Diarrhoeal disease is the second leading cause of mortality in children, killing approximately 1·5 million children worldwide, annually(1). Weaning is a particularly vulnerable period, as the removal of maternal immune components coincides with the increased exposure to pathogens. Escherichia coli is a common causative agent of diarrhoea in infants and young children; numerous E. coli virotypes including enterotoxigenic E. coli (ETEC), enterohaemorrhagic, enteroinvasive and enteropathogenic E. coli exist(Reference Nweze2). ETEC induces diarrhoea via the expression of a choleragen-like enterotoxin that adheres to intestinal epithelial cells and induces oversecretion of electrolytes into the intestinal lumen. The piglet has been used extensively as a model for infant E. coli infection(Reference Nabuurs3).
A recent study has indicated that weaning suppressed adaptive immunity immediately after weaning and subsequently for about a week(Reference Juul-Madsen, Jensen and Nielsen4). In contrast, variables of the innate immune system seem to be stimulated immediately after weaning(Reference Juul-Madsen, Jensen and Nielsen4). The post-weaning period involves significant changes in the composition of immune cells in the blood and intestine(Reference Bianchi, Zwart and Jeurissen5–Reference Vega-Lopez, Bailey and Telemo8). At weaning, there is a change in the number and proportion of T-cells in the blood, which is associated with a reduced ability to respond to various challenges(Reference Bianchi, Zwart and Jeurissen5, Reference Bailey, Miller and Telemo9, Reference Darwich, Segales and Domingo10). Many antigens encountered by the immune system gain access to the body through mucosal surfaces such as the intestine and respiratory tract. Gut-associated lymphoid tissue is the largest immune organ of the body and is responsible for handling large quantities of potentially harmful antigens (reviewed in Forchielli & Walker(Reference Forchielli and Walker11)). Although gut-associated lymphoid tissue is an important immune tissue and is the first and most significant contact the immune system has with dietary antigens, few studies have examined the effect of incorporating specific nutrients into the weaning diet on the function of this tissue.
Experimental studies have shown that feeding glutamine (Gln) to infants(Reference van den Berg, van Elburg and Westerbeek12) and young animals(Reference Chamorro, de Blas and Grant13, Reference Yi, Carroll and Allee14) reduces the incidence of infections and infectious morbidity. These effects have primarily been attributed to the effect of Gln on the health of the intestinal epithelial cell(Reference DeMarco, Li and Thomas15). We have previously demonstrated that Gln modifies immune cells in the mesenteric lymph nodes of newly weaned piglets by supporting a Th1-type cytokine response after T-cell stimulation(Reference Johnson, Ball and Baracos6). Gln has also been shown to stimulate intestinal epithelial cell proliferation(Reference Scheppach, Dusel and Kuhn16) and reduce apoptosis(Reference Evans, Jones and Ziegler17). Gln is also an energy substrate for lymphocytes(Reference Wu, Field and Marliss18) and macrophages(Reference Wu, Field and Marliss19), is important for the optimal function of T- and B-cells (reviewed in Newsholme(Reference Newsholme20)) and is required for neutrophil bactericidal function(Reference Ogle, Ogle and Mao21). Gln increases intestinal expression of genes related to growth and antioxidant function, and preserves epithelial barrier function in the distal ileum of 21-d-old piglets during infection(Reference Dugan and McBurney22, Reference Wang, Chen and Li23). These observations have lent support for the concept that this amino acid may become transiently essential during periods of immune stress (reviewed in Field et al. (Reference Field, Thomson and Van Aerde24)), notably, the weaning period. When provided orally to rodents, Gln preserved intestinal metabolism, structure and function by accelerating healing of the gut mucosa in irradiated animals through increased mitosis in the proliferative zone of the villous crypts(Reference Klimberg, Salloum and Kasper25). Similarly, Gln supplementation has been shown to prevent villous atrophy in early-weaned piglets(Reference Wu, Meier and Knabe26).
Despite the important roles attributed to Gln in clinical nutrition(Reference Dhaliwal and Heyland27), supplementation during the vulnerable weaning period has received little attention. The objective of the present study was to determine the ability of dietary supplementation with Gln (4·4 %, w/w) to improve immune and gastrointestinal function and defence against an ETEC challenge in the early post-weaning period in a piglet model. As the development of a reproducible orally induced model of ETEC infection in the piglet has proved problematic(Reference Nabuurs3, Reference Sarmiento, Casey and Moon28), an in vivo closed intestinal loop model of ETEC infection was used to produce the early signs of ETEC infection.
