Brain ageing is accompanied by cognitive decline and mood disorders, which impose a considerable socio-economic burden. The functions most affected, which also cause elderly people to complain, are cognitive changes related to attention, memory and learning. Although little is known about the risk factors for neurobiological and cognitive deterioration in elderly subjects, there has been considerable interest in the role of dietary fat in recent years(Reference Bourre1–Reference Donini, De Felice and Cannella3). A high-fat intake has been associated with impaired cognitive function(Reference Molteni, Barnard and Ying4–Reference Winocur and Greenwood6). It has also been suggested that the typical diet in most industrialised western societies, rich in saturated fat and refined sugar, and more generally, obesity and overweight in early adulthood and middle age, significantly increase the risk of cognitive decline and dementia in old age(Reference Donini, De Felice and Cannella3, Reference Gorospe and Dave7). It is thus extremely important at present to understand how dietary fat affects neural function, increasing vulnerability to numerous neurological diseases and cognitive deficits associated with ageing. Fatty acids, notably PUFA, exert their physiological effects on brain function through various mechanisms, including some that are involved in the modulation of gene transcription(Reference Chalon, Vancassel and Zimmer8–Reference Sampath and Ntambi13). PUFA are important modulators of gene expression in various tissues in response to nutritional modifications(Reference Jump12, Reference Jump and Clarke14). Their transcriptional activity is mediated by nuclear receptors (NR), such as the PPAR, which are ligand-inducible transcription factors(Reference Chambon15) that confer on the cell the ability to display a genic response to fatty acids.
Tenuous relationships have been described between the retinoid and fatty acid signalling pathways. The PPAR as well as the retinoid NR (retinoic acid receptor, RAR, and retinoid X receptor, RXR) belong to the NR superfamily. Retinoid NR occupy a key position among the signalling pathways mediated by NR. Indeed, the RXR is the common dimerisation partner of several other receptors, particularly PPAR (RAR/RXR and PPAR/RXR)(Reference Khan and Vanden Heuvel16). Moreover, the ability of several PUFA, including DHA (22 : 6n-3) and arachidonic acid (20 : 4n-6), as well as oleic acid (18 : 1n-9), to bind and activate RXR at supra-physiological levels, has been highlighted(Reference de Urquiza, Liu and Sjoberg17, Reference Lengqvist, Mata De Urquiza and Bergman18). These data indicate that fatty acid ligands exert significant effects on RXR-mediated gene transcription, suggesting that RXR plays a crucial role in vivo as a fatty acid sensor. This is supported by the fact that, in many animal tissues, the retinoid signalling pathway has been shown to be sensitive to the supply of fatty acids(Reference Lengqvist, Mata De Urquiza and Bergman18–Reference Delage, Bairras and Buaud20) and, consequently, to the activity level of their signalling pathway. In this context, the brain retinoid signalling pathway may also be assumed to be responsive to dietary fatty acid content. The potential consequences of this phenomenon are extremely important, as retinoids have been shown to be strongly involved in maintaining synaptic plasticity and memory performance in aged animals(Reference Etchamendy, Enderlin and Marighetto21). Vitamin A and, similarly, retinoic acid (RA) play a significant role in the function of the mature brain(Reference Malik, Blusztajn and Greenwood22, Reference Lane and Bailey23), by controlling the expression of numerous genes, including those involved in neurite growth(Reference Prince and Carlone24), synaptic plasticity(Reference Chiang, Misner and Kempermann25), memory and cognitive processes(Reference Ikegaya, Ishizaka and Matsuki26), through their NR. Among the RA target genes identified in the brain are those coding for two neuron-specific protein kinase substrates implicated in the molecular mechanisms underlying synaptic plasticity and memory formation: neuromodulin (GAP-43) and neurogranin (RC3). These two proteins are expressed on both sides of the synaptic cleft(Reference Watson, Szijan and Coulter27, Reference Gerendasy and Sutcliffe28), thus constituting good markers of dendritic spine density. GAP-43 plays a fundamental role in controlling axonal growth(Reference Piontek, Regnier-Vigouroux and Brandt29) and regeneration(Reference Chen, Chai and Cao30) in the adult brain, while RC3 is involved in synaptogenesis and neuronal plasticity(Reference Iniguez, Morte and Rodriguez-Pena31). Knockout studies have shown that decreased GAP-43 expression is associated with reduced neuronal plasticity and impaired learning(Reference Rekart, Meiri and Routtenberg32), and that the lack of the RC3 gene induces deficits in hippocampal synaptic plasticity and spatial learning impairments(Reference Pak, Huang and Li33).
The present study therefore investigates the possible effects of a high-fat diet (HFD) on the brain retinoid signalling pathway in young adult rats and the probable neurobiological consequences for synaptic plasticity. To achieve this goal, the expression of retinoid and fatty acid NR as well as that of RC3 and GAP-43 was measured in the striatum and the hippocampus, two brain areas essential to synaptic plasticity, learning and memory processes(Reference Fasano and Brambilla34, Reference White and McDonald35). The impact of a HFD on the bioavailability of fatty acids was also studied by the assessment of the fatty acid composition of plasma lipids and brain phosphatidylethanolamine (PE), one of the brain phospholipids richest in DHA in the mammalian brain(Reference Crawford, Bloom and Broadhurst36).
