Dietary fibres are important constituents of diet which have been reported to have remarkable beneficial effects that include their hypolipidaemic property (Anderson, Reference Anderson1985). These substances of plant origin include a number of NSP materials like cellulose, hemicellulose, β-glucans, pectins, mucilages and gums and the non-polysaccharide lignin. These fibres have unique chemical structures and characteristic physical properties (Kay, Reference Kay1982; Van Horn, Reference Van Horn1997; Anderson & Hanna, Reference Anderson and Hanna1999). The physical characteristics of dietary fibres include high water-holding capacity, viscosity, fermentability and adsorption/binding to different biomolecules and metallic ions (Schneeman & Tietyen, Reference Schneeman, Tietyen, Shills, Olson and Shike1994). Fibres with high water-holding capacity, such as pectin, gums and mucilages, have been referred to as soluble fibre.
Different classes of fibres differ in their potential to lower lipid levels (Kritchevsky & Stony, Reference Kritchevsky, Stony and Spiller1986; Anderson et al. Reference Anderson, Jones and Riddell-Mason1994). Certain fibres, particularly soluble fibres capable of forming gels, appear to be effective in lowering serum levels of total and LDL cholesterol in normal subjects as well as in those with hyperlipidaemia. Specific mechanisms involved in the hypolipidaemic effect of dietary fibre remain inconclusive. Recent research provides evidence that the hypocholesterolaemic effect of dietary fibre may be due to fibre-induced alterations of intestinal absorption, intestinal or pancreatic hormone secretion, lipoprotein metabolism, bile acid metabolism, or by-products of fermentation and their effect on hepatic cholesterol synthesis. Gallaher et al. (Reference Gallaher, Hassel, Lee and Gallaher1993) and Carr et al. (Reference Carr, Gallaher, Yang and Hassel1996) showed that the viscosity of dietary fibre is related to its cholesterol-lowering effect in hamsters fed a cholesterol-supplemented diet. Fermentation of fibre can lead to an enhanced production of SCFA, particularly propionate, which may be involved in the hypocholesterolaemic effect of certain soluble plant fibres (Chen et al. Reference Chen, Anderson and Jennings1984). SCFA produced by caecal fermentation of dietary fibre have been reported to suppress cholesterol synthesis in rat liver and intestine (Hara et al. Reference Hara, Haga, Aoyama and Kiriyama1999). Within the same class of dietary fibre, there are differences in physiological activity, which may be attributed, at least partially, to differences in chemical nature (Evans et al. Reference Evans, Hood, Oakenfull and Sidhu1992); but this aspect has not been investigated in detail.
Mucilages are synthesized by plant secretory cells and help prevent desiccation of seed endosperm. Mucilaginous fibres such as pectin, guar and psyllium seed colloid have consistently been shown to increase bile acid excretion (Kay, Reference Kay1982). In order to study how the serum lipid-lowering effect of the dietary fibre is related to its chemical nature, mucilages having different chemical natures were assessed for their hypolipidaemic effect. Relative hypolipidaemic effect and the mechanism of action of a glucomannan, a galactomannan and an arabinogalactan, mucilages of different chemical nature isolated from three different sources, were investigated and the results are presented here.
Materials and methods
Chemicals
Eagles minimum essential medium, type IV collagenase, penicillin, streptomycin and protein-A-sepharose were purchased from Sigma (St Louis, MO, USA). [14C]Leucine (uniformly labelled with high specific activity), [14C]acetate (specific activity 1·5466 GBq/mmol) and [35S]methionine (specific activity 37·0 TBq/mmol) were purchased from BRIT (Mumbai, India). Plastic tissue culture dishes were purchased from NUNC (Denmark). Anti-rat apoB antisera in rabbit, prepared in our laboratory as described earlier, was used (Anil et al. Reference Anil, Abraham, Kumar, Sudhakaran and Kurup1992). All other chemicals used were of high purity analytical grade.