Methods
Animals and diets
The study was reviewed and approved by the Faculty of Agriculture, Forestry and Home Economics Animal Policy and Welfare Committee, and was conducted in accordance with the Canadian Council on Animal Care guidelines. A total of four litters (ten piglets per litter) of Dutch Landrace (Genex Swine Group, Heartland Livestock Services, Regina, SK, Canada) piglets were obtained at weaning (21 d) from the University of Alberta Swine Research and Technology Centre (Edmonton, AB, Canada), and were housed individually in metabolism crates, each fitted with water nipples and creep feeders. Upon receipt, piglets in each litter were randomly assigned to one of the two diet treatments: (1) basal diet with a control mixture of amino acid supplement (CTL); (2) basal diet with Gln supplement (Gln) (Table 1). These diets were formulated to meet 110 % of the requirements for piglets weighing 5–10 kg as specified by the National Research Council of Canada. The supplement portion of the Gln diet (60 g/kg) consisted of 43·8 g Gln, and the CTL diet consisted of an isomolar, isonitrogenous mixture of amino acids (alanine, glycine and serine), which have limited or no metabolic interaction with Gln and are not known to limit the growth or immune function in piglets of this age(Reference Wu29). Gln supplementation level was designed to be consistent with an approximate amount (on a diet proportion) that has been added in adult human clinical studies that found beneficial effects on immune function and infection rates(Reference Field, Johnson and Pratt30, Reference Field31). The variable portion of the diet was isonitrogenous and was made isoenergetic by balancing the supplement with sucrose. Piglets were fed the test diets for 14 d from weaning. Body weight and feed intake (FI) were recorded daily during the feeding trial.
Table 1 Nutrient composition of the weaning diets
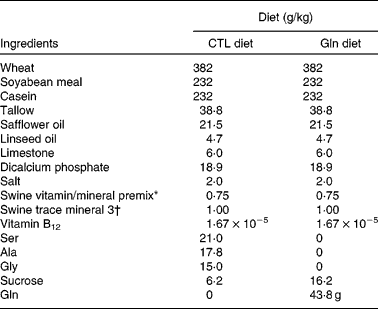
CTL, control; Gln, glutamine.
* Vitamin premix contained (%, w/w): protein, 3·8912 %; fat, 0·99 %; moisture, 2·701 %; digestible energy, 359·8 kJ/kg; metabolisable energy, 343·1 kJ/kg; Ca, 25·7937 %; P, 0·225 %; available P, 0·0765 %; Mg, 0·1282 %; Na, 0·0328 %; Fe, 642·2313 mg/kg; vitamin A, 7 000 014·2 IU(100 004·3 μg)/kg; vitamin D3, 700 014·2 IU(17 500·4 μg)/kg; vitamin E, 20 000·0117 IU(181 818 288 163 μg)/kg; vitamin K, 1500·0179 mg/kg; biotin, 40·0001 mg/kg; folic acid, 399·9008 mg/kg; niacin, 20 000·0117 mg/kg; pantothenic acid, 7499·9741 mg/kg; pyridoxine, 533·9845 mg/kg; riboflavin, 3000·0051 mg/kg; thiamin, 580·5812 mg/kg; vitamin B12, 10 mg/kg.
† Trace mineral premix contained (%, w/w): fat, 0·99 %; moisture, 0·001 %; digestible energy, 359·8 kJ/kg; metabolisable energy, 343·1 kJ/kg; Ca, 15·1874 %; Mg, 0·0729 %; Na, 0·0283 %; S, 4·756 %; Co, 351 mg/kg; Cu, 5000 mg/kg; I, 749·3 mg/kg; Fe, 75 365·1328 mg/kg; Mn, 25 020 mg/kg; Se, 150 mg/kg; Zn, 75 024 mg/kg; choline, 100·001 g/kg.
In situ surgical procedure
At approximately 35 d of age, animals underwent an in situ closed intestinal loop ETEC infection procedure. A single blood sample was taken from each piglet before receiving anaesthesia for isolation of peripheral blood mononuclear cells. Piglets received intramuscular injections of Torbugesic (0·2 mg/kg), Ketamine (11 mg/kg), Rompun (2·2 mg/kg) and Robinul (0·01 mg/kg). Anaesthesia was maintained with 1·0–1·5 % (v/v) halothane delivered with oxygen (3 litres/min). The abdominal wall was opened by a midline incision, and the ileum was exteriorised and gently flushed with PBS to remove the intestinal contents. Intestinal loops (each 10 cm in length and 50 cm apart) were ligated with braided umbilical tape (Baxter International, Inc., Deerfield, IL, USA). The first intestinal loop was located 15 cm from the ligament of Treitz. Each loop was injected with either an ETEC suspension (K88AC or K88 wild-type (WT), approximately 1 × 109 colony-forming units/ml, described later) or PBS (negative control). Following an incubation period of 4 h, euthanasia was induced by cardiac injection of pentobarbital (Euthanyl; 2 ml/4·5 kg body weight), and the gut sections were removed and immersed in ice-cold PBS. The amount of fluid inoculated into each loop was recorded, and the volume recovered from each loop was measured at the end of the experiment. Mucosal scrapings from each segment were snap-frozen in liquid N2 and stored at − 80°C. Mesenteric lymph nodes were excised adjacent to the distal ileum (10–20 cm before the ileocaecal junction), immersed in ice-cold PBS and stored on ice until processing.
Bacterial preparation
E. coli cultures were prepared fresh from frozen stock immediately before each surgery. We chose two representative swine ETEC strains: one well-characterised strain known to express the K88AC fimbrial antigen and one K88+ WT field isolate. To ensure that we included a strain that was infective to piglets, we also used a WT strain, isolated from infected piglets. K88AC ETEC was kindly provided by Dr Marquardt (University of Manitoba, Winnipeg, MB, Canada). K88WT field isolate was kindly provided by Dr Nick Nation (VPL Laboratories, Edmonton, AB, Canada). To prepare fresh ETEC cultures, ETEC frozen culture was inoculated into 6 ml of brain heart infusion medium (Oxoid Limited, Basingstoke, Hampshire, England, UK) and grown for 24 h at 37°C with shaking, then subcultured for 24 h. The final culture was prepared by 12 h incubation of a 1:100 dilution of the subculture into brain heart infusion media. Bacteria were diluted in PBS to a final concentration of approximately 1 × 109 colony-forming units/ml. The presence of K88 fimbriae was confirmed in each culture by a latex agglutination test (Vetway Fimbrex K88; Central Veterinary Laboratory, Addlestone, Surrey, UK) immediately before the surgical procedure.
