Chronic liver disease is a common pathology worldwide. Non-alcoholic fatty liver disease (NAFLD) has become increasingly common, indicating that obesity or ectopic fat induces an innate immune response with subsequent recruitment of immune cells such as macrophages and T cells, leading, ultimately, to the development of insulin resistance and non-alcoholic steatohepatitis (NASH). Thus, NAFLD can be defined as lipotoxic liver damage and can impair insulin resistance in the whole body as well as progress to NASH( Reference Loguercio, De Girolamo and Federico 1 – Reference Cusi 3 ). Antioxidant micronutrients such as carotenoids and tocopherols exist in abundance in fruits and vegetables and have been known to contribute to the body’s defence against reactive oxygen species( Reference Gutteridge 4 , Reference Rock, Jacob and Bowen 5 ). It is known that antioxidant vitamins and carotenoids are reduced in several liver diseases such as hepatitis and cirrhosis( Reference Munoz, Heubi and Balisteri 6 – Reference Rocchi, Casalgrandi and Ronzoni 10 ). Oxidative stress is thought to play a key role in the pathogenesis of liver injury. Therefore, antioxidant micronutrients such as α-tocopherol and β-carotene can be expected to protect against liver injury. In fact, some recent intervention studies indicate that a diet high in antioxidants including vitamins C and E and carotenoids or pharmacological supplements of vitamins C and E reduced serum liver enzymes( Reference Valtueña, Pellegrini and Franzini 11 – Reference Arendt and Allard 13 ). These recent findings have attracted attention to the idea that nutritional approaches rather than medication therapy might be readily accepted as a recommended strategy for preventing liver diseases. Thus, there is a wide range of studies on the relationship between nutrition and liver disease. On the other hand, an epidemiological approach concerning the association between antioxidant carotenoid levels and liver disease has not been well tested.
However, one large cross-sectional study has been reported. Ruhl & Everhart( Reference Ruhl and Everhart 14 ) revealed that the risk for apparent liver injury was associated with decreased antioxidants, particularly carotenoids, in the Third National Health and Nutrition Examination Survey in the USA. Furthermore, we previously found that serum carotenoids were inversely associated with serum liver enzymes( Reference Sugiura, Nakamura and Ikoma 15 , Reference Sugiura, Nakamura and Ikoma 16 ). On the basis of these previous studies, we cross-sectionally examined the association of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltransferase with the serum carotenoid concentrations and found that serum provitamin A carotenoids, especially β-carotene and β-cryptoxanthin, were inversely associated with these serum liver enzymes. These findings support our hypothesis that antioxidant carotenoids may act as suppressors against the development of liver injury. However, these data were from cross-sectional analyses. Therefore, only limited inferences can be made regarding temporality and causation. To determine whether antioxidant carotenoids are beneficial micronutrients for preventing liver disease in Japanese subjects, further cohort studies will be required.
The objective of this study was to longitudinally investigate whether the risk of developing elevated serum ALT is associated with basal serum carotenoid concentrations in middle-aged and older Japanese subjects. The associations of six serum carotenoid concentrations – lutein, lycopene, α-carotene, β-carotene, β-cryptoxanthin and zeaxanthin – with elevated serum ALT were evaluated longitudinally. This is the first longitudinal cohort study to investigate the association between serum carotenoids and the risk for developing elevated serum liver enzymes.
Methods
Ethics statement
This study was carried out in accordance with the Declaration of Helsinki and was approved by the ethics committee of the NARO Institute of Fruit Tree Science and the Hamamatsu University School of Medicine. We obtained written informed consent from all the participants involved in our study.
Study population
This was a prospective survey involving participants of the Mikkabi cohort study conducted in the town of Mikkabi, Shizuoka Prefecture, Japan. The Mikkabi cohort study involved two cohorts, one recruited in 2003 (cohort I) and the other in 2005 (cohort II). The study design has been described previously( Reference Sugiura, Nakamura and Ogawa 17 ).