Preparation of mucilages
Fenugreek (Trigonella foenum-graecum) seeds were washed with water, dried and finely powdered. The powder was extracted with 95 % ethanol, until it became colourless. The dry defatted powder was then extracted with distilled water at 4°C. It was centrifuged and cold TCA was added to a final concentration of 5 %, mucilage was precipitated from the supernatant by the addition of three volumes of 95 % ethanol. This was then washed with 95 % ethanol, dissolved in water, dialysed against water, and the mucilage was reprecipitated with three volumes of ethanol, then dried and powdered. To isolate mucilages from Colocasia esculenta and Dioscorea esculenta, 25 g tuber flour was extracted with cold 10 % TCA, centrifuged and to the supernatant four volumes of acetone was added, the precipitated mucilage was collected by centrifugation at 10 000 rpm, dissolved in water, dialysed against water and reprecipitated with four volumes of acetone. The mucilages were dried and powdered.
Characterization of mucilages
The yield of mucilage was determined as the weight of dry mucilage powder obtained. The purity of the mucilage was assessed by analysing carbohydrate by the phenol sulphuric acid method (Kochert, Reference Kochert, Hellebust and Craigie1978) and testing protein by the method of Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951).
For chemical analysis, an aqueous solution of mucilage was hydrolysed by heating with 1 m-H2SO4 in a sealed tube under N2 atmosphere in a boiling water-bath for 4–6 h. The solution was neutralized with barium carbonate, centrifuged and the supernatant was filtered through 0·2 μm filter and used for determination of monosaccharide components both by descending paper chromatography (butanol–acetic acid–water, 4:1:5, by vol.) and HPLC. HPLC was carried out using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) with acetonitrile–water (80:20, v/v) as mobile phase using a Supelco-LC-NH2 column and a RI detector. The R t values were compared with those of standard sugars, the peak areas were noted and the relative content of sugars calculated.
Measurement of viscosity
Relative viscosities of mucilage solutions were measured using an Ostwald's viscometer held in a temperature-controlled water-bath. Time of flow was measured in triplicate, the average values taken and the relative viscosity calculated.
Determination of water-holding capacity
For the determination of the water-holding capacity of mucilages the modified method of McConnell (McConnell et al. Reference McConnell, Eastwood and Mitchell1974) was employed. Sample (0·5 g) was transferred to a preweighed 50 ml polypropylene centrifuge tube with screw cap. To each tube, 30 ml distilled water were added, the tube was placed in a forced-air oven at 37°C with moderate shaking for 16 h. The tubes were then centrifuged at 2000 g for 15 min and excess water was decanted. Each tube was weighed. The amount of water held was calculated by subtracting the weight before and after water treatment and was expressed on a dry weight basis.
Animals and diet
Twenty-four male albino rats (Sprague-Dawley strain, weighing 100–120 g) were randomly divided into four groups of six rats each. Animals were housed in plastic cages individually in a temperature- and light-controlled room and fed ad libitum on standard laboratory feed (approximate composition: protein, 221 g/kg; fat, 40 g/kg; fibre, 36 g/kg; minerals, 50 g/kg; energy, 15·16 kJ/g) and water. For oral administration, the required amount of mucilage was suspended in water and shaken on a rocker for about an hour until it dissolved to form a homogeneous solution. Aqueous solutions (2 ml) of galactomannan, arabinogalactan and glucomannan, isolated from fenugreek seeds, Colocasia and Dioscorea tubers, respectively, were administered orally by intubation at a dose of 4 mg/100 g body weight per d to one group each and the fourth group of animals maintained under identical conditions receiving 2 ml distilled water/d, served as control. The mucilage solution was administered immediately before feeding. The experiments were carried out with the approval of the Institutional Animal Ethics Committee. The duration of the experiment was 8 weeks. Body weights were recorded weekly during the experimental period.