Epithelial monolayer barrier integrity measurements
Within 1 h of death, a small segment from each intestinal loop was removed, transported in ice-cold PBS, stripped of the serosa and mounted in an Ussing chamber. Once mounted, sections were bathed in a bicarbonate Ringer's solution (143 mm-Na+, 5 mm-K+, 1·1 mm-Mg, 1·25 mm-Ca2+, 25 mm-HCO3− , 123·7 mm-Cl− , 0·3 mm-HPO4− and 20 mm-fructose) with 95 % O2 and 5 % CO2 at 37oC, pH 7·4. Permeability was measured via scintillation counter (β-ray scintillation; Beckman Coulter, Fullerton, CA, USA) to determine the flux of [3H]mannitol (37 MBq; 1 mCi) across individual ileal specimens. The spontaneous transepithelial potential difference (PD) and short-circuit current (I sc) were determined for all segments, and tissue conductance (G) was calculated from PD and I sc according to Ohm's law. At the end of the sampling period, forskolin (10 μl; Sigma) was added to the serosal chamber, and the peak change in I sc was recorded, in order to assess tissue viability.
RNA isolation and PCR analysis of cytokines
Mucosal samples were ground to a fine powder under liquid N2 using mortar and pestle on dry ice. RNA was extracted using TRIzol reagent (Invitrogen, Burlington, ON, Canada), following the manufacturer's instructions with a slight modification. RNA was precipitated with isopropanol and linear glycogen overnight at − 20°C. The total RNA concentration of each sample was quantified spectrophotometrically using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). DNA-free kit (Ambion, Streetsville, ON, Canada) was used to remove any DNA contamination. First-strand complementary DNA synthesis using SuperScript II RT (Invitrogen) on 3 μg RNA in a 30 μl total volume with random primers was done following the manufacturer's guidelines. Real-time PCR was performed on a 7900 HT fast real-time PCR system using 1 μl (approximately 100 ng) complementary DNA, 8 μl diethylpyrocarbonate water, 10 μl TaqMan Fast Universal PCR Master Mix and 1 μl TaqMan Gene Expression Assay. Cycle threshold (C t) values were determined using SDS 2.3 software. The gene of interest was normalised to the C t value of our endogenous reference gene, cyclophilin, using the ΔC t method described by Pfaffl(Reference Pfaffl and Bustin32, Reference Pfaffl33). The primer/probe sequences are listed in Table 2.
Table 2 RT-PCR primers 5′–3′

IFN, interferon; TGF-β, transforming growth factor-β.
Western blotting
Protein lysates were prepared from intestinal mucosal scrapings by PARIS™ Protein and RNA Isolation System (Ambion, Austin, TX, USA). The protein concentration of the lysates was determined by bicinchoninic acid assay (Sigma-Aldrich Canada Limited, Oakville, ON, Canada). Equal amounts of protein from each treatment (20 μg) were separated by SDS-PAGE on 7·5 % (Zona occludens-1) and 10 % (claudin-1 and occludin) polyacrylamide gels. ECL DualVue™ Western blotting markers (Amersham Biosciences, Baie D'Urfe, QC, Canada) were used to monitor protein separation. Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Amersham Biosciences). Even protein loading and transfer was confirmed by staining with Ponceau S (Sigma-Aldrich Canada Limited). Membranes were blocked for 1 h at room temperature with TBST (10 mm-Tris–HCl, pH 7·4, 150 mm-NaCl, Tween-20 (1 ml/l)) and powdered milk (50 g/l). Primary antibodies to claudin-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and occludin (Santa Cruz Biotechnology, Inc.) were diluted (1:750 and 1:1500, respectively) in TBST containing powdered milk (50 g/l) and incubated with membranes for 1 h at room temperature. Membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (BD Biosciences, Mississauga, ON, Canada) diluted in TBST containing powdered milk (50 g/l) for 1 h at room temperature. Membranes were developed using an enhanced chemiluminescence (ECL Plus™) detection kit (Amersham Biosciences) and visualised using a Typhoon Trio™ Imager (GE Healthcare, Baie D'Urfe, QC, Canada). Densitometric values for protein bands were determined using ImageQuant™ software (GE Healthcare).
Statistical analysis
All statistical analyses were completed using the SAS statistical package (version 8.1; SAS Institute, Cary, NC, USA). Diet effect was tested using ANOVA, blocked for litter. For epithelial barrier integrity measures and fluid recovery, the effect of diet and loop was determined using a mixed model, with loop comparisons performed by least-squares means, with blocking for piglet litter. Repeated-measures analysis was also performed on Ussing chamber data. For all measurements, a probability of P < 0·05 was accepted as being statistically significant. Significant differences between groups were identified by least-square means. All results are presented as means with their standard errors. All measured parameters were tested for normal distribution. Values that were not normally distributed were log transformed before statistical analysis.