After a baseline survey (2003 or 2005), subjects were invited to participate in follow-up surveys in 2005 (for cohort I), 2006, 2007, 2008, 2009 and 2013. Subjects in cohort I were followed-up from 2005 and subjects in cohort II from 2006 through September 2013. In this manner, 2-year follow-up data were obtained from forty participants. In the same way, 3-, 4-, 5-, 6-, 8- and 10-year follow-up data were obtained from forty-one, ninety-five, forty-seven, 161, ninety-four and 432 participants, respectively. In total, from the six follow-up surveys, 910 subjects (295 males and 615 females) took part in a follow-up survey at least one time. The follow-up rate was 84·9 %. The person-years of follow-up were calculated for each subject from the starting point to the date of the onset of elevated serum ALT. For this study, we excluded subjects suffering from elevated serum ALT (>30 IU/l) at the baseline survey as defined by the Japan Society of Ningen Dock( 18 ). We also excluded persons with other common causes of liver disease (≥60 g of alcohol intake/d and positive serum hepatitis B surface antigen). In addition, those who reported a history of infection with hepatitis C and/or medication use for liver disease in the self-administered questionnaire at the baseline survey were also excluded. As a result, a total of 213 male and 574 female subjects were included for further data analysis.
Blood and anthropometric measurements
In the baseline survey, concentrations of six serum carotenoids – lutein, lycopene, α-carotene, β-carotene, β-cryptoxanthin and zeaxanthin – were analysed by reverse-phase HPLC using β-apo-8'-carotenal as an internal standard at the Laboratory of Public Health and Environmental Chemistry, Kyoto Biseibutsu Kenkyusho (Kyoto, Japan), as described previously( Reference Sugiura, Nakamura and Ikoma 15 ). All blood parameters measurements, except for serum carotenoid concentrations, were obtained at the laboratory of the Seirei Preventive Health Care Center (Shizuoka, Japan). Each subject’s height and body weight were measured by trained public-health nurses. BMI was calculated as body weight (kg) divided by height (m2). Blood pressure was measured using an automated sphygmomanometer, model BP-103iII (Nihon Colin Inc.).
Self-administered questionnaire
A self-administered questionnaire was used to collect information about subject history regarding chronic disease, medication, lifestyle and dietary intake as described previously( Reference Sugiura, Nakamura and Ogawa 17 ). The assessment of diet was a modification of the validated self-administered 121-item simple FFQ developed especially for the Japanese. Information about alcohol intake and the daily intake of nutrients from foods was estimated from monthly food intake frequencies using the FFQ analysis software package for Windows (Food Frequency Questionnaire System; System Supply Co. Ltd) as described previously( Reference Sugiura, Nakamura and Ogawa 17 ).
Ascertainment of elevated serum alanine aminotransferase
The primary end point was the development of elevated serum ALT. Elevated serum ALT was ascertained by the results of a follow-up health examination during the 10-year period after the baseline survey. At the six follow-up surveys in 2005 (for cohort I), 2006, 2007, 2008, 2009 and 2013, elevated serum ALT was defined on the basis of the recommended cut-off value (>30 IU/l) according to the Japan Society of Ningen Dock definition( 18 ). The six follow-up surveys were conducted in April 2005, 2006, 2007, 2008 and 2009 and in September 2013. In our survey, blood samples were obtained in the morning after the subjects had fasted overnight. Serum was separated from blood cells by centrifugation, and the serum ALT levels of each subject were measured immediately at the laboratory of the Seirei Preventive Health Care Center (Shizuoka, Japan).
We also measured serum AST in our survey. Although the AST test is often part of an initial screening for liver disease, this enzyme is found in many tissues – not only in the liver but also in the heart, muscles, kidneys and brain. If any of these organs or tissues is affected by disease or injury, AST is released into the bloodstream. This means that AST is not as specific an indicator of liver damage as ALT. Therefore, in our study, we examined the association of serum carotenoids with ALT as a specific indicator of liver damage.