Materials and methods
Animals
The study was conducted in accordance with European Community Council Directives (861609/EEC). All experiments conformed to the Guidelines for the Handling and Training of Laboratory Animals. Seventy-two male Wistar rats (7 weeks old) purchased from Harlan (France) were maintained with unrestricted access to water and food, under controlled temperature (21 ± 1°C), humidity and airflow conditions, with a fixed 12 h light–dark cycle. Before experimentation, they were fed with standard laboratory chow (A04-type pellets, UAR, Epinay sur Orge, France).
Dietary manipulation
After 1 week of acclimatisation, rats were randomly assigned to one of two experimental groups (Table 1). The first group of rats (n 36) received standard laboratory chow for 8 weeks (control diet). Over the same period, the second group (n 36) received a HFD composed of a selection of highly palatable human foods, containing by weight (per 100 g diet) 28·5 g of ham pâté, and 14·3 g each of the following ingredients: bacon; chocolate; potato chips; biscuits; standard laboratory chow. The food in the animal cages was changed every day. Food intake was recorded daily and each animal weighed three times per week during the experimental period. At the end of the 8-week period, food was withdrawn overnight and animals were sacrificed by decapitation the following morning. Blood was collected from the sectioned jugular vein and rapidly centrifuged to obtain plasma, which was stored at − 80°C until use. The brain was rapidly removed, and individual brain regions (whole striatum and hippocampus) dissected out, rapidly frozen and stored at − 80°C for subsequent analysis.
Table 1 Composition of experimental diets
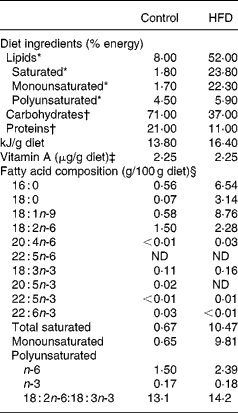
HFD, high-fat diet; ND, not detected.
* Lipids were extracted from food according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley42), transmethylated and subjected to GC.
† Energy supplied was estimated from the composition of the two experimental diets.
‡ Vitamin A levels were determined by normal-phase HPLC according to NF EN 12 823-1 (ITERG, Pessac, France). The HFD was supplemented with retinyl palmitate to equal the vitamin A content of the control diet.
§ Minor fatty acids made content up to 100 %.
Quantitative real-time PCR
Total RNA from the whole striatum and hippocampus was extracted using a kit (RNA plus, Q.BIOgene, Illkirch, France) according to the manufacturer's suggested protocol. cDNA was synthesised with Superscript II RT (Invitrogen, Cergy Pontoise, France) as previously described by Husson et al. (Reference Husson, Enderlin and Alfos37). Real-time PCR was carried out using a LightCycler system (Roche Diagnostics, Mannheim, Germany), which combines the processes of amplification and detection (by fluorescence) of a PCR product, thereby enabling online and real-time detection. To detect target gene amplification products, LightCycler DNA Master SYBR Green I was used(Reference Husson, Enderlin and Alfos37). The oligonucleotide primers (Proligo, Paris, France) for RARβ, RXRβγ, PPARδ, RC3 and GAP-43 mRNA are shown in Table 2. Target gene mRNAs were co-reverse transcribed with glyceraldehyde-3-phosphate-dehydrogenase mRNA, except for PPARδ, which was co-reverse transcribed with cyclophilin B (peptidylprolyl isomerase B) mRNA. Data were analysed using the LightCycler analysis software, version 3.5 (Roche Diagnostics, Mannheim, Germany) as previously described(Reference Husson, Enderlin and Alfos37). Results were normalised by calculating the ratio of the concentration of the target gene to that of the reference gene glyceraldehyde-3-phosphate-dehydrogenase or peptidylprolyl isomerase B in the same sample.
Table 2 Primers used for real-time PCR
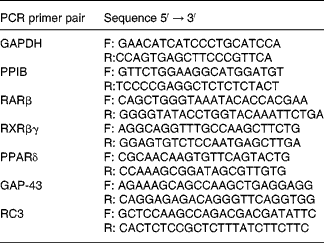
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse; PPIB, peptidylprolyl isomerase B; RAR, retinoic acid receptor; RXR, retinoid X receptor; GAP-43, neuromodulin; RC3, neurogranin.
Western blot analysis
Western blot analysis was performed with the whole striatum and hippocampus of twelve rats from each group, according to the protocol described by Husson et al. (Reference Husson, Enderlin and Alfos37, Reference Husson, Enderlin and Alfos38). β-Actin and RC3 were labelled using a monoclonal mouse anti-β-actin antibody (1:8000, Sigma no. A-5441) and a polyclonal rabbit anti-neurogranin antibody (diluted 1:3000, Affinity Research Product, Le Perray en Yvelines, France, no. NA 1300), respectively. GAP-43 was labelled using a polyclonal rabbit antibody (1:4000, Affinity Research Product, no. GA 1330). The staining intensity of protein bands was determined using Quantity One quantification software (BioRad Laboratories, Hercules, CA, USA). The levels of RC3 and GAP-43 proteins in HFD rats were calculated relative to the same proteins (percentage) in control rats. The level of β-actin was verified and found to be identical in the two groups (data not shown).