Results
Animal weight gain and food intake
There was no effect of diet on final body weight, weight gain or food intake (Table 3).
Table 3 Body weight (BW) and food intake of piglets fed the glutamine (Gln) and control (CTL) diets
(Mean values with their standard errors)

Water
Analysis of fluid recovery data revealed a significant loop effect in which K88AC- and K88WT-inoculated loops were significantly different than the loops that were not inoculated with ETEC (P < 0·0001; Fig. 1). Gln did not significantly impact fluid secretion in non-inoculated, K88AC- or K88WT-inoculated loops (P = 0·069, 0·70 and 0·09, respectively).
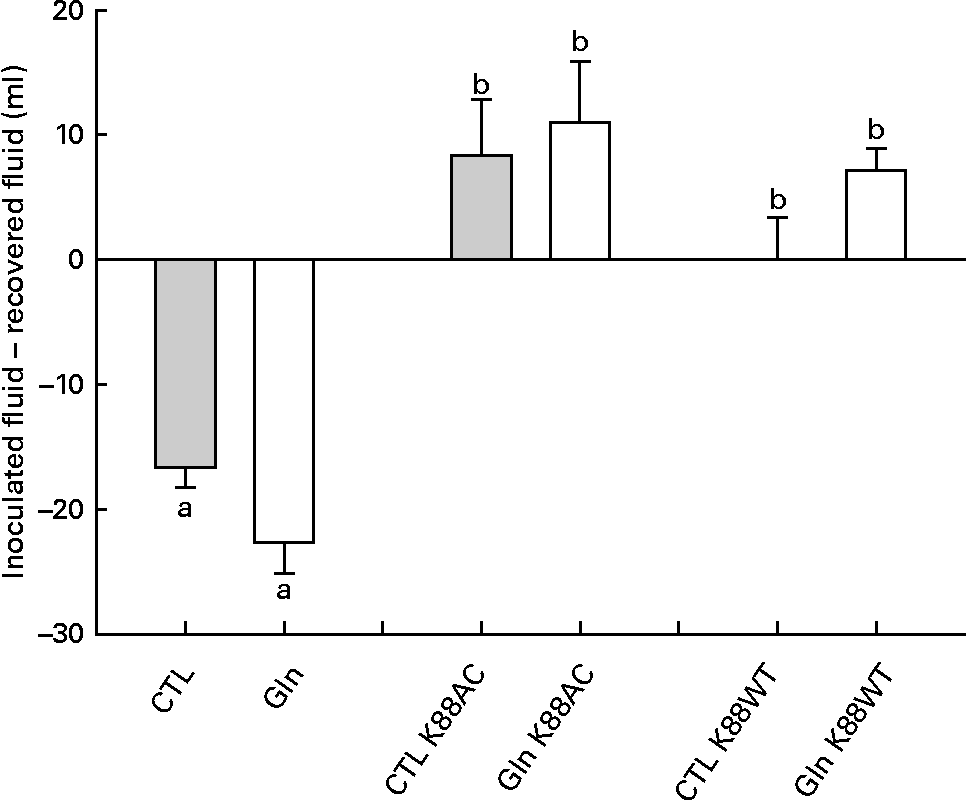
Fig. 1 Fluid recovery from intestinal loops. Fluid volume change in isolated intestinal loops inoculated with or without Escherichia coli from piglets fed the glutamine (Gln) or control (CTL) diets. Values are means of recovered fluid − inoculated fluid, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
Epithelial monolayer barrier integrity measurements
Within both groups, mannitol flux was significantly higher in intestinal segments inoculated with ETEC (K88AC and K88WT) than non-ETEC segments (P < 0·0001; Fig. 2(A)). Diet did not influence mannitol permeability in either the non-inoculated loops or the ETEC-inoculated loops (Fig. 2(A)).