Statistical analysis
The serum carotenoid concentrations, fasting plasma glucose levels and serum TAG were skewed towards higher concentrations. These values were loge (natural)-transformed to improve the normality of their distribution. An ANCOVA adjusted for age followed by a Bonferroni multiple comparison test was used to compare the means of continuous variables in the three groups stratified by the baseline serum total carotenoid concentrations. All variables were presented in original scale. The data are expressed as mean values and standard deviations, geometric means and 95 % CI or percentages.
To assess the relationship between the serum carotenoid concentrations at baseline and the development of elevated serum ALT, Cox proportional hazards regression analyses were performed after excluding subjects whose serum ALT levels were >30 IU/l, who were heavy drinkers (≥60 g of alcohol/d), who had positive serum hepatitis B surface antigen and who had a history of infection with hepatitis C and/or medication use for liver disease according to the self-administered questionnaire at the baseline survey. Participants were divided into three categories according to tertiles of serum baseline carotenoid concentrations after being stratified by sex, because serum carotenoid concentrations differ substantially between male and female subjects. Hazard ratios (HR) and 95 % CI were calculated for the categories of serum carotenoid concentrations at baseline in tertiles, with the lowest tertile as the reference, by using the Cox proportional hazards model and adjusting for potential confounding variables. In these data analyses, we used the time from baseline until the first determination of a high ALT value (for cases) and time to the last determination of ALT in non-cases, and a Cox proportional hazards model was constructed with time-on-study as the time scale with stratification on birth cohort (10-year intervals) not using baseline age as a covariate. We also assessed linear associations by using the mean values of serum carotenoid concentrations at baseline for each tertile. We did not adjust each carotenoid concentration in the multivariate models because Pearson’s correlation analyses of serum carotenoid concentrations revealed significant positive correlations among all combinations of the six carotenoids. All statistical analyses were performed using statistical software package for Windows (SPSS version 12.0J, SPSS Inc.) on personal computers.
Results
Baseline characteristics of study subjects
Table 1 shows the characteristics of the study subjects at the baseline survey according to the tertile of the baseline serum total carotenoid concentrations. The percentages of male subjects and current cigarette smokers were significantly low, in accordance with the tertile of the baseline serum total carotenoid concentrations. Serum total cholesterol and all six serum carotenoid concentrations at baseline were significantly positively associated with the baseline serum total carotenoid concentration. In contrast, serum TAG was significantly inversely associated with the baseline serum total carotenoid concentration. Although protein, fat, carbohydrate and total energy intakes were not different among the three groups, the fibre and fruits and vegetable intakes of the middle and highest tertiles were significantly higher than that of the lowest group. In contrast, ethanol and tea and/or coffee intakes of the middle and highest tertiles were significantly lower than those of the lowest group.
Table 1 Characteristics of the study subjects at baseline survey according to tertiles of baseline serum total carotenoid concentrations (Mean values and standard deviations; geometric means and 95 % confidence intervals or percentages)
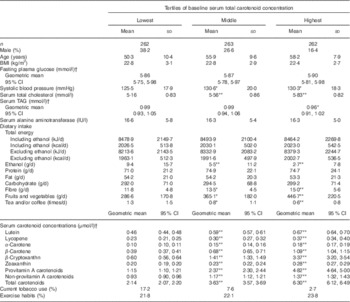
* P<0·05, ** P<0·001 v. the lowest tertile of serum total carotenoid concentration by ANCOVA adjusted for age followed by Bonferroni multiple comparison test.
† These variables were represented as original scale after analysis by loge (natural)-transformed values.
Risk of elevated serum alanine aminotransferase according to tertiles of baseline serum carotenoid concentrations
During the six follow-up surveys, subjects who newly started taking medication for liver disease (but had no elevated serum ALT) were not present. In our study, the overall incidence rate of elevated serum ALT was 13·8/1000 person-years.
