













