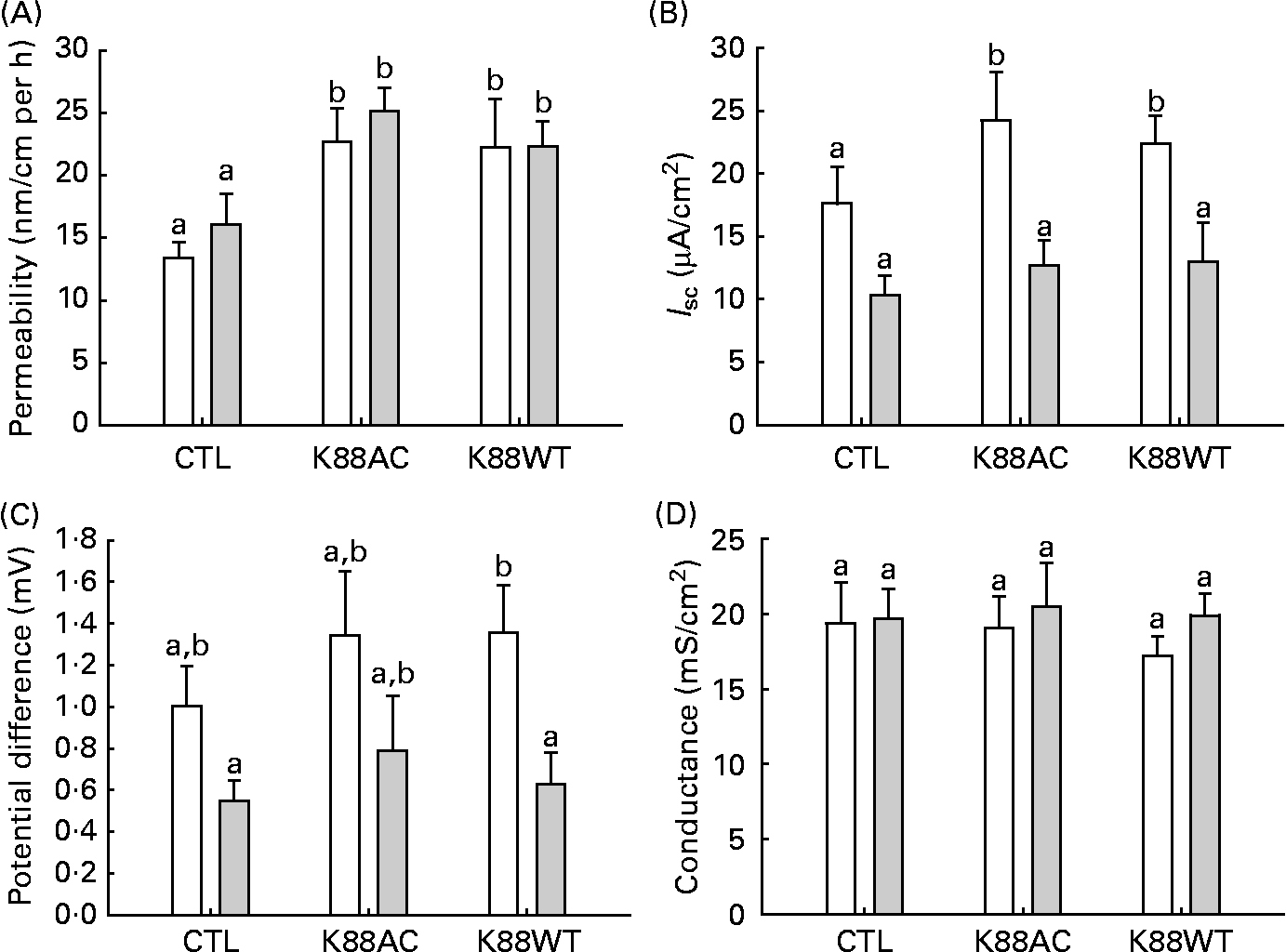
Fig. 2 Ussing chamber analysis. (A) Jejunal mannitol permeability, (B) short-circuit current (I sc), (C) potential difference from piglets fed the glutamine (Gln, ![]() , n 10) and control (CTL, □) diets (n 10) and (D) conductance. Loops were incubated without Escherichia coli (CTL) or with one of the two E. coli strains (K88AC or K88WT). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
, n 10) and control (CTL, □) diets (n 10) and (D) conductance. Loops were incubated without Escherichia coli (CTL) or with one of the two E. coli strains (K88AC or K88WT). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
Electrical measurements of intestinal loop tissue mounted in Ussing chambers revealed that inoculation with K88AC caused an increase in I sc in control-fed animals (Fig. 2(B)) compared with the CTL loops. Gln feeding resulted in decreased I sc values in both K88AC- and K88WT-inoculated loops (Fig. 2(B)).
CTL animals had a higher PD in the ETEC-inoculated loops compared with Gln animals, which reached statistical significance in the K88WT-inoculated loops (Fig. 2(C)). All groups responded to forskolin equally at the end of the incubation period, indicating similar viability (change in I sc; data not shown). No differences in conductance (G) were observed between the groups (Fig. 2(D)). These results confirm that both E. coli strains produced the expected effects on the gut physiology, therefore only the well-characterised K88AC E. coli strain was used to study the effect of Gln on cytokines and tight-junction proteins.
Intestinal cytokine expression
Neither Gln nor the E. coli (K88AC) challenge significantly influenced the expression of IFN-γ in the mucosa of the intestinal loops (Fig. 3). The presence of ETEC stimulated TNF-α and IL-1β expression in both Gln and CTL diets compared with uninfected loops (P < 0·01; Fig. 3). The expression of IL-6 was significantly increased in ETEC-infected loops of control-fed animals compared with uninfected loops, but there was no difference in IL-6 expression in Gln-fed animals between the control and ETEC loops. Transforming growth factor-β and IL-10 were significantly higher in the ETEC-challenged intestinal loops compared with the control loop in control-fed animals, but in Gln-fed animals (P < 0·05), no differences were observed in these cytokines between the ETEC-infected and control loops (Fig. 3). Gln supplementation did not significantly alter the expression of any measured cytokine in ETEC-infected loops compared with the CTL diet.