In situ hybridisation
After decapitation, brains were removed and fixed overnight in 2 % (w/v) paraformaldehyde in 0·1 m phosphate buffer (pH 7·4) and then immersed in the same buffer with 30 % sucrose for 2 d at 4°C. Brains were then rapidly frozen in cooled isopentane and stored at − 80°C. Serial coronal sections (20 μm) were cut using standard microtome techniques, thaw mounted onto gelatine-coated slices and stored at − 80°C until processing. The distribution of RC3 and GAP-43 mRNA was analysed using a 60-mer oligodeoxyribonucleotide probe complementary to positions 40–99 of transcript 140(Reference Rhyner, Borbély and Mallet39) and a 50-mer oligodeoxyribonucleotide probe complementary to bases 220–270 of the rat GAP-43 coding sequence(Reference Basi, Jacobson and Virag40), respectively. Probes were end labelled with α[35S]-deoxy-ATP (ICN Pharmaceuticals, Orsay, France) using terminal deoxynucleotidyl transferase (Amersham, Arlington Heights, IL, USA). For the following steps, hybridisations were carried out as previously described by Husson et al. (Reference Husson, Enderlin and Alfos38). For an assessment of the relative amounts of RC3 and GAP-43 mRNA in various areas of the rat brain, X-ray autoradiographs were digitised using an image analysis system (Autoradiography V4.03; Samba Technologies, Meylan, France). Optical density measurements within a particular brain region were carried out using three consecutive sections per animal. Background optical density was subtracted from each image. mRNA densities for each region in HFD rats were expressed as a percentage of the mean mRNA density observed in the control group within the same brain region.
Lipid analyses
Plasma lipids
Total esterified fatty acids from plasma were methylated according to the method of Lepage & Roy(Reference Lepage and Roy41). Briefly, 2 ml of methanol–benzene (4:1, v/v) and 200 μl of acetyl chloride were added to 400 μl of plasma for 1 h at 100°C. To stop the reaction, 5 ml of 6 % (w/v) Na2CO3 were added to the mixture. After centrifugation, the upper phase containing fatty acid methyl esters (FAME) was removed, evaporated to dryness under a stream of nitrogen, redissolved in hexane and then stored at − 20°C until further analysis.
Preparation of phosphatidylethanolamine from brain
Total brain lipids were extracted using the method of Folch et al. (Reference Folch, Lees and Sloane Stanley42), with 20 volumes of chloroform–methanol (2:1, v/v) per g of tissue. Extraction was carried out under agitation at room temperature; after 1 h, 0·2 volumes of KCl (0·8 % in water, w/v) were added per volume of extraction mixture. The hydroalcoholic and chloroform phases were separated by centrifugation. The hydroalcoholic phase was removed and the chloroform phase washed with a mixture of chloroform–methanol–0·8 % KCl in water (3:48:47, by vol.). After centrifugation, the chloroform phase was filtered with chloroform–methanol (2:1, v/v), and the solvent evaporated under vacuum at room temperature using a rotary evaporator. The extract was redissolved in chloroform and filtered. The solvent was evaporated under nitrogen and the dry extract was redissolved in chloroform–methanol (2:1, v/v).
Total phospholipids from brain tissue were separated by TLC using plates pre-coated with 0·35 mm silica gel 60 H (Merck, Fontenay-sous-Bois, France). An aliquot of the solution obtained above was evaporated to dryness under a stream of nitrogen. The lipids were redissolved in an appropriate volume of chloroform–methanol (2:1, v/v) and deposited on the silica gel. The solvent system used for separation was a mixture of chloroform–methanol–acetic acid–water (75:45:12:6, by vol.). After migration and revelation with dichlorofluorescein (0·2 % in ethanol, w/v), the silica gel area corresponding to PE was visualised under UV (254 nm), removed from the TLC plate and transferred to a glass tube for FAME preparation.
Preparation of fatty acid methyl esters and dimethylaldehydes
Total fatty acid chains of brain PE were methylated according to the method of Morrison & Smith(Reference Morrison and Smith43). A quantity of 1 ml of boron trifluoride–methanol solution (14 %; w/v; Sigma Chemical Co., St Louis, MO, USA) was added to the silica gel area corresponding to brain PE in a glass tube maintained at 90°C for 20 min. After the addition of 1 ml of NaOH (5 m), the FAME and dimethylaldehydes obtained were extracted three times with 2 ml of hexane. The hexane phases were concentrated, washed with 1 ml of water and stored at − 20°C until gas chromatographic analysis.