The HR of elevated serum ALT associated with the tertiles of the six serum carotenoid concentrations at the baseline survey, after adjusting for confounding factors, are shown in Table 2. After adjusting for sex and BMI, a significantly lower hazard ratio for elevated serum ALT was observed in the highest group (T3) of serum β-carotene. After further adjusting for current tobacco use, exercise habits, fasting plasma glucose, total cholesterol, TAG and intake of total energy, excluding ethanol, fat, fibre, fruits and vegetables and tea and/or coffee, significantly lower HR for elevated serum ALT were observed in the highest groups (T3) of serum β-carotene, β-cryptoxanthin, total provitamin A carotenoids and the total of the six serum carotenoids. Although a significantly lower hazard ratio was observed in the second tertile (T2) of serum lycopene after multiple adjustments, a significant linear trend was not observed. Furthermore, although a significantly lower hazard ratio was not observed in the highest group (T3) of serum α-carotene, a significant linear trend was observed (P for trend=0·049). On the other hand, other serum carotenoid concentrations such as lutein, zeaxanthin and the total of non-provitamin A carotenoid concentrations showed no inverse associations with a risk for developing elevated serum ALT.
Table 2 Tertiles of baseline serum carotenoid concentrations on the incidence of elevated serum alanine aminotranseferase among total subjectsFootnote * (Hazard ratios (HR) and 95 % confidence intervals of tertiles of baseline serum carotenoid concentrations on incidence of elevated serum alanine aminotranseferase among total subjects)
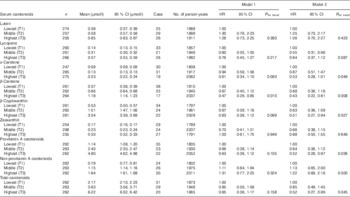
* Elevated alanine aminotransferase was a level >30 U/l. Cox proportional hazards model was constructed with time-on-study as the time scale with stratification on birth cohort (10-year intervals) not using baseline age as a covariate. Model 1: sex and BMI were adjusted. Model 2: current tobacco use, exercise habits, fasting plasma glucose, TAG, total cholesterol and intakes of total energy excluding ehtanol, ethanol, fat, fibre, fruits and vegetables, and tea and/or coffee were further adjusted.
Risk of elevated serum alanine aminotransferase according to quartiles of baseline fruit and vegetable intake and serum carotenoids
Next, study subjects were divided into four groups according to median values of fruit and vegetable intake and/or basal serum β-carotene and β-cryptoxanthin concentrations. Subsequently, we analysed the associations of fruit and vegetable intakes and/or serum β-carotene and β-cryptoxanthin concentrations with the risk for elevated serum ALT. Although significant inverse associations were observed among serum β-carotene and β-cryptoxanthin with the risk for developing elevated serum ALT, significant inverse associations were not observed between fruit and vegetable intake and the risk for elevated serum ALT (Fig. 1).
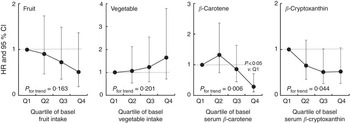
Fig. 1 Hazard ratios (HR) and 95 % CI of quartiles of baseline fruit and vegetable intakes and serum carotenoids on incidence of elevated serum alanine aminotransferase (ALT). Elevated ALT was at a level>30 U/l. The Cox proportional hazards model was constructed with time-on-study as the time scale with stratification on birth cohort (10-year intervals) not using baseline age as a covariate. Sex, BMI, current tobacco use, exercise habits, fasting plasma glucose, TAG, total cholesterol and intake of total energy excluding ethanol, fat, fibre and tea and/or coffee were adjusted in the model for fruit and vegetable intakes. Intakes of fruits and vegetables were further adjusted in the model for serum carotenoids.