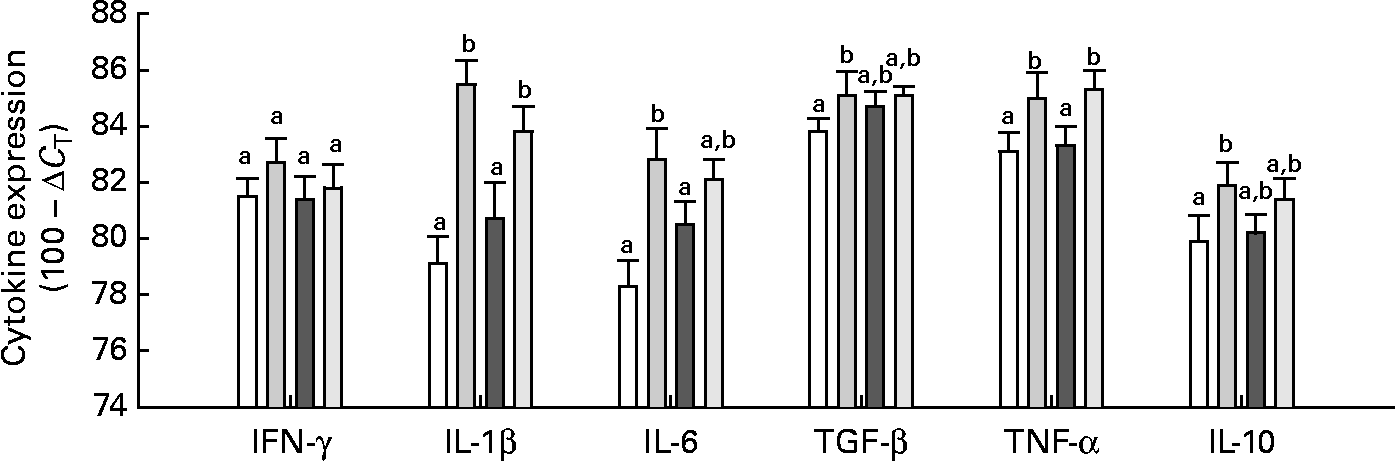
Fig. 3 Intestinal cytokine expression. Values are means with their standard errors represented by vertical bars (gene-specific cytokine RNA expressed as 100 − ΔC T (eight per group)). ΔC T was calculated by correcting to the level of 18S (which was not significantly affected by diet or Escherichia coli). a,b Mean values with unlike letters were significantly different as determined by a repeated-measures ANOVA and least-square means (P ≤ 0·05). IFN, interferon; TGF-β, transforming growth factor-β; □, control (CTL); ![]() , CTL+E. coli;
, CTL+E. coli; ![]() , Gln;
, Gln; ![]() , Gln+E. coli.
, Gln+E. coli.
Effects on tight-junction proteins
There was a significant decrease in the levels of occludin and claudin-1 in the mucosa from E. coli-challenged loops (P < 0·002; Fig. 4). There was no significant effect of diet on the expression of claudin-1 in the mucosa. After the ETEC challenge, there was a trend (P = 0·06) towards a higher expression of occludin in Gln-fed piglets, compared with CTL piglets.
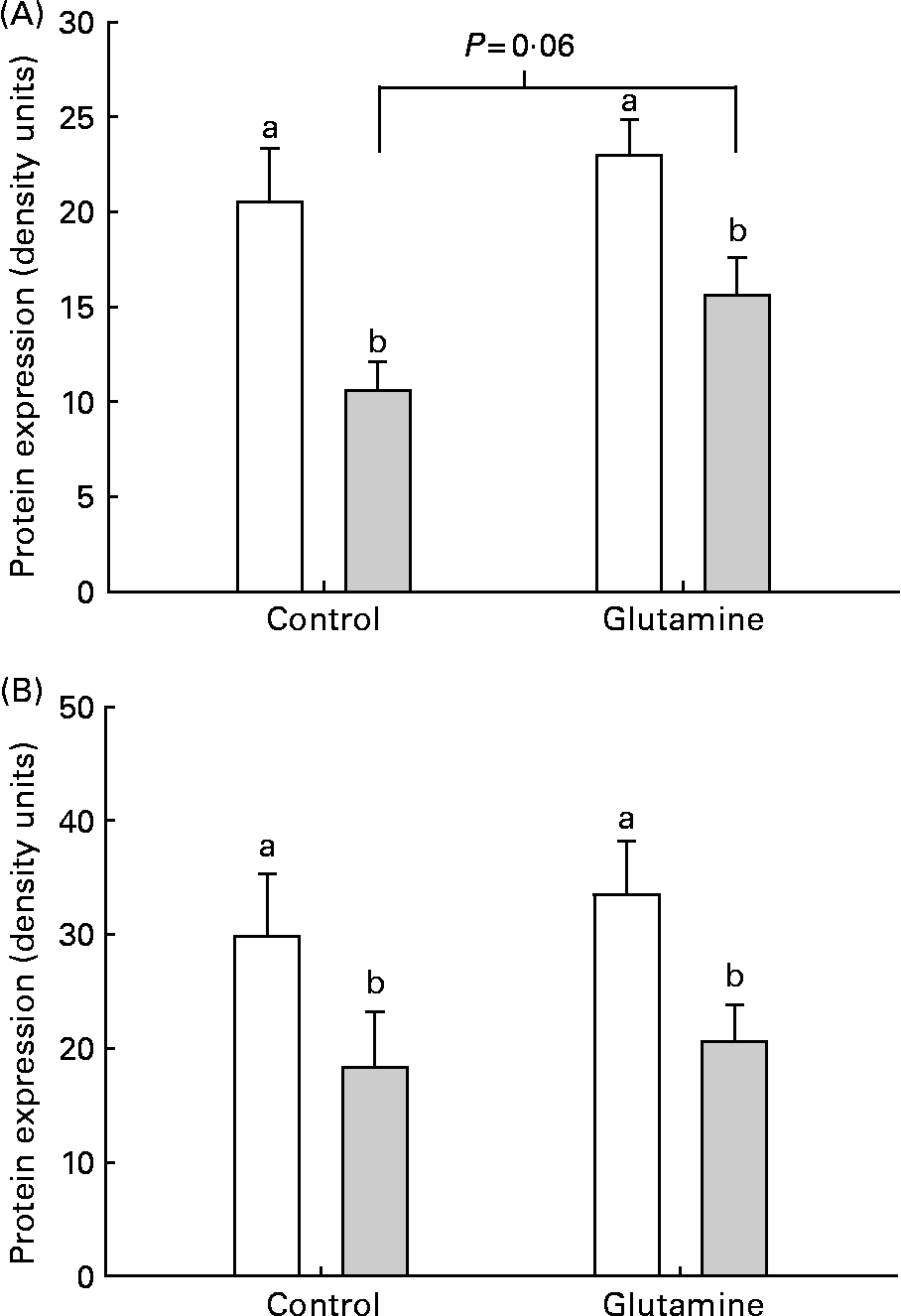
Fig. 4 (A) Occludin and (B) claudin-2 and protein expression in intestinal mucosa from piglets fed the glutamine (Gln) or control (□) diets. a,b Mean values were significantly different in the levels of occludin and claudin-1 in the mucosa from Escherichia coli (![]() )-challenged loops (P < 0·002). There was no significant effect of Gln on the expression of claudin-1 in the mucosa.
)-challenged loops (P < 0·002). There was no significant effect of Gln on the expression of claudin-1 in the mucosa.
Discussion
In the present study, we investigated the impact of feeding Gln on immune and intestinal barrier function in a piglet model of weaning-associated ETEC infection. We found that piglets fed Gln before E. coli infection had decreased intestinal PD and I sc compared with control-fed animals. We also observed a significantly increased expression in mucosal cytokine mRNA expression (IL-6, transforming growth factor-β and IL-10) in response to ETEC infection in control-fed animals, but not in Gln-fed animals. Fluid secretion and tight-junction protein expression were both negatively altered by E. coli infection in both control-fed and Gln-fed piglets.