Sampling procedures
During the last 2 d of the experimental period, faecal samples were collected from each rat and stored at − 20°C. At the end of the experimental period, rats were deprived of food for 16 h and then anaesthetized with ether inhalation and killed by decapitation. Blood samples were collected into tubes without an anticoagulant, kept at room temperature for 1 h, and serum was separated by centrifugation at 4°C for 20 min at 1500 g. Serum was stored at − 20°C until analysed. Livers were perfused with cold PBS to remove blood and individual livers were taken, sliced and portions equivalent to about 500 mg were taken for analysis. Adventitial fat was removed from aorta, washed in cold PBS and whole aorta was used for extraction and analysis of lipids. Tissues from each animal were analysed separately.
Analytical procedures
Serum total cholesterol was determined by the cholesterol oxidase method (Zlatkis et al. Reference Zlatkis, Zak and Boyle1953). Triacylglycerols in the serum were estimated by the glycerol phosphate oxidase method (Fossati & Prencipe, Reference Fossati and Prencipe1982). Serum glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) were estimated by the method of Reitman & Frankels (Reference Reitman and Frankel1957). Total protein and albumin to globulin ratio was determined by the Biuret method. These estimations were done with commercially available reagent kits (Qualigen's Diagnostics, Mumbai, India). Serum VLDL+LDL was precipitated using heparin–MnCl2 solution (Gidez et al. Reference Gidez, Miller, Burstein, Slagle and Eder1982) and the lipids in the VLDL+LDL fraction were extracted (Folch et al. Reference Folch, Lees and Sloane-Stanley1957) and cholesterol estimated (Carr & Drektor, Reference Carr and Drektor1956). ApoB in the VLDL+LDL fraction was precipitated using heparin–MnCl2 (Gidez et al. Reference Gidez, Miller, Burstein, Slagle and Eder1982), subjected to SDS–PAGE using 5–15 % gradient gel (Laemmli, Reference Laemmli1970), stained with Coomassie Brilliant Blue R-250 and the intensity of bands was measured using the Quantity One program in a BioRad gel doc. Lipids were extracted from tissues (Folch et al. Reference Folch, Lees and Sloane-Stanley1957) and estimated for cholesterol (Carr & Drektor, Reference Carr and Drektor1956) and triacylglycerols (Van Handel & Zilversmith, Reference Van Handel and Zilversmith1957). Faecal samples from rats of all groups were homogenized with an equal volume of water and lyophilized to a fine powder. From this powder faecal neutral sterols and bile acids were extracted (Grundy et al. Reference Grundy, Ahrens and Miettinen1965) and estimated as described earlier (Menon & Kurup, Reference Menon and Kurup1976).
Isolation and culture of hepatocytes
Hepatocytes were isolated from rats of all four groups, which were starved overnight, by collagenase perfusion (Seglen, Reference Seglen and Prescott1976) as described earlier (Sudhakaran et al. Reference Sudhakaran, Sinn and von Figura1980). The isolated hepatocytes were examined by light microscopy and the viability was tested by the ability of cells to exclude 0·5 % trypan blue. Cell preparations with more than 90 % viability were used for experiments and they retained their viability in culture during the experimental period. Hepatocytes in minimum essential medium (approximately 2·5 × 106 cells/ml) were seeded on 35 mm plastic culture dishes. After 4 h, unattached cells were removed and the monolayers of attached cells were used for the study.
Metabolic labelling of hepatocytes
The cells in culture were metabolically labelled by incubating with serum-free leucine-deficient medium containing [14C]leucine (185 kBq/ml). The cells were also incubated separately with serum-free medium containing [14C]acetate (185 kBq/ml). Cells were maintained at 37°C in a 95 % air and 5 % CO2 atmosphere for different time intervals. At the end of the incubation period, the medium and cells were collected separately for analysis.