Discussion
The objective of this study was to longitudinally investigate whether the incidence of risk for elevated serum ALT is associated with serum carotenoid concentrations in middle-aged and older Japanese subjects. The results indicated that higher serum α- and β-carotene and β-cryptoxanthin at baseline were significantly associated with a lower risk of developing elevated serum ALT. In our data analysis, after excluding heavy drinkers (≥60 g of alcohol/d), serum hepatitis B surface antigen-positive subjects and those with a history of hepatitis C infection and/or medication use for liver disease according to the self-administered questionnaire from the baseline survey, a significant positive association of the risk for developing elevated serum ALT with alcohol intake levels was not observed among study subjects (data not shown). On the other hand, in our study population including heavy drinkers, a significantly higher hazard ratio was observed among heavy drinkers (≥60 g of alcohol/d) than among the non-drinkers used for reference (HR 2·36; 95 % CI 1·00, 5·59). Therefore, we concluded that the elevated serum ALT in our survey was caused by non-alcoholic mild liver dysfunction. This is the first longitudinal cohort study to examine the association of serum carotenoid concentrations with the risk of developing elevated serum liver enzymes. Numerous antioxidant vitamins and carotenoids are contained in fruits and vegetables, and several recent studies have shown inverse associations of antioxidant vitamins and carotenoids with liver dysfunction( Reference Munoz, Heubi and Balisteri 6 – Reference Arendt and Allard 13 ). Our results further support the hypothesis that antioxidant carotenoids, especially provitamin A carotenoids such as α- and β-carotene and β-cryptoxanthin, might help prevent the development of liver disease among middle-aged and older Japanese subjects.
NAFLD has emerged as a major public health problem worldwide. Considerable recent evidence has shown that NAFLD is associated with the metabolic syndrome and that it is present in lean glucose-tolerant subjects as well as in obese diabetic subjects( Reference Pagano, Pacini and Musso 19 ). Thus, NAFLD can be defined as a lipotoxic liver injury, and it can impair whole-body insulin resistance and progress to NASH( Reference DeFronzo 2 , Reference Cusi 3 ). Insulin resistance and increased oxidative stress are believed to be major causes for the progression to NASH. Recently, many agents including insulin sensitisers have been tested with disappointing results for the management of NASH. Only vitamin E has yielded some promise in the treatment of patients with NASH( Reference Sanyal, Chalasani and Kowdley 12 ). The lipophilic antioxidant vitamin E was associated with reduced hepatic steatosis and lobular inflammation. Furthermore, β-carotene is the most abundantly stored carotenoid in the liver( Reference Dimitrov, Meyer and Ullrey 20 , Reference Rock and Swendseid 21 ). In particular, hepatic stellate cells are considered to be the major site of storage and metabolism of retinoids and carotenes( Reference Martucci, Ziulkoski and Fortuna 22 ). Furthermore, it has been reported that the concentrations of all antioxidant liposoluble vitamins such as retinol, tocopherol and β-carotene are reduced in both the plasma and liver tissue of liver cirrhosis patients( Reference Munoz, Heubi and Balisteri 6 , Reference Rocchi, Borghi and Paolillo 7 , Reference Von Herbay, De Groot and Hegi 9 , Reference Rocchi, Casalgrandi and Ronzoni 10 ). On the basis of the knowledge concerning the associations of oxidative stress and metabolic disorders with liposoluble antioxidant vitamins and carotenoids, eating a diet rich in antioxidants might be readily accepted as a strategy for preventing liver dysfunction.
