Considerable interest has been focused on the influence of dietary fatty acids on CVD risk(Reference Mente, de Koning and Shannon1), with attention being centred on the value of dietary fat quality(Reference Mensink, Zock and Kester2–Reference Eckel, Kris-Etherton and Lichtenstein4). Evidence from prospective cohort studies and controlled clinical trials supports the use of dietary unsaturated fatty acids for the reduction of CVD risk factors(Reference Mente, de Koning and Shannon1–Reference Hu, Manson and Willett3, Reference Hu, Stampfer and Manson5). Therefore, dietary guidelines with a focus on cardiovascular health have recommended replacing SFA intakes with unsaturated fats(6). Increased consumption of novel dietary oils rich in MUFA and α-linolenic acid (ALA) may improve the fatty acid imbalance typical of modern Western diets (WD), high in SFA and the n-6:n-3 fatty acid ratio(Reference Kris-Etherton, Taylor and Yu-Poth7). Recent advances in the edible oil industry have produced dietary oils with nutritionally superior fatty acid profiles(Reference Tarrago-Trani, Phillips and Lemar8). High-oleic rapeseed (canola) oil (HOCO) is rich in MUFA, low in SFA and exhibits a low ratio of n-6:n-3 fatty acids. With enhanced oxidative stability, HOCO is an attractive oil replacement for high SFA–high trans oil varieties currently used in the food industry. Furthermore, recommendations have been made to increase dietary n-3 fatty acid intake(Reference Kris-Etherton, Taylor and Yu-Poth7). Flaxseed oil is a rich source of ALA; however, as flaxseed oil is less commonly consumed, blending flaxseed oil with other dietary oils provides a viable option to increase ALA intakes in WD.
Dyslipidaemia, specifically elevated LDL-cholesterol, is a primary risk factor in predicting CVD events and a major target of dietary intervention(Reference Lichtenstein and Appel9). Recently, elevated concentrations of circulating inflammatory biomarkers have been associated with cardiovascular events(Reference Ridker and Silvertown10–Reference Blankenberg, Rupprecht and Bickel12). C-reactive protein (CRP) and pro-inflammatory cytokines, such as IL-6, initiate the development of atherosclerosis by up-regulating endothelial expression of adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin(Reference Ross13). Therefore, the reduction of both circulating LDL-cholesterol levels and inflammatory biomarkers is important in ameliorating CVD risk.
To date, the efficacy of HOCO consumption in modulating established biomarkers of CVD risk has not been investigated in a human clinical study. Additionally, although a high dose of flaxseed oil consumption has been reported to reduce inflammatory biomarkers in at-risk subjects(Reference Prasad14), the effects of flaxseed oil on serum lipids have been inconsistent(Reference Prasad14, Reference Pan, Yu and Demark-Wahnefried15). Therefore, the objectives of this human clinical study were to evaluate the efficacy of HOCO and a flaxseed/high-oleic rapeseed oil (FXCO) blend in modulating circulating lipids and inflammatory biomarkers associated with CVD risk as compared with a typical WD.
Experimental methods
Subjects
Thirty-nine individuals (fourteen males and twenty-five females) were recruited using flyers and media advertisements. Subjects were screened for LDL-cholesterol after 12 h of fasting, and detailed blood chemistry analyses were performed. Inclusion criteria for the study were serum LDL-cholesterol>3·0 mmol/l, age 18–65 years and BMI between 22 and 36 kg/m2. Before study enrolment, the subjects underwent a routine physical examination by the study physician. Exclusion criteria were documented atherosclerotic disease, inflammatory disease, diabetes mellitus, uncontrolled hypertension, kidney disease, cancer, tobacco smoking, use of lipid-lowering medications for at least 3 months before starting the study, alcohol consumption>2 servings/d or excessive exercise expenditure of>16 735 kJ (4000 kcal)/week. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Bannatyne Campus Research Ethics Board (Protocol no. B2007:071) and the St Boniface General Hospital Research Review Committee (Ref no. RCC/2007/0862). A written informed consent was obtained from all the subjects. The study is registered in the ClinicaTrials.gov registry (identifier #NCT00927199).
Experimental design
A randomised, single-blind, crossover, controlled diet clinical trial was conducted at the Clinical Research Unit at the Richardson Centre for Functional Foods and Nutraceuticals, University of Manitoba. The study was designed as three phases with 28 d per phase separated by 4–8-week washout periods during which the subjects consumed their habitual diets. The subjects were randomised to the three experimental diets using a 3 × 3 Latin-square design. Diets were individualised to meet daily energy requirements for weight maintenance for each subject as determined by the Mifflin equation(Reference Mifflin, St Jeor and Hill16), multiplied by a factor of 1·7 for medium physical activity. The study diets were prepared in the metabolic kitchen of the Richardson Centre Clinical Nutrition Research Unit and the food ingredients were weighed within 0·5 g. Diets consisted of three isoenergetic meals prepared according to a 3 d cycle menu providing a variety of foods. In order to ensure stability of the flaxseed oil, experimental oils were added to cold foods; provided in milkshakes at breakfast and puddings at lunch and dinner. The subjects consumed one of three daily meals (breakfast) at the Richardson Centre Clinical Nutrition Research Unit under supervision, while other meals (lunch and dinner) were prepared and cold packed for take out. The subjects were instructed to consume only foods and beverages provided by the Richardson Centre Clinical Nutrition Research Unit and to refrain from alcoholic and caffeinated beverages during the intervention periods. The subjects were advised to maintain their typical physical activity level and asked to report any symptoms or changes in health and medications throughout the study. Subjects' body weights were measured under supervision every morning before breakfast using a medical scale (Detecto, Webb City, MO, USA) to monitor weight stability.