Analysis of fatty acid methyl esters and dimethylaldehydes
Analysis of FAME and dimethylaldehydes was carried out on a gas chromatograph equipped with a flame ionisation detector and a split injector. A fused silica capillary column (BPX 70, 60 m × 0·25 mm internal diameter, 0·25 μm film; SGE, Courtaboeuf, France) was used with H2 as the carrier gas (inlet pressure: 1 bar). The split ratio was 1:70. The column temperature was programmed to increase from 150 to 200°C at 1·5°C/min for 25 min, then from 200 to 225°C at 20°C/min and held at 225°C until completion of the analysis (20 min). The injection port and detector were maintained at 250 and 280°C, respectively. The GC peaks were integrated using an SP 4400 integrator (Spectra Physics, San Jose, CA, USA). FAME and dimethylaldehydes were identified by comparison with the retention times of standards eluted under the same conditions (Sigma Chemical Co., Saint Quentin Fallavier, France).
Statistical analysis
Results are expressed as mean values with their standard errors. Statistical comparisons were carried out between the two dietary groups. Statistically significant differences between groups were determined by the Fisher's F test (to verify for the homogeneity of variance) followed by the Student's t test. A P value of less than 0·05 was taken to indicate a statistically significant difference.
Results
Body weight gain
As seen in Table 3, during the experiment, the HFD group exhibited a greater increase in body weight than the control group. Indeed, at the end of the 8-week period, the body weight of HFD rats was significantly higher than that of controls (P < 0·05). The average difference in weight gain between the two groups was 41 g. This excess weight gained by HFD rats reached 16 % of their initial weight, i.e. overweight. Moreover, the present data show that the weight gain of HFD rats was due to the fact that they ate more food than controls (+18 %, P < 0·05) because of the highly palatable composition of the HFD. Consequently, the mean caloric intake was 40 % higher in the HFD group than in controls (P < 0·05).
Table 3 Dietary characteristics and body weights of rats fed a control diet or a high-fat diet (HFD)
(Mean values with their standard errors for thirty-six animals per experimental group)

Mean values were significantly different from those of the control group: * P < 0·05 (Student's t test).
Plasma lipid and brain phosphatidylethanolamine fatty acid composition
Table 4 shows the fatty acid composition of plasma lipids and brain PE as a percentage of total fatty acid content in control and HFD rats at the end of 8 weeks of feeding. The plasma of HFD rats exhibited a significant increase in SFA and MUFA levels. Indeed, the stearic acid (18 : 0) content doubled (+106 %, P < 0·001) and that of oleic acid (18 : 1n-9) also increased markedly (+66 %, P < 0·001). The total PUFA content of plasma was significantly lower ( − 23 %, P < 0·001). This phenomenon was due to the concomitant decline in total n-3 ( − 59 %, P < 0·001) and n-6 fatty acids, including linoleic acid (18 : 2n-6, − 31 %, P < 0·001). The diet-related decrease in n-3 fatty acids concerned both the precursor α-linolenic acid (18 : 3n-3, − 70 %, P < 0·001), and its long-chain derivatives, EPA (20 : 5n-3, − 77 %, P < 0·001), docosapentaenoic acid (22 : 5n-3, − 53 %, P < 0·001), and DHA ( − 51 %, P < 0·001). The HFD also affected the fatty acid composition of brain PE but less markedly than in the plasma. The main variations were a marked increase in docosapentaenoic acid (22 : 5n-6,+46 %, P < 0·001) associated with decreases in both 22 : 5n-3 ( − 21 %, P < 0·01) and DHA ( − 5 %, P < 0·01).
Table 4 Fatty acid composition of plasma lipids and brain phosphatidylethanolamine (PE) of rats fed a control diet or a high-fat diet (HFD)
(Mean values with their standard errors for six animals per experimental group)

ND, not detected.
Mean values were significantly different from those of the control group: * P < 0·05, ** P < 0·01, *** P < 0·001 (Student's t test).
† Minor fatty acids made content up to 100 %.
Retinoic acid receptor β, retinoid X receptor βγ and PPARδ mRNA expression in the striatum and hippocampus
The data summarised in Table 5 reveal that the HFD rat striatum contains a significantly higher amount of RXRβγ mRNA (+52 %, P < 0·001) and a significantly lower amount of RARβ mRNA ( − 14 %, P < 0·05) compared to the control group, as revealed by real-time PCR. In addition, PPARδ mRNA expression was significantly decreased ( − 13 %, P < 0·05). In contrast to the effects observed in the striatum, HFD did not modify mRNA expression levels of the three NR in the hippocampus.
Table 5 mRNA expression of nuclear receptors (retinoid X receptor (RXR)βγ, retinoic acid receptor (RAR)β and PPARδ), and mRNA and protein expression of synaptic plasticity genes (RC3 and GAP-43) in the striatum and hippocampus of rats fed a control diet or a high-fat diet (HFD)
(Mean values with their standard errors for twelve animals per experimental group for real-time PCR and Western blot analyses)
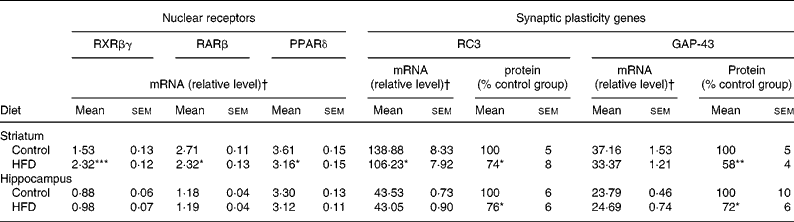
RC3, neurogranin; GAP-43, neuromodulin.