We previously found that serum β-carotene and β-cryptoxanthin concentrations were inversely associated with serum liver enzymes from the cross-sectional Mikkabi study( Reference Sugiura, Nakamura and Ikoma 15 , Reference Sugiura, Nakamura and Ikoma 16 ). Furthermore, significant inverse associations of serum β-carotene and β-cryptoxanthin with risks for the metabolic syndrome( Reference Sugiura, Nakamura and Ogawa 23 ) and insulin resistance( Reference Sugiura, Nakamura and Ikoma 24 ) were observed. From the experimental approach using animal models, Ni et al. ( Reference Ni, Nagashimada and Zhan 25 ) recently found that administration of β-cryptoxanthin attenuated insulin resistance, excessive hepatic lipid accumulation, peroxidation, increased M1-type macrophages/Kupffer cells and activated stellate cells, as well as induced fibrosis in mice fed a high-cholesterol and high-fat diet. On the basis of these findings, β-cryptoxanthin might be a useful dietary antioxidant for preventing earlier pathogenesis of liver dysfunction and also NAFLD and related metabolic disorders.
β-Cryptoxanthin is a xanthophyll carotenoid that is particularly abundant in the Japanese mandarin orange( Reference Goodner, Rouseff and Hofsommer 26 , Reference Holden, Eldridge and Beecher 27 ) and is relatively abundant in human plasma( Reference Sugiura, Nakamura and Ikoma 15 – Reference Sugiura, Nakamura and Ogawa 17 ). Previously, we found that serum β-cryptoxanthin levels increased significantly in accordance with increased intake of Japanese mandarins( Reference Sugiura, Matsumoto and Kato 28 ). Our Mikkabi cohort study was conducted in the town of Mikkabi, Shizuoka Prefecture, Japan. Fruit trees are the key industry in Mikkabi, which is an important producer of Japanese mandarin oranges. The subjects in this survey were residents of an area in which the Japanese mandarin orange is considerably more popular than in the rest of Japan. The average amount of fruit intake in the group with the highest tertile of serum β-cryptoxanthin was about 247 g/d and was approximately equal to three pieces of the Japanese mandarin orange. Therefore, the serum concentrations of β-cryptoxanthin in our study population were widely distributed. Interestingly, Montonen et al. ( Reference Montonen, Knekt and Järvinen 29 ) found that, among the six main carotenoids, only β-cryptoxanthin intake was significantly associated with a reduced risk for type 2 diabetes. On the basis of these findings, β-cryptoxanthin might be a useful dietary antioxidant for preventing metabolic disorders. However, we concluded that most liposoluble antioxidant carotenoids might be effective in the prevention of earlier pathogenesis in liver dysfunction and related metabolic disorders, such as NAFLD, insulin resistance and type 2 diabetes, because all carotenoids except for lutein showed inverse association with the risk of developing elevated serum ALT in our survey. α-Carotene, β-carotene and β-cryptoxanthin are provitamin A carotenoids, which are converted to retinol in the body. Retinoic acid is synthesised intracellularly from retinol and plays a regulatory role in lipid/glucose homoeostasis and type 2 diabetes( Reference Rhee and Plutzky 30 ). Among the six main carotenoids, provitamin A carotenoids might be more effective against liver dysfunction and its related metabolic disorders than other non-provitamin A carotenoids such as lycopene, lutein and zeaxanthin.