Diets
Experimental diets were designed as typical WD containing 50 % of energy as carbohydrate, 15 % as protein and 35 % as fat, of which 70 % was provided by the experimental oil. Diets were identical in composition throughout each phase, except for the type of experimental oil. Macronutrient profiles of experimental diets (Table 1) were analysed using the nutrient composition software FOOD PROCESSOR (Food Processor version 7.81; Salem, OR, USA). Experimental oils tested included (1) HOCO (approximately 70 % oleic acid; Canola Harvest HiLo®; Richardson Oilseed Limited, Lethbridge, AB, Canada); (2) a 1:1 blend of the HOCO and flaxseed oil (FXCO) (approximately 55 % ALA and no lignans; Bioriginal Food & Science Corporation, Saskatoon, SK, Canada); (3) a blend of oils typical of a WD including non-salted butter (12 %), extra-virgin olive oil (35 %), vegetable lard (35 %) and sunflower oil (>60 % linoleic acid) (18 %). Fatty acid profiles of experimental oils are reported in Table 2.
Table 1 Macronutrient profile of the three experimental diets*
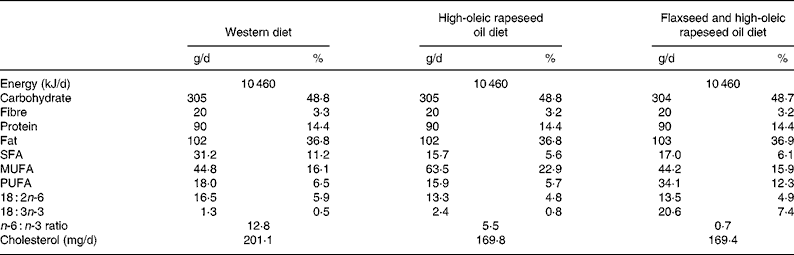
* The macronutrient profile of the three experimental diets was estimated using FOOD PROCESSOR software (version 7.81; Food Processor, Salem, OR, USA).
Table 2 Fatty acid composition of the three experimental dietary oils*
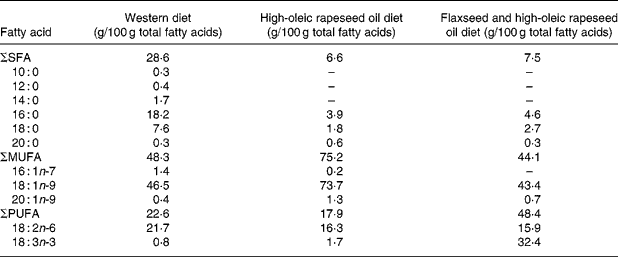
–, Undetected fatty acid.
* Values were determined by GC of triplicate samples of the dietary oil blends.
Blood sampling and serum lipid analysis
On days 1, 2, 28 and 29 of each phase, 12 h fasted serum and EDTA plasma samples were collected. Within 1 h of blood collection, serum, plasma and erythrocyte fractions were separated by centrifugation at 3000 rpm for 20 min at 4°C, aliquoted and immediately stored at − 80°C until further analysis.
Serum total cholesterol (TC), HDL-cholesterol, TAG and glucose levels were determined by automated enzymatic methods on a Vitros-350 chemistry analyser (Ortho-Clinical Diagnostics, Markham, ON, Canada). Serum LDL-cholesterol levels were calculated by the Friedewald equation(Reference Friedewald, Levy and Fredrickson17).
Plasma inflammatory biomarker and cell adhesion molecule analysis
Plasma CRP levels were measured using quantitative colorimetric sandwich ELISA according to the manufacturer's guidelines (R&D Systems, Minneapolis, MN, USA). IL-6 levels were measured by high-sensitivity ELISA (R&D Systems, Minneapolis, MN, USA). The intra-assay and inter-assay CV values were 2·31 and 4·26 %, and 2·51 and 8·04 %, for CRP and IL-6, respectively.