FI was slightly lower in the present study than that in a similar study of Gln supplementation (0–1 %) of 21 d weaned piglets(Reference Wu, Meier and Knabe26). In the present study, average daily FI was approximately 174 g/d over the 14 d experimental period, whereas the above-mentioned study reports FI in two periods: the first week post-weaning (about 140 g/d) and the second week post-weaning (about 350 g/d). This makes the direct comparison difficult; however, it does appear that there was probably a lower FI in the present study. Given that the control-fed and Gln-fed piglets consumed the same amount of feed in the present study, it seems unlikely that it is due to Gln supplementation, which was significantly higher in the present study (4·38 v. 1 %). This difference may be attributable to the breed selected – the present study was performed using Dutch Landrace cross piglets, whereas Wu et al. (Reference Wu, Meier and Knabe26) utilised Yorkshire × Landrace sows and Duroc × Hampshire boars.
ETEC causes diarrhoea by adhering to intestinal epithelial cells via pili or fimbriae and subsequently producing enterotoxins (heat-stable and heat-labile) (reviewed in Gyles(Reference Gyles34)), which lead to a decrease in the absorption of electrolytes by the villus cells and an increase in Cl− secretion by the crypt cells. This rapid increase in the rate of secretion of electrolytes and water from the intestine leads to diarrhoea. The immune system becomes activated by the presence of ETEC on intestinal cells, and inflammation ensues(Reference Al-Sadi, Boivin and Ma35, Reference Duchmann, Neurath and Marker-Hermann36). This process was in agreement with the present study, as shown by the higher fluid absorption in non-ETEC-exposed loops (demonstrated by the lower recovery of liquid from these loops). We found that dietary Gln supplementation before an E. coli challenge did not prevent the increase in fluid secretion. In contrast, Silva et al. (Reference Silva, Santos-Neto and Soares37) used a rabbit model (cholera toxin-infected intestinal loop sections) and found that Gln-supplemented oral rehydration solution was capable of reducing water secretion. Similarly, PG-induced secretion has been reported to be reduced in the presence of Gln infusion in human subjects(Reference Coeffier, Hecketsweiler and Hecketsweiler38). These discrepancies may be attributable to the use of Gln after infection is initiated, whereas in the present study, Gln supplementation occurred as a dietary pre-treatment to E. coli infection, rather than an intestinal infusion at the time of induction of secretion. Furthermore, since the precise ionic composition of the luminal contents is unknown in the present study, the aetiology of altered fluid secretion is unknown.