On the other hand, in our data analyses, although significant inverse associations were observed among serum β-carotene and β-cryptoxanthin with the risk for developing elevated serum ALT, significant inverse associations were not observed between fruit and vegetable intake and the risk for elevated serum ALT (Fig. 1). A few possible reasons for this are considered. First, although in our survey diet was assessed with a modified validated simple FFQ developed especially for the Japanese, the limitation of the FFQ validity cannot be denied. Second, we previously found that the highest intake among the six carotenoids was that of lutein (1·92 mg daily) in our study population( Reference Sugiura, Nakamura and Ogawa 17 ). The second was β-carotene (1·7 mg daily) and zeaxanthin was third (0·7 mg daily). Lutein, β-carotene and zeaxanthin are carotenoids present abundantly in vegetables, but these carotenoids were not associated with the risk for developing elevated serum ALT in our present study except for β-carotene (Table 2). Therefore, it might be difficult to discover the association of the intake of vegetables rich in lutein and zeaxanthin with the risk of elevated serum ALT. In contrast, in our study subjects, fruit intake was significantly correlated with serum β-carotene (r 0·312, P<0·001) and β-cryptoxanthin (r 0·481, P<0·001), and lower HR for elevated serum ALT were observed in the second, third and highest quartiles of fruit intake; however, these were not significant. Finally, we previously found that cigarette smoking and alcohol drinking might reduce serum carotenoid concentrations( Reference Sugiura, Nakamura and Ogawa 17 ). In our previous report, we found that cigarette smoking and >1 g of daily alcohol intake may reduce serum α- and β-carotene and β-crypktoxanthin concentrations synergistically. On the basis of these facts, we assumed that we could not find out the significant inverse association of fruit and vegetable intakes with the risk for developing elevated serum ALT because these three effective carotenoids would be reduced in current smokers and/or alcohol drinkers. Next, therefore, we re-examined this association in non-smokers among non-drinkers after excluding current smokers among alcohol drinkers (>1 g of daily alcohol intake). As a result, the risk of elevated serum ALT showed a tendency to lower according to the intake of fruits and vegetables; however, this association was not significant, because the sample size was too small (thirty-two males and 419 females) and the statistical power might be decreased. In our study, therefore, we could not assess the dietary status and the risk of elevated serum ALT; however, data on serum carotenoid levels provide a relatively accurate measure of the actual amount of carotenoids present in the body, which therefore gives a more detailed account of the association between carotenoid levels and the risk of chronic liver disease.
This study had some limitations. First, in our survey, we only examined the association of basal serum carotenoid concentrations with the risk for developing elevated serum ALT. We could not evaluate the liver histology of subjects who developed elevated serum ALT. Therefore, it remains unclear whether liposoluble antioxidant carotenoids such as α- and β-carotene and β-cryptoxanthin are a truly beneficial functional food factor against the development of liver disease from mild liver dysfunction to chronic hepatitis such as NAFLD, NASH and/or cirrhosis. Second, we could not evaluate the association of the blood levels of other antioxidants such as vitamins C and E with the risk for developing elevated serum ALT. It would be necessary to measure the blood levels of vitamins C and E in order to examine the association of these antioxidant vitamin concentrations with elevated serum ALT. Third, as we used a single measurement of serum carotenoid concentrations at baseline, subjects’ dietary changes were not considered during follow-up surveys. Misclassification of serum carotenoid concentrations relative to long-term average levels was expected. Fourth, we could not evaluate the influence of other drugs except for liver dysfunction medication. It might be difficult to ignore that other drugs affect serum liver enzymes. Finally, in our study, the sample size was not particularly large, and thus had less statistical power. Additional studies on a larger scale will be required.
In conclusion, this longitudinal cohort study among middle-aged and older Japanese subjects showed that the risk of developing elevated serum ALT was inversely associated with baseline serum α- and β-carotene and β-cryptoxanthin concentrations. Our findings further support the hypothesis that antioxidant carotenoids, especially provitamin A carotenoids such as α- and β-carotene and β-cryptoxanthin, might help prevent the development of liver disease. However, further evidence is needed before a definitive conclusion on this issue can be drawn.
Acknowledgements
The authors are grateful to the participants in the survey and to the staff of the health examination programme for residents of the town of Mikkabi, Shizuoka, Japan. The authors are also grateful to the staff of the Seirei Preventive Health Care Center (Shizuoka, Japan).
This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries for a food research project titled ‘Research Project on the Development of Agricultural Products and Foods with Health-Promoting Benefits (NARO)’ and a grant from the Council for the Advancement of Fruit Tree Science.
M. S. was responsible for the study design, data collection and data management, carried out the data analysis and wrote the manuscript. M. N. was responsible for study design, data collection and data management and assisted in manuscript preparation. K. O., Y. I. and M. Y. were involved in data collection and assisted in manuscript preparation.
The authors have declared that they have no competing interests.