Plasma soluble adhesion molecules (sVCAM-1, sICAM-1 and sE-selectin) were measured simultaneously by flow cytometry using multianalyte profiling performed on a Luminex-100 IS system (Luminex Corporation, Austin, TX, USA). Plasma concentrations of sVCAM-1, sICAM-1 and sE-selectin were determined using a MILLIPLEX MAP human CVD panel-1 (3-plex) kit according to the manufacturer's guidelines (HCVD1-67AK; Millipore Corporation, Billerica, MA, USA). Acquired median fluorescent intensity data were analysed using a weighted 5-parameter logistic curve by the IS 2.3 software (Luminex Corporation, Austin, TX, USA). The sensitivity of the assay reported by the manufacturer had a minimum detectable concentration of 0·016, 0·009 and 0·079 ng/ml for sVCAM-1, sICAM-1 and sE-selectin, respectively. The intra-assay and inter-assay CV values were 7·4 and 10·9 %, 8·8 and 11·3 %, and 6·0 and 7·4 % for sVCAM-1, sICAM-1 and sE-selectin, respectively.
For the analyses of inflammatory biomarkers by ELISA and adhesion molecules by LUMINEX, controls (low, medium and high) supplied by the respective assay manufacturer and the subject plasma samples were assayed in duplicate by a single laboratory technician with all the samples for each subject run in one assay.
Plasma fatty acid profile
Plasma total lipids were extracted by the Folch method(Reference Folch, Lees and Slone Stanley18) using chloroform–methanol (2:1, v/v) containing 0·01 % BHT (Sigma-Aldrich, Oakville, ON, Canada) and heptadecanoic acid as an internal standard (Sigma-Aldrich, Oakville, ON, Canada). Extracted fatty acids were methylated with methanolic HCl. Fatty acid methyl esters were separated on a Supelcowax 10 column (30 m × 0·25 mm with 0·25 μm film thickness; Supelco, Bellefonte, PA, USA) using an Agilent 6890N gas chromatograph equipped with a flame ionisation detector (Agilent Technologies, Mississauga, ON, Canada). The oven was programmed from 70 to 240°C in four temperature steps (70°C for 1 min, rise of 25°C/min, 180°C for 2 min, rise of 3°C/min, 220°C for 10 min, rise of 20°C/min, 240°C for 15 min). Samples were run with a 10:1 split ratio, and He was used as the carrier gas with a column flow rate of 1·0 ml/min. Temperatures for the injector and detector were set at 280 and 300°C, respectively. Individual fatty acids were identified by comparison with known standards (NuChek Prep, Inc., Elysian, MN, USA). Individual fatty acids were calculated according to the peak area relative to the total area and expressed as the percentage of total fatty acids.
Intima-media thickness assessment
A subset of study subjects (n 18; randomised selection from study population) underwent clinical endothelial health assessment at the onset of the study (phase 1; days 1–3) and at the end of each treatment phase (days 24–26) by common carotid arterial ultrasound to assess changes in intima-media thickness (IMT). Assessment of common carotid IMT was performed with the use of an annular array ultrasound imaging system (9L probe; GE Vivid 7, Milwaukee, IL, USA). The subjects were examined in the supine position. Ultrasound scans of the right and left common carotid arteries were performed at the bifurcation of the first proximal centre of internal carotid arteries as described previously(Reference Lorenz, Markus and Bots19). All the measurements were made offline of the longitudinal carotid IMT scans using dedicated computer software (GE Echopac BT 08; Milwaukee, IL, USA). Average and maximal IMT values of each segment were measured as described previously(Reference Lorenz, Markus and Bots19). All ultrasound scans were performed by two trained sonographers, and the recorded ultrasound images were analysed blindly at the Institute of Cardiovascular Sciences, St Boniface General Hospital Research Centre, Winnipeg, Canada.
Statistical analyses
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Results are expressed as means with their standard errors unless otherwise noted. For variables with non-normal distribution, as determined by a Shapiro–Wilk test value < 0·05, statistical analyses were conducted after a logarithmic (base 10) transformation. Data on inflammatory biomarkers and adhesion molecules were not normally distributed and are reported as the median and 25th and 75th percentiles. Effects of dietary treatment were examined using a mixed model ANOVA procedure with diet, sequence and phase as fixed factors and subject as a random factor in the model. Baseline values were inserted into the model as covariates for serum lipid measurements. Significant diet effects were examined with the Bonferroni adjustment for multiple comparisons. For serum lipids, percentage change from baseline for each group was analysed with a two-tailed paired Student's t test. Pearson correlation analyses were conducted to test the associations between lipid levels and inflammatory biomarkers. Statistical significance was set at P < 0·05 for all the analyses. For all the data, baseline and endpoint values are reported as averages of days 1 and 2, and days 28 and 29, respectively.
Results
Subject characteristics
Baseline characteristics of the subjects who completed the study are presented in Table 3. Thirty-six subjects (thirteen males and twenty-three females; five premenopausal) completed the study. Two subjects withdrew from the study due to relocation of residence, and one withdrew due to work-related issues. All the subjects showed good tolerance to experimental diets and reported consuming all meals provided to them. No side effects were associated with the experimental diets. The subjects reported no change in physical activity, and no significant differences were observed in body weight after the consumption of the three experimental diets.