Pulse chase experiment
The attached monolayer of hepatocytes were pulse labelled with [35S]methionine (740 kBq/ml) for 3 h. After removing the radioactive medium, the cells were maintained in non-radioactive medium. The radioactivity associated with apoB that secreted into the medium was chased at different time intervals (2 and 4 h).
Immunoprecipitation of cellular and secreted apoB
After separation of hepatocytes and media, the cell pellet was resuspended in 1 ml 20 mm-PBS, pH 7·4. Cell protein was estimated by the method of Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951). The medium was centrifuged at 10 000 g for 5 min to remove any cell debris. ApoB was immunoprecipitated from cells and media and the incorporation of radioactivity into apoB was determined as described previously (Anil & Sudhakaran, Reference Anil and Sudhakaran1994) using anti-rat apoB.
TLC separation of lipids in lipoproteins
Lipids associated with the lipoprotein that was secreted into the medium and that associated with the cell layer were extracted by the method of Folch et al. (Reference Folch, Lees and Sloane-Stanley1957). Individual lipids were separated by TLC (Skipski et al. Reference Skipski, Good, Barclay and Reggio1968) on silica gel G plates with a solvent mixture of hexane–diethyl ether–acetic acid (80:20:1, by vol.). Authentic lipid standards were run concurrently and the TLC plates were developed using iodine vapour. Individual lipids were located as spots corresponding to standards. Silica gel corresponding to different lipids were scraped, extracted with chloroform and the radioactivity associated with each lipid was measured separately by liquid scintillation counting.
Statistical analysis
All values are expressed as means and standard deviations. Data were analysed by one-way ANOVA, and significant differences among groups were determined by Duncan's multiple range test (P < 0·05). The statistical analyses were done using SPSS Statistical Package for Windows version 10.0 (SPSS, Chicago, IL, USA).
Results
Isolation of mucilages
The yield of mucilages was as follows: fenugreek, 8 g/100 g seeds; Colocasia, 1 g/100 g dry flour; Dioscorea, 0·8 g/100 g dry flour. Dry powder of fenugreek mucilage contained 92 % carbohydrate and 3 % protein. Dioscoria mucilage contained 89 % carbohydrate and 5 % protein whereas Colocasia mucilage contained 90 % carbohydrate and 5 % protein. No starch was detected in any of the mucilages. Acid hydrolysis followed by separation of sugars by paper chromatography and HPLC showed that fenugreek mucilage was a galactomannan containing galactose and mannose in the ratio 1:1, Colocasia mucilage was an arabinogalactan containing galactose and arabinose in the ratio 2:1 and Dioscorea mucilage was a glucomannan with a trace (less than 5 %) amount of glucose. These mucilages were resistant to degradation by salivary amylase in vitro.
Animal growth
Although animals receiving different mucilages showed some reduction in feed consumption during the early few days of the experiment, there was no statistically significant difference in body weight gain when compared to control animals and among different groups. The weight gain for control, galactomannan, arabinogalactan and glucomannan groups were 112 (sd 5), 109 (sd 5), 112 (sd 4) and 109 (sd 4) g/8 weeks, respectively.
Serum glutamate oxaloacetate transaminase, glutamate pyruvate transaminase and proteins
In order to assess hepatic function, markers of liver function such as the activities of serum GOT and GPT, and the amount of total protein and albumin/globulin ratios were determined and the results are given in Table 1. There was no significant difference in the activities of serum GOT and GPT in rats of different diet groups when compared with control rats. The amount of total protein and the albumin:globulin ratio also did not show any statistically significant difference in rats of different diet groups when compared with control.
Table 1 Serum glutamate oxaloacetate transaminase (GOT) and serum glutamate pyruvate transaminase (GPT) activities, and serum total protein concentration and albumin:globulin ratio in rats fed different mucilages* (Mean values and standard deviations for six rats per group)
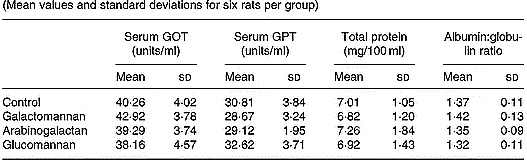
* Rats were divided into four groups and fed normal diet. Animals of three groups were fed galactomannan, arabinogalactan and glucomannan 4mg/100g body weight daily for 8 weeks and the serum was collected and analysed. For details of procedures, see p. 1023. There was no significant differences among different diet groups: P < 0·05.