Mean values were significantly different from those of the control group: * P < 0·05, ** P < 0·01, *** P < 0·001 (Student's t test).
† Target mRNAs are expressed as a percentage of glyceraldehyde-3-phosphate dehydrogenase mRNA, except for PPARδ which is expressed as a percentage of peptidylprolyl isomerase B mRNA.
RC3 and GAP-43 mRNA and protein expression in the striatum and hippocampus
As presented in Table 5, the amount of RC3 in the striatum of the HFD group decreased at both mRNA and protein levels ( − 24 and − 26 %, respectively, P < 0·05). The amount of GAP-43 mRNA was slightly lower in the striatum of HFD rats than in controls ( − 10 %, P < 0·10), whereas the amount of protein expressed decreased considerably ( − 42 %, P < 0·05). Interestingly, in the hippocampus of HFD rats, GAP-43 and RC3 expression decreased only at the protein level, by approximately − 25 % (P < 0·05), without any significant modification at the mRNA level under our conditions. RC3 and GAP-43 mRNA levels were also studied in several subfields of the striatum and the hippocampus by in situ hybridisation. The results, summarised in Fig. 1, show a slight alteration in both RC3 and GAP-43 expression in the dorsal striatum of HFD rats when compared to controls ( − 12 and − 22 %, respectively, P < 0·10). A significant reduction in RC3 mRNA was also observed in two hippocampal areas – the CA1 and the dentate gyrus ( − 13 %, P < 0·001, and − 12 %, P < 0·01, respectively; Fig. 2).
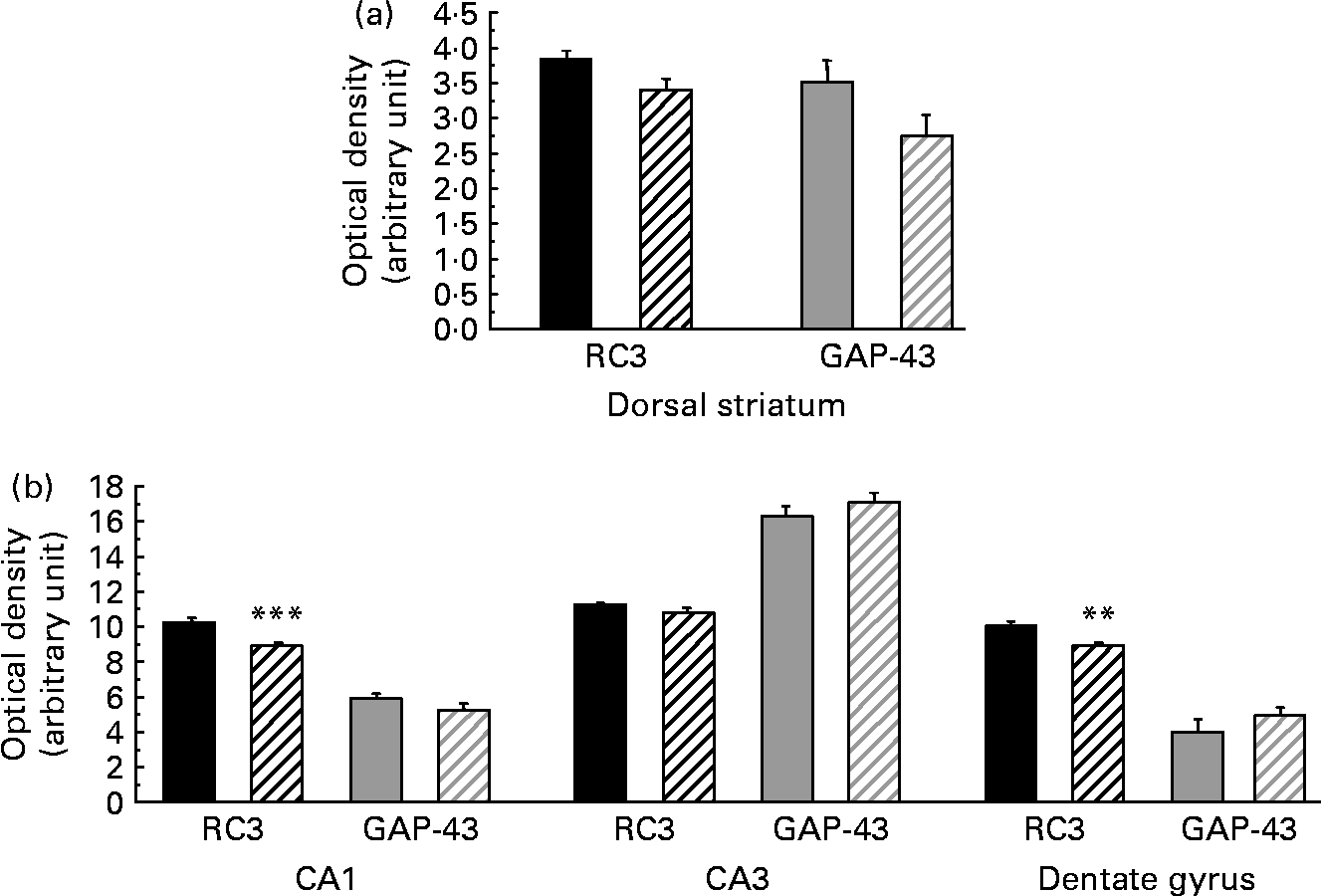
Fig. 1 Levels of neurogranin (RC3) and neuromodulin (GAP-43) mRNA (optical density in arbitrary unit) in different subfields of the striatum (a) and hippocampus (b) of rats fed a control diet (□) or a high-fat diet (HFD, ![]() ). CA1, field CA1 of Ammon's horn, pyramidal layer; CA3, field CA3 of Ammon's horn, pyramidal layer. Values are means of six animals per group, with standard errors represented by vertical bars. Mean values were significantly different from those of the control group: ** P < 0·01, *** P < 0·001 (Student's t test).