Table 3 Baseline characteristics of the subjects
(Mean values and standard deviations; median values with their 25th and 75th percentiles)
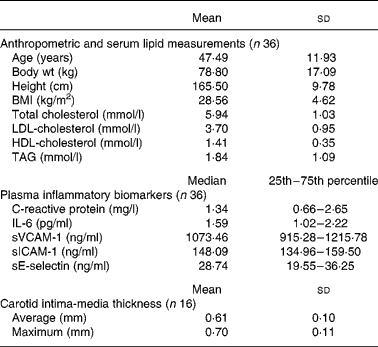
sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; sE-selectin, soluble E-selectin.
Plasma fatty acids
After consumption of the experimental diets, changes in the plasma fatty acid concentrations (Table 4) reflected the fatty acid profile of the experimental diets (Table 1), verifying subjects' compliance with the experimental diets. As expected, plasma total MUFA, specifically 18 : 1n-9, was higher after the consumption of the HOCO diet than after the consumption of both the FXCO diet (P < 0·001) and the WD control (P < 0·001). Plasma total PUFA and total n-3 PUFA (including 18 : 3n-3, 20 : 5n-3 and 22 : 5n-3) were higher after the consumption of the FXCO diet than after the consumption of both the HOCO diet (P < 0·001) and the WD control (P < 0·001). No change in plasma DHA (22 : 6n-3) content was observed after the consumption of the FXCO diet compared with the WD control (P = 0·683); however, there was a slight decrease in plasma DHA content after the consumption of the FXCO diet compared with the HOCO diet (P = 0·025). Plasma total SFA, total n-6 PUFA (specifically 18 : 2n-6) and n-6:n-3 ratios were lower after the consumption of both the HOCO and FXCO diets than after the consumption of the WD control (P < 0·001 for all). Furthermore, plasma n-6:n-3 ratio was lower after the consumption of the FXCO diet than after the consumption of the HOCO diet (P < 0·001). No significant differences in the baseline fatty acid concentrations across the groups indicated no carryover effect and adequate washout periods between the treatment phases (data not shown).
Table 4 Plasma fatty acid concentration at the end of each of the three experimental diets
(Mean values with their standard errors)

a,b,c Mean values within a row with unlike superscript letters were significantly different between treatment groups (P < 0·05).
* P values are shown for the treatment effect analysed by mixed model ANOVA (with the Bonferroni adjustment for multiple comparisons).
Serum lipid concentrations
The concentrations of fasting serum lipids and glucose at the end of each treatment phase are presented in Table 5. Serum lipid percentage change from baseline is presented in Fig. 1. After the 28 d treatment phase, serum TC concentrations were reduced when the subjects consumed the HOCO diet (5·27 (sem 0·14) mmol/l; P < 0·001) and the FXCO diet (5·12 (sem 0·13) mmol/l; P < 0·001) compared with the WD control (5·65 (sem 0·16) mmol/l). TC percentage change from baseline was reduced by 3·5 % (P = 0·002) and 11·0 % (P < 0·001) when subjects consumed the HOCO and FXCO diets, respectively, compared with the WD control. Furthermore, TC endpoint values (P = 0·025) and percentage change from baseline (7·5 %; P = 0·015) were lower when the subjects consumed the FXCO diet compared with the HOCO diet.
Table 5 Serum lipid and glucose concentrations at the end of each of the three experimental diets
(Mean values with their standard errors)

a,b,c Mean values within a row with unlike superscript letters were significantly different between treatment groups (P < 0·05).
* P values are shown for the treatment effect analysed by mixed model ANOVA (with the Bonferroni adjustment for multiple comparisons).

Fig. 1 Percentage change in serum lipids from baseline in response to the three treatment diets: ■, Western diet; ![]() , high-oleic rapeseed oil diet; □, flaxseed/high-oleic rapeseed oil diet. Values are presented as means with their standard errors (n 36). a,b,c Mean values with unlike letters between treatment groups are significantly different at P ≤ 0·05 (mixed model ANOVA followed by the Bonferroni adjustment for multiple comparisons). Mean values were significantly different when compared within treatment group from baseline: *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001 (two-tailed paired-Student's t test). TC, total cholesterol.
, high-oleic rapeseed oil diet; □, flaxseed/high-oleic rapeseed oil diet. Values are presented as means with their standard errors (n 36). a,b,c Mean values with unlike letters between treatment groups are significantly different at P ≤ 0·05 (mixed model ANOVA followed by the Bonferroni adjustment for multiple comparisons). Mean values were significantly different when compared within treatment group from baseline: *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001 (two-tailed paired-Student's t test). TC, total cholesterol.