Serum lipids
To compare the effect of different mucilages on serum lipids, serum collected from rats of all four groups were analysed for total cholesterol, HDL/VLDL+LDL cholesterol and triacylglycerols. The results are given in Table 2. There was significant reduction in total cholesterol in the serum of rats fed galactomannan and glucomannan (P < 0·05) compared to control rats. Arabinogalactan-fed rats showed no statistically significant change in serum total cholesterol levels. Cholesterol in the VLDL+LDL fraction of the lipoprotein decreased significantly in mucilage-fed rats when compared to control. But there was no difference in cholesterol level in these lipoproteins in any of the three different mucilage diet groups. However, serum HDL/VLDL+LDL cholesterol ratio was found to be increased significantly in rats fed galactomannan, arabinogalactan and glucomannan compared to control rats (P < 0·05). Serum triacylglycerols decreased significantly in all the mucilage groups compared to control (P < 0·05). Glucomannan-fed rats showed more effect followed by arabinogalactan and galactomannan (Table 2). ApoB, the principal apoprotein in VLDL and LDL, also decreased.
Table 2 Changes in serum, liver and aortic lipids in rats fed different mucilages* (Mean values and standard deviations for six rats per group)
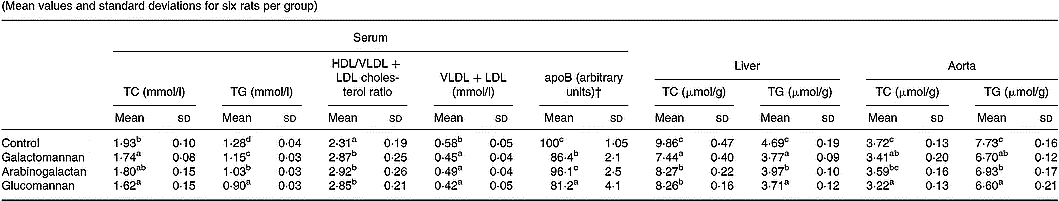
TC, total cholesterol; TG, triacylglycerols.
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Rats were divided into four groups and fed normal diet. Animals of three groups were fed galactomannan, arabinogalactan and glucomannan (4mg/100g body weight) daily for 8 weeks and the serum and tissues were collected and analysed for lipids. For details of procedures, see p. 1023.
† Intensity corresponding to apoB bands are expressed as a percentage of that of the control.
Tissue lipids
Lipids were extracted from liver and aortic tissues and amounts of cholesterol and triacylglycerols were estimated and the results are shown in Table 2. Liver cholesterol and triacylglycerols were significantly reduced in all the mucilage groups compared to control (P < 0·05). Aortic cholesterol decreased significantly in galactomannan- and glucomannan-fed rats compared to control; a greater decrease in aortic cholesterol was observed in glucomannan-fed rats (P < 0·05) followed by galactomannan-fed rats (P < 0·05). Aortic triacylglycerols were found to be decreased significantly in all the three mucilage groups, glucomannan-fed rats showed a greater effect followed by galactomannan and arabinogalactan, compared to control rats.
Faecal bile acids and neutral sterols
Faecal bile acid and neutral sterols were estimated and the results are given in Fig. 1. Faecal bile acid excretion increased significantly in rats of all mucilage groups, namely rats fed galactomannan (P < 0·05), arabinogalactan (P < 0·05) and glucomannan (P < 0·05) compared to control rats. Faecal neutral sterol excretion also increased significantly in all the diet groups; namely rats fed galactomannan (P < 0·05), arabinogalactan (P < 0·05) and glucomannan (P < 0·05) compared to control rats. But there was no significant difference in the amount of bile acids and neutral sterols excreted by animals among the various mucilage-fed groups.