We also observed solute movement across the paracellular pathway, via measurement of the unidirectional serosal-to-mucosal flux of mannitol. A significantly higher mannitol permeability was found in loops inoculated with ETEC (K88AC and K88WT), indicating an increased intestinal permeability in the presence of ETEC. An increase in permeability due to the presence of pathogens has previously been reported in studies of in vivo infection by transmissible gastroenteritis virus(Reference Keljo, Butler and Hamilton39) and rotavirus(Reference Isolauri, Kaila and Arvola40) in piglets and rats, respectively. Reduced enterotoxin-induced enterocyte death(Reference Haynes, Li and Li41) and decreased bacterial translocation(Reference Gianotti, Alexander and Gennari42) have also been reported following oral administration of Gln. In the present study, there was no significant effect of Gln supplementation on mannitol permeability before or after an intestinal E. coli challenge. Dietary Gln has been shown in numerous studies to exert trophic effects on the intestinal epithelium, leading to increased villus height, improved mucosal integrity and cell proliferation(Reference Wang, Chen and Li23, Reference Baskerville, Hambleton and Benbough43, Reference Domeneghini, Di and Bosi44). Since an increase in surface area would also increase mannitol permeability, diet-induced changes could potentially be masked if enterocyte proliferation and surface area were increased.
In the present study, jejunal intestinal sections did not demonstrate a significant change in G in response to ETEC in control-fed or Gln-fed piglets. The forskolin response (change in I sc) was significantly higher in the intestinal loops from Gln-fed piglets infected with both K88AC and K88WT with ETEC exposure compared with the CTL-fed piglets. Similarly, PD was higher in K88WT ETEC-infected loops from Gln-fed animals compared with CTL-fed animals. Tissue I sc in Ussing chambers is a measure of the net movement of several actively transported ions, which significantly increases following the addition of heat-stable enterotoxin to rabbit distal ileal sections(Reference Guandalini, Rao and Smith45). PD is also related to ion movement but measures changes in the electrochemical gradient across the intestinal epithelium that is generated by electrogenic ion pumps in epithelial cell membranes, mainly the Na–K-ATPase(Reference Armstrong, Taylor and Torrence46). Although the movement of individual ions was not measured in the present study, the higher I sc and PD values in ETEC-infected loops of CTL-fed piglets compared with Gln-fed piglets are suggestive of an increased Cl− secretion caused by ETEC. It is possible that enterocytes of Gln-fed piglets were not influenced to the same extent by the presence of ETEC, either by being less responsive to secreted enterotoxins or because ETEC was not able to bind and release enterotoxins to the same magnitude. The decrease in intestinal cytokine production that we observed could similarly be attributed to reduced binding and enterotoxin production of ETEC.
Alternately, Gln may have had a direct effect on intestinal cytokine production. Feeding Gln has been shown to reduce the production of the pro-inflammatory cytokines IL-6 and IL-8, in response to IL-1β stimulation in human intestinal mucosa(Reference Coeffier, Marion and Ducrotte47). Consistent with the present study, Gln supplementation has also been shown to reduce plasma inflammatory cytokine concentrations (TNF-α and IL-6) in response to E. coli lipopolysaccharide-induced shock, via induction of HSP70 expression(Reference Jing, Wu and Wang48). In that study, Gln also enhanced the production of the regulatory cytokine, IL-10(Reference Guandalini, Rao and Smith45). In the present study, there was an increase in IL-10 in the intestines from Gln-fed piglets compared with control-fed piglets.
Although it has been demonstrated that feeding Gln can reduce the risk of enteric infections in piglets(Reference Yi, Carroll and Allee14) and infants(Reference van den Berg, van Elburg and Westerbeek12), the relative contribution of dietary Gln to immune development and health has not been clearly established. In a previous study by our group, healthy piglets feeding a weaning diet supplemented with Gln exhibited enhanced immune function, including increased proliferation of peripheral blood mononuclear cells and mesenteric lymph node cells after an antigen challenge and prevention of an increase in antigen-naive CD4+ cells(Reference Johnson, Ball and Baracos6). To prevent post-weaning ETEC-induced diarrhoea, an activated mucosal immunity system is required(Reference Snoeck, Huyghebaert and Cox49). The process by which animals meet the immediate response to infection is influenced by inflammatory cytokines produced primarily by macrophages and professional antigen-presenting cells(Reference Murtaugh and Foss50). It is possible that this activated mucosal immunity in turn alters tight-junction protein expression, as pro-inflammatory cytokines are known to induce endocytosis of tight-junction proteins and subsequently cause increased permeability(Reference Al-Sadi, Boivin and Ma35).
In summary, the results of the present study suggest that Gln supplementation during the weaning period is useful in reducing early steps in weaning-related gastrointestinal infections by suppressing the inflammatory and regulatory cytokine response in the gut and decreasing damage to tight junction proteins and intestinal electrolyte movement.
Acknowledgements
The present study was supported by the Canadian Institutes of Health Research and Alberta Pork with the Alberta Agriculture Research Institute. The authors would like to acknowledge the technical assistance provided by Susan Goruk, Michelle Tavernini and Grace Chen, and the excellent animal care provided by the outstanding technical assistance of the animal technicians Brenda Tchir and Charlene Gorsak at the University of Alberta Metabolic Unit. J. B. E. participated in the data analysis, and drafted and edited the manuscript. G. K. M. designed the PCR probes, conducted the cytokine analysis and participated in the data analysis. I. R. J. carried out the studies and conducted the preliminary data analysis. K. L. M. participated in the study design and carried out the Ussing chamber analyses. C. J. F. conceived the study, participated in its design, coordination and supervision. There are no financial or personal conflicts of interest to report.