Similarly, endpoint serum LDL-cholesterol concentrations were reduced after the consumption of the HOCO diet (3·10 (sem 0·12) mmol/l; P < 0·001) and the FXCO diet (3·08 (sem 0·12) mmol/l; P < 0·001) compared with the WD control (3·53 (sem 0·14) mmol/l). LDL-cholesterol percentage change from baseline was reduced by 7·4 % (P < 0·001) and 15·1 % (P < 0·001) after the consumption of the HOCO and FXCO diets, respectively, compared with the WD control. However, no differences were observed in endpoint or percentage change from baseline in LDL-cholesterol concentrations between the FXCO and HOCO diets.
No differences were observed in endpoint TAG concentrations between the treatment groups (P = 0·060; trend). With respect to percentage change from baseline, no differences were observed for serum TAG concentrations between the treatment groups.
Endpoint serum HDL-cholesterol concentrations were reduced after the consumption of the FXCO diet (1·28 (sem 0·06) mmol/l) compared with the HOCO diet (1·33 (sem 0·06) mmol/l; P = 0·008) and the WD control (1·37 (sem 0·06) mmol/l; P < 0·001). The FXCO diet reduced HDL-cholesterol concentrations from baseline by 6·6 % (P = 0·006) and 8·5 % (P < 0·001) compared with the HOCO diet and WD control, respectively. No differences were observed in endpoint or percentage change from baseline in HDL-cholesterol concentrations between the HOCO diet and WD control.
Endpoint LDL:HDL-cholesterol ratios were reduced after the consumption of the HOCO diet (2·49 (sem 0·14); P < 0·001) and FXCO diet (2·62 (sem 0·17); P = 0·018) compared with the WD control (2·76 (sem 0·17)). Both the HOCO and FXCO diets reduced the LDL:HDL-cholesterol ratio from baseline by 5·7 % (P = 0·002) and 7·5 % (P = 0·008), respectively, compared with the WD control. Endpoint and percentage change from baseline in serum TC:HDL-cholesterol ratios did not differ after the treatment periods. Endpoint non-HDL-cholesterol was reduced after the consumption of the HOCO diet (3·94 (sem 0·14); P = 0·003) and FXCO diet (3·84 (sem 0·14); P < 0·001) compared with the WD control (4·28 (sem 0·17)). Both the HOCO and FXCO diets reduced non-HDL-cholesterol from baseline by 3·9 % (P = 0·004) and 11·7 % (P < 0·001), respectively, compared with the WD control. Furthermore, non-HDL-cholesterol endpoint values (P = 0·031) and percentage change from baseline (7·8 %; P = 0·030) were lower when the subjects consumed the FXCO diet compared with the HOCO diet.
No significant effects were observed in fasting serum glucose endpoint levels between the treatment groups, nor were changes from baseline values observed.
Plasma inflammatory biomarkers and adhesion molecule concentrations
The results for measures of inflammatory biomarkers by ELISA and adhesion molecules by LUMINEX were within the detection limits of the assay. No significant differences were observed in endpoint concentrations for CRP or IL-6 between the treatment groups (Table 6). A decrease in endpoint E-selectin concentrations was observed after the consumption of the FXCO diet compared with the WD control (P = 0·023), however, not in comparison with the HOCO diet (P = 0·34). No significant changes were observed in endpoint concentrations for sVCAM-1 and sICAM-1 between the treatment groups (Table 6). As compared with the WD control, after the subjects consumed the FXCO diet, the change in endpoint E-selectin concentrations was directly associated with changes in TC (r 0·413; P = 0·012), LDL-cholesterol (r 0·383; P = 0·021) and non-HDL-cholesterol (r 0·340; P = 0·042) concentrations (Table 7). However, changes in E-selectin concentrations following the consumption of the FXCO diet compared with the WD control did not correlate with other lipid parameters or plasma fatty acid concentrations (data not shown). There were no correlations between changes in lipid concentrations after the consumption of the HOCO diet and changes in inflammatory biomarkers.
Table 6 Plasma inflammatory biomarker concentrations and carotid intima-media thickness (IMT) at the end of each of the three experimental diets
(Median values with their 25th and 75th percentiles for inflammatory biomarkers; mean values with their standard errors for IMT)

CRP, C-reactive protein; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; sE-selectin, soluble E-selectin; IMT, intima-media thickness.
a,b Mean values within a row with unlike superscript letters were significantly different between treatment groups (P < 0·05).
* P values are shown for the treatment effect analysed by mixed model ANOVA (with the Bonferroni adjustment for multiple comparisons).
Table 7 Correlation coefficients among the change in plasma E-selectin and the changes in serum lipids when the subjects consumed the flaxseed and high-oleic rapeseed oil diet compared with the Western diet*
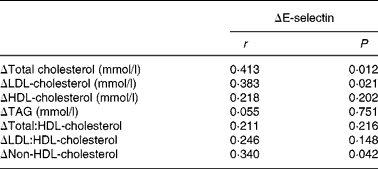
* Pearson correlation analyses were conducted to test associations.