). CA1, field CA1 of Ammon's horn, pyramidal layer; CA3, field CA3 of Ammon's horn, pyramidal layer. Values are means of six animals per group, with standard errors represented by vertical bars. Mean values were significantly different from those of the control group: ** P < 0·01, *** P < 0·001 (Student's t test).

Fig. 2 Distribution pattern of neurogranin (RC3) mRNA in different subfields of the striatum and hippocampus of rats fed a control diet or a high-fat diet (HFD). Different subfields of the striatum and the hippocampus are shown in (a) and (b). DS, dorsal striatum; VS, ventral striatum; CA1, field CA1 of Ammon's horn, pyramidal layer; CA3, field CA3 of Ammon's horn, pyramidal layer; CA4, field CA4 of Ammon's horn, pyramidal layer; DG, dentate gyrus, granular layer; SB, subiculum. The HFD did not modify RC3 mRNA levels in the dorsal striatum ((c): control, (d): HFD) but significantly reduced them in the hippocampus ((e): control, (f): HFD).
Discussion
The relationship between lifestyle and disease that develops later in life has attracted growing attention in the last 10 years. Our eating habits, particularly the consumption of SFA, are increasingly known to be responsible for the rising prevalence of obesity and the development of correlated diseases such as atherosclerosis and type 2 diabetes with age. Previous research in both animals and human subjects has demonstrated an association between SFA intake and cognition. Some authors have found using rat models that the level of SFA in HFD contributes to cognitive deficits in certain tasks that require the hippocampus and frontal cortex(Reference Winocur and Greenwood6, Reference Greenwood and Winocur44). Wainwright et al. (Reference Wainwright, Bulman-Fleming and Lévesque45) have observed that a diet rich in SFA reduces the complexity of dendritic arborisation in cortical neurons during the development of the mouse brain. In accordance with this finding, epidemiological studies have demonstrated that a high SFA and cholesterol intake is associated with cognitive decline(Reference Kalmijn, van Boxtel and Ocke46, Reference Solfrizzi, D'Introno and Colacicco47).
The aim of the present study was to enhance our understanding of the mechanisms by which a HFD could alter synaptic plasticity.
To assess our nutritional model and objectify the nutritional impact of HFD, we analysed the fatty acid composition of plasma lipids and brain PE. We were particularly interested in the total fatty acid composition of plasma, as it is an indicator of the NEFA available for use by the brain. Indeed, Spector(Reference Spector48), in a study investigating the potential sources of PUFA for the brain, has demonstrated that the plasma-free fatty acid pool is its primary source of fatty acids. In the present experiment, the plasma of HFD rats exhibited a significant increase in total SFA and MUFA. This was explained by the fact that HFD rats consumed more SFA (3·2 v. 0·2 g/d) and MUFA (3·0 v. 0·2 g/d) than controls. Although HFD rats ate more PUFA (0·8 v. 0·4 g/d), including more precursors (0·74 v. 0·42 g/d), than control rats, a decrease in the proportion of PUFA was observed in their plasma. The two precursors linoleic acid and α-linolenic acid are commonly used to synthesise their long-chain derivatives, such as arachidonic acid and docosapentaenoic acid of the n-6 family and EPA and DHA of the n-3 family. However, the HFD provided more linoleic acid than α-linolenic acid (0·69 v. 0·05 g/d). In view of the competition between the two species in relation to the desaturase enzymes, and the fact that SFA may interfere with their activity(Reference Das49), the production of long-chain derivatives could occur to the detriment of the n-3 series. This could result in a deficit of α-linolenic acid and lower proportions of long-chain n-3 PUFA (EPA, 22 : 5n-3 and DHA) in the plasma of the HFD group.