Intima-media thickness assessment
A subset of sixteen subjects (age, 48·7 (sem 11·9) years; BMI, 30·53 (sem 4·64) kg/m2; four males and twelve females (four premenopausal)) completed the assessment of common carotid IMT. Two subjects withdrew due to relocation of residence. For endpoint measures, there were no significant changes detected in right posterior wall or left posterior wall average or maximum IMT between the dietary treatments or from baseline values at the study entry (Table 6).
Discussion
The present results are the first to demonstrate the lipid-lowering efficacy of low-SFA diets enriched with novel HOCO alone or blended with ALA-rich flaxseed oil. Compared with the WD control, we observed substantial decreases in TC for both the HOCO and the FXCO diets after 28 d, with the FXCO diet further reducing TC beyond that of the HOCO diet (Table 5; Fig. 1). The present study observed similar reductions in LDL-cholesterol concentrations after the consumption of the HOCO and FXCO diets compared with the WD control. Reports examining the lipid-lowering action of PUFA-rich v. MUFA-rich diets support the notion that PUFA-rich diets reduce TC and LDL-cholesterol concentrations comparable with MUFA-rich diets, and that PUFA oils elicit a slight TAG-lowering effect(Reference Mensink, Zock and Kester2, Reference Kris-Etherton20–Reference Dai, Su and Bartell22). Similarly, compared with the HOCO diet, the FXCO diet and WD control, both higher in dietary PUFA content, tended to reduce endpoint TAG concentrations; however, due to large individual variation, there was no difference in percentage change in TAG levels from baseline between the dietary interventions examined.
The ability of HOCO to reduce TC and LDL-cholesterol, as well as to preserve HDL-cholesterol, is of particular interest since to date the efficacy of HOCO in modulating blood lipids has not been assessed. Furthermore, it has previously been reported that not all MUFA-rich oils elicit the same effects on plasma cholesterol concentrations(Reference Truswell and Choudhury23), suggesting the importance of other oil-derived fatty acid and non-lipid components. Reports suggest that ALA-rich flaxseed oil interventions fail to modify TC and LDL-cholesterol levels when compared with other dietary interventions(Reference Prasad14, Reference Pan, Yu and Demark-Wahnefried15). However, these results could be confounded by the use of MUFA and n-6 PUFA dietary controls. Limited work has directly compared dietary flaxseed oil with MUFA-rich oils. Whereas Singer et al. (Reference Singer, Jaeger and Berger24) observed a reduction in TAG, as well as in TC and LDL-cholesterol levels after 2-week supplementation with 60 ml/d of flaxseed oil but not with olive oil, Li et al. (Reference Li, Sinclair and Wilson25) failed to find differences in plasma lipids after 4-week supplementation of a rapeseed oil or flaxseed oil-enriched diet. In the present study, substitution of 50 % HOCO with flaxseed oil in the FXCO treatment group was effective in further reducing TC compared with the HOCO treatment group.
FXCO reduced HDL-cholesterol from baseline, resulting in lower endpoint HDL-cholesterol levels than the WD control (Table 5; Fig. 1). Previous studies administering high doses of flaxseed oil to hypercholesterolaemic subjects have observed reductions in HDL-cholesterol levels(Reference Rallidis, Paschos and Liakos26–Reference Wilkinson, Leach and Ah-Sing29). Generally, dietary strategies replacing SFA with PUFA result in a reduction in plasma TC and LDL-cholesterol and a parallel decrease in plasma HDL-cholesterol concentrations. Although concern exists that the cardioprotection associated with low LDL-cholesterol is diminished with simultaneous reductions in HDL-cholesterol, it has been shown that rates of cholesterol efflux from macrophage cells to serum are not affected(Reference Kralova Lesna, Suchanek and Kovar30). Furthermore, endpoint LDL:HDL-cholesterol ratios were reduced in response to the HOCO and FXCO diets compared with the WD control (Table 5). The LDL:HDL-cholesterol ratio is valuable in evaluating CVD risk across many populations(Reference Fernandez and Webb31). As well, non-HDL-cholesterol provides a single measure of the atherogenic apo B-containing lipoproteins and can thus provide a tool for cardiovascular risk assessment(6, Reference Fernandez and Webb31). After the consumption of the FXCO diet, non-HDL-cholesterol levels decreased beyond that of the HOCO diet and the WD control. Therefore, the additive effects of ALA and oleic acid in the FXCO diet may have provided additional hypolipidaemic effects that extend beyond those incurred by the HOCO diet alone.
In addition to dyslipidaemia, elevated CRP levels associate with clinical manifestations of atherosclerosis and CVD risk(Reference Ridker and Silvertown10). The intricate communication between inflammatory stimuli and endothelial cell adhesion molecules regulates inflammatory responses and the progression of atherosclerosis(Reference Ross13). Thus, a direct association may exist between plasma concentrations of VCAM-1, ICAM-1 and E-selectin and the extent of atherosclerosis and incidence of CVD risk(Reference Hwang, Ballantyne and Sharrett11, Reference Blankenberg, Rupprecht and Bickel12). In vitro studies have shown the ability of oleic acid to inhibit cytokine-induced expression of VCAM-1, ICAM-1 and E-selectin in endothelial cells(Reference Carluccio, Massaro and Bonfrate32, Reference Massaro, Carluccio and Paolicchi33). Although human clinical trials have yet to specifically investigate effects of HOCO on inflammatory biomarkers, Keogh et al. (Reference Keogh, Grieger and Noakes34) failed to observe any effect of a MUFA-rich diet on serum CRP or plasma adhesion molecules in forty healthy adults. Likewise, the consumption of the HOCO-rich diet for 28 d did not affect inflammatory biomarker measures. Results of the clinical trials investigating the effects of flaxseed oil on inflammatory biomarkers and adhesion molecules are inconsistent(Reference Prasad14). It has been suggested that the discrepancy may be dose related, as intakes exceeding 14 g/d of ALA from flaxseed oil have been shown to be more effective. In the present study, after 4-week supplementation of 21 g/d (7·5 % energy) of ALA in the FXCO diet, a reduction was observed in E-selectin compared with the WD control; however, no reductions in other inflammatory biomarkers were observed. In a 6-week randomised crossover trial that examined hypercholesterolaemic subjects consuming 6·5 % ALA supplemented from walnuts and flaxseed oil daily, Zhao et al. (Reference Zhao, Etherton and Martin28) observed significant reductions in serum CRP, VCAM-1, ICAM-1 and E-selectin, compared with an average American diet. Similarly, decreases in CRP, VCAM-1, as well as in IL-6, have been reported with the supplementation of 15 ml/d flaxseed oil (8·1 g ALA/d) for 12 weeks(Reference Rallidis, Paschos and Liakos26, Reference Paschos, Rallidis and Liakos35, Reference Rallidis, Paschos and Papaioannou36); however, no effects on ICAM-1 or E-selectin were observed(Reference Paschos, Rallidis and Liakos35, Reference Rallidis, Paschos and Papaioannou36). In contrast, recently Nelson et al. (Reference Nelson, Stevens and Hickey37) failed to observe decreases in CRP or IL-6 in healthy abdominally obese subjects consuming 5 % of energy from ALA for 8 weeks. Similar to the latter study, we observed no change in plasma CRP or IL-6 concentration following the consumption of the FXCO diet.
Unlike VCAM-1 and ICAM-1, E-selectin activity is specific to the surface of stimulated endothelial cells, mediating the rolling of monocytes along the cell surface(Reference Roldan, Marin and Lip38). Furthermore, the expression of E-selectin directly associates with dyslipidaemia. It was previously shown that effective lipid-lowering intervention reduced plasma E-selectin concentrations in dyslipidaemic subjects; however, the lipid-lowering effect was not associated with a reduction in VCAM-1 or ICAM-1(Reference Hackman, Abe and Insull39). Of interest, in the present study, a significant correlation was observed between changes in plasma E-selectin and TC, LDL-cholesterol and non-HDL-cholesterol concentrations when subjects consumed the FXCO diet compared with the WD control (Table 7). However, albeit the reduction in serum lipids following the consumption of the FXCO and HOCO diets, there was no change in VCAM-1 or ICAM-1 concentrations. Since the FXCO diet resulted in reductions in TC and non-HDL-cholesterol concentrations beyond that of the HOCO diet (Table 5), we speculate that the acute effects of FXCO consumption on E-selectin concentrations may be attributed to the magnitude of reductions in circulating lipids.
The discrepancy between the present results and those of previous studies that reported reductions in inflammatory biomarkers may be related to subject baseline levels of those biomarkers. In the present study, the subject baseline levels of inflammatory biomarkers CRP and IL-6 were in the healthy range compared with those of subjects examined previously(Reference Rallidis, Paschos and Liakos26, Reference Paschos, Rallidis and Liakos35, Reference Bemelmans, Lefrandt and Feskens40). Similarly, studies that failed to observe an effect of ALA intervention on inflammatory biomarkers have attributed the absence of response to a ‘floor effect’; the inability to detect changes due to low baseline levels(Reference Nelson, Stevens and Hickey37, Reference Barcelo-Coblijn, Murphy and Othman41). Another consideration may be the duration of the present study. Although a 4-week intervention is typically sufficient to observe significant alterations in blood lipids, previous studies reporting reductions in inflammatory markers were of 6–12 weeks in duration(Reference Rallidis, Paschos and Liakos26, Reference Zhao, Etherton and Martin28, Reference Paschos, Rallidis and Liakos35, Reference Rallidis, Paschos and Papaioannou36). Similarly, the limited study duration may also explain the absence of treatment effects on carotid IMT. Bemelmans et al. (Reference Bemelmans, Lefrandt and Feskens40), using a parallel-arm design and a 2-year dietary intervention, found that 4·5 g/d of ALA yielded no significant effect on IMT progression. The present study focused on examining whether a high dose of ALA, approximately 3·5-fold greater than that used in Bemelmans et al. (Reference Bemelmans, Lefrandt and Feskens40), utilising a crossover design, would have acute effects on IMT progression; however, no positive action was observed.