As brain membranes are known to be sensitive to the type and amount of dietary fatty acids(Reference Marteinsdottir, Horrobin and Stenfors50), we measured the fatty acid profile of brain PE. Total SFA and MUFA proportions of brain PE did not differ between the two groups, suggesting that the unsaturation index of cerebral membranes was not modified by 8 weeks of HFD. Interestingly, the present results revealed modifications in PUFA content, with HFD rats exhibiting a significantly higher percentage of arachidonic acid and docosapentaenoic acid, concomitant with a lower proportion of n-3 PUFA, notably DHA. Eight weeks of HFD were thus sufficient to lead to an n-3 fatty acid deficit in young adult brain membranes. A similarly lower DHA content has already been described by Greenwood & Winocur(Reference Greenwood and Winocur44) in brain phosphatidylserine of rats fed a diet rich in saturated fat. Several authors have described the consequences of such a deficit on brain function. For instance, Horrocks & Farooqui(Reference Horrocks and Farooqui51) have reported on the consequences of a DHA deficiency on gene expression and synaptic plasticity. Additionally, DHA deficiency apparently plays an important role in neurodegenerative processes, as lower DHA levels in the brain increase the vulnerability of dendrites to β-amyloid deposition(Reference Calon, Lim and Yang52). In the blood, an epidemiological study has reported that the plasma levels of EPA, DHA and total n-3 fatty acids, as well as the n-6:n-3 ratio are lower in Alzheimer's patients, patients with other kinds of dementias and patients who are cognitively impaired but not demented. Interestingly, the decreased plasma level of DHA is not limited to Alzheimer's patients but appears to be common in cognitive impairment with ageing(Reference Conquer, Tierney and Zecevic53). Furthermore, in the present study, the fatty acid composition of red blood cell membranes after 8 weeks of HFD, i.e. a lower percentage of n-3 PUFA and a higher percentage of stearic acid and n-6 PUFA (data not shown), is comparable to the one described in human subjects as increasing the risk of cognitive decline(Reference Heude, Ducimetière and Berr54).
In view of data indicating that the retinoid signalling pathway is susceptible to the dietary intake of fatty acids and to the activity level of their PPAR-mediated signalling pathway in many animal tissues(Reference Khan and Vanden Heuvel16, Reference Delage, Bairras and Buaud20), we studied the effect of the HFD on brain retinoid signalling and on retinoid target genes involved in synaptic plasticity.
The present results demonstrate that HFD induces significant variations in the NR expression pattern of the retinoid (RAR and RXR) and fatty acid (PPAR) signalling pathways in the striatum, with a decrease in both RARβ and PPARδ expression together with a marked increase in RXRβγ expression. Several studies have already demonstrated a link between a decrease in RARβ transcripts and the deterioration of synaptic plasticity, notably in rats and mice during ageing or vitamin A deficiency(Reference Husson, Enderlin and Alfos38, Reference Féart, Mingaud and Enderlin55), as well as in RARβ knockout mice, which exhibit a deficit in long-term potentiation (the most widely studied form of synaptic plasticity, thought to underlie memory formation)(Reference Chiang, Misner and Kempermann25). The variation in RARβ expression is often due to its positive responsiveness to RA(Reference Yamagata, Momoi and Kumagai56), but in our model, there is no evidence to indicate a reduction in retinol bioavailability, i.e. no difference in the retinol content of plasma samples from HFD and control rats (data not shown), although HFD rats consumed more retinol. Thus, it could be assumed that the decrease in RARβ expression was probably induced by overall changes in the balance between the RA and fatty acid pathways, already reported for other tissues(Reference Bonilla, Redonnet and Nöel-Suberville57–Reference Groubet, Pallet and Delage59). These pathways involve RXR, a receptor that interacts directly with RAR and PPAR. RXR is the common dimerisation partner of the other two NR. In the present work, concomitant with the decrease in RARβ expression, there was an increase in the amount of RXRβγ mRNA. This pattern of receptor expression has previously been reported in the liver(Reference Bonilla, Redonnet and Nöel-Suberville57), colonic mucosa(Reference Groubet, Pallet and Delage59) and adipose tissue(Reference Redonnet, Groubet and Noël-Suberville60) of animals fed with a similar diet. In the present situation, we suggest that the modification in RXRβγ expression at the transcriptional level is directly induced by components of the diet, including fatty acids. The main ligand of RXR has been identified as 9-cis RA. Several studies have demonstrated that supra-physiological levels of DHA are required for efficient RXR activation, but that other naturally occurring PUFA as well as oleic acid activate RXR at similar levels(Reference de Urquiza, Liu and Sjoberg17, Reference Lengqvist, Mata De Urquiza and Bergman18). RXR is now recognised as an opportunistic NR capable of binding several ligands, including fatty acids derived from arachidonic acid metabolism, with differential affinities and transcriptional activity. Hence, the marked increase in RXR expression observed in the present experiment was probably due, at least in part, to the HFD-related increase in the n-6 fatty acid content of brain membranes. The HFD also induced significant modifications in PPARδ expression in the striatum, concomitant with that of RXRβγ. Both in vitro and in vivo observations have confirmed that PPARδ is the prevalent isoform in the brain(Reference Basu-Modak, Braissant and Escher61). The PPAR, including PPARδ, are currently recognised as generalised sensors of fatty acid levels, coupling fluxes in fatty acid levels with the transcriptional