The plasma fatty acid concentrations reflected fatty acid profiles of the experimental oils, indicating compliance with the dietary interventions(Reference Baylin and Campos42, Reference Hodson, Skeaff and Fielding43). After consumption of the ALA-rich FXCO diet, an approximate 5-fold increase in plasma ALA (18 : 3n-3) concentrations and 3-fold increase in EPA (20 : 5n-3) concentrations were observed compared with the HOCO diet and WD control. However, there were no differences in plasma DHA (20 : 6n-3) concentrations between the FXCO diet and the WD control. These results are consistent with previous stable isotope tracer studies demonstrating the linear relationship between dietary ALA intakes and plasma EPA, with no direct relationship between ALA intakes and plasma DHA due to limited conversion rates(Reference Burdge44). Nonetheless, the increase in plasma concentration of ALA, EPA and DPA after the consumption of the FXCO diet may be cardioprotective as an inverse association has been found between plasma concentrations of combined EPA and DHA, as well as of ALA, and risk of fatal IHD(Reference Lemaitre, King and Mozaffarian45). Furthermore, the higher plasma MUFA concentration after the consumption of the HOCO diet may provide cardiovascular benefits, as MUFA has been shown to be resistant to oxidative modifications of LDL-cholesterol(Reference Moreno, Lopez-Miranda and Perez-Martinez46).
A potential limitation of the present study is that the experimental diets were not balanced for dietary cholesterol levels; however, it has been reported that in human subjects, dietary fatty acids are primary determinants of serum cholesterol, whereas dietary cholesterol has minimal effect on modulating serum cholesterol levels(Reference Hu, Manson and Willett3, Reference Grundy and Denke47, Reference Jones, Pappu and Hatcher48). Furthermore, the average daily intake of cholesterol in each experimental diet was considerably lower than the AHA recommendation of < 300 mg/d(Reference Lichtenstein and Appel9). Moreover, the feasibility of incorporating both HOCO and FXCO into typical diets requires further consideration. In order to maintain total fat energy intake, it is crucial to target fat substitution v. fat supplementation of the diet. The high-stability properties of HOCO make it a practical substitution for trans fat-rich partially hydrogenated vegetable oils in food processing, frying and culinary purposes(Reference Tarrago-Trani, Phillips and Lemar8). Increased dietary ALA intake can be achieved by fortifying dressings, spreads and margarines with the FXCO blend as a replacement of traditional products. Currently, the US Food and Drug Administration (FDA) has authorised a qualified health claim stating that rapeseed (canola) oil (approximately 19 g/d) may reduce the risk of CHD due to its unsaturated fat content, recommending direct energetic replacement of dietary SFA with rapeseed (canola) oil(49). Therefore, increased compliance with dietary recommendations and targeting a reduction in CHD risk would be possible by replacing a proportion of commonly used dietary oils and spreads in the WD with HOCO alone or blended with flaxseed oil.
In conclusion, the present study is the first human clinical trial to investigate effects of HOCO on serum lipids and other markers of CVD risk. HOCO alone or when blended with flaxseed oil effectively reduced serum TC and LDL-cholesterol compared with a WD. Moreover, the ALA-rich FXCO may further target inflammation and atherogenic pathways by reducing plasma E-selectin. Substitution of dietary fats common to the WD with both HOCO and flaxseed oil is a feasible option to target dietary recommendations and risk factors for CVD.
Acknowledgements
We thank the personnel at the Richardson Centre for Functional Foods and Nutraceuticals including Kimberley Robinson as a clinical coordinator, Darren Speziale, Katherine Leung and the other staff of the Metabolic Kitchen for preparation of the controlled meals, and Joan Richardson and ultrasound sonographers at St Boniface General Hospital for conducting the carotid ultrasound scans. The present work was funded in part by Flax Canada 2015, Canola Council of Canada and Agri-Food Research & Development Initiative. We acknowledge the kind contribution of the flaxseed oil and the HOCO from Bioriginal Food & Science Corporation and Dow AgroSciences LLC, respectively. L. G. G. was supported by a doctoral studentship award from the Manitoba Health Research Council. P. J. H. J. was responsible for the conception and design of the project, submission for ethical approval and seeking financial support. L. G. G. was responsible for subject recruitment, management of the clinical trial, data collection and laboratory analysis, statistical analysis and writing the manuscript. J. A. G. contributed to subject recruitment and was clinical coordinator for the trial. S.-Y. H. and D. S. J. coordinated and analysed IMT scans. All the authors contributed to revisions of the manuscript and reviewed the final version. The authors declare no conflict of interest.