regulation of genes implicated in lipid and glucose homoeostasis. However, similar to RARβ, PPARδ activation in neurons may directly affect neuronal viability and differentiation(Reference Park, Lee and Kang62, Reference Smith, Monteith and Robinson63). It also promotes differentiation, myelin maturation and turnover in oligodendrocytes(Reference Dehmer, Lindenau and Haid64, Reference Cimini, Bernardo and Cifone65). Even a slight decrease in the expression of this master transcriptional factor could thus have neurobiological consequences. Some authors consider that the deleterious effects of HFD on memory performance are due to the development of insulin resistance(Reference Greenwood and Winocur5). It is now generally accepted that the PPAR act primarily by regulating energy homoeostasis, driving lipid and glucose metabolism and affecting insulin sensitivity(Reference Desvergne and Wahli66–Reference Heneka and Landreth69). Variations in the PPAR content of the brain may also be assumed to affect brain glucose metabolism, thus contributing to the induction of a deficit in memory performance. Taken together, the present results show that in rats fed a HFD, a modified expression pattern of the NR is associated with a decrease in the expression of the RA target genes RC3 and GAP-43, which code for neural proteins involved in synaptic plasticity. Comparable modifications in synaptic plasticity, i.e. reduced GAP-43 expression in the hippocampus associated with a deterioration in learning and memory, have been reported with respect to a high-fat, refined-sugar diet(Reference Molteni, Barnard and Ying4). The present study also revealed a HFD-related decrease in hippocampal GAP-43 expression at the protein level, without any modification in the amount of mRNA. This type of post-transcriptional regulation of GAP-43 has previously been described in the hippocampus(Reference Namgung and Routtenberg70). GAP-43 has been implicated in several forms of synaptic plasticity, including neurite outgrowth, regeneration and long-term potentiation(Reference Routtenberg, Cantallops and Zaffuto71). In vitro, a failure to induce GAP-43 has been shown to inhibit neuronal differentiation, resulting in cell death(Reference Mani, Shen and Schaefer72).
When the effect of the HFD on RC3 mRNA brain levels in each individual subfield was analysed separately, a significant diet-related effect was found in the CA1 and dentate gyrus. Thus, after 8 weeks of HFD, a marked decrease in RC3 expression was observed in the striatum, but only slight changes in the hippocampus. No difference between the two groups was observed in the cerebral cortex (data not shown). Interestingly, these results are similar to those reported in older animals, where a reduction in RC3 mRNA is first observed in the striatum, followed at a more advanced age by changes in the hippocampus(Reference Féart, Mingaud and Enderlin55). This region-specific regulation of RC3 may be due to a combinatorial distribution of transcription factors, as previously evoked(Reference Bernal, Guadano-Ferraz and Morte73). Indeed, the striatum is known to have large numbers of RA receptors, unlike the hippocampus, where RARβ mRNA and RXRβγ mRNA expression levels are almost undetectable(Reference Husson, Enderlin and Alfos38). This region-specific regulation of RC3 supports the proposal outlined by Zetterström et al. (Reference Zetterström, Lindqvist and Mata de Urquiza74) that retinoids play a predominant role in gene regulation events in the adult striatum, as demonstrated by the specific expression patterns of retinoid binding protein and aldehyde dehydrogenase, as well as the presence of RA in this region. Moreover, aged mice that exhibit a decreased expression of RARβ and RC3 in the brain also demonstrate age-specific memory loss, i.e. deficits in relational memory and hippocampal long-term potentiation(Reference Etchamendy, Enderlin and Marighetto21).
Conclusion
NR are now generally considered to be master transcription factors that act in precise combinations to orchestrate the maintenance of the neurobiological properties underpinning memory processes. Moreover, it has now been clearly established that the same NR also act as sensors, assessing the vitamin and lipid contents of the diet and controlling the metabolic response to it. Several reports have confirmed the occurrence of age-related modifications in the expression pattern of the RA NR (lower expression) in various tissues(Reference Féart, Mingaud and Enderlin55, Reference Pallet, Azais-Braesco and Enderlin75). In the brain, such events lead to neurobiological changes – especially a deterioration in synaptic plasticity, observable at the level of the transcription of molecular marker genes as well as by modifications in long-term potentiation – and are thus at least partly responsible for age-related memory decline(Reference Etchamendy, Enderlin and Marighetto21). The present study demonstrates that a HFD rich in saturated fat induces in the brain of young adult rats, a pattern of n-3 PUFA deficiency associated with changes in the expression of the retinoid NR similar to those that occur in the aged brain(Reference Etchamendy, Enderlin and Marighetto21).
Acknowledgements
The present study was partly funded by the Région Aquitaine, FranceAgriMer and Association de Coordination Technique pour l'Industrie Agro-alimentaire. The authors wish to thank L. Caune for animal care, and L. Fonseca and S. Serrano for technical assistance. None of the authors has any conflict of interest to report. B. B. and V. P. drafted the manuscript. B. B. performed the different analyses with the help of L. E., and was involved in their interpretation. C. V., S. A., N. C., P. H. and V. P. actively contributed to the interpretation of the results. All authors contributed to the interpretation and discussion of the results and saw and approved the final version of the present manuscript.