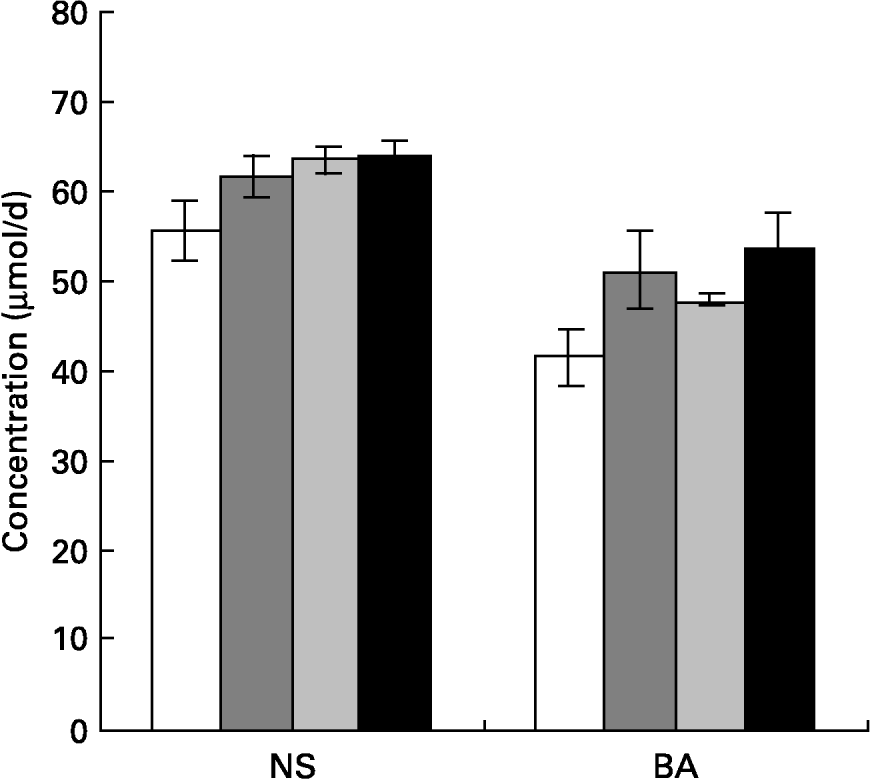
Fig. 1 Concentrations of total neutral sterols (NS) and total bile acids (BA) in faeces of rats fed different mucilages. Rats were fed control diet (□), galactomannan (), arabinogalactan () or glucomannan (■) for 8 weeks at a concentration of 4 mg/100 g body weight per d. Stool samples (24 h) were collected and analysed for NS and BA. For details of procedures, see p. 1023. Values are means with standard deviations depicted by vertical bars (n 6). All mean values were significantly different from those of the control group: P < 0·05. There were no significant differences among the different mucilage groups for either NS or BA.
Synthesis and secretion of apoB by hepatocytes
The synthesis and secretion of VLDL was studied using hepatocytes isolated from livers of animals fed mucilage and maintained in culture. This was studied by metabolic labelling of apoB, the major apoprotein of VLDL, and [14C]acetate into lipids. The incorporation of [14C]leucine into apoB secreted into the medium, at different time intervals, was found to be less in cells derived from the livers of rats of mucilage diet group when compared to control rats (Fig. 2). The reduction was more in hepatocytes isolated from glucomannan-fed rats (P < 0·05), followed by galactomannan-fed (P < 0·05) and arabinogalactan-fed (P < 0·05) rats. However, incorporation of [14C]leucine into the total TCA-precipitable protein secreted into the medium or that associated with the cell layer was not significantly different (data not shown), indicating that the decrease in [14C]apoB was not a non-specific effect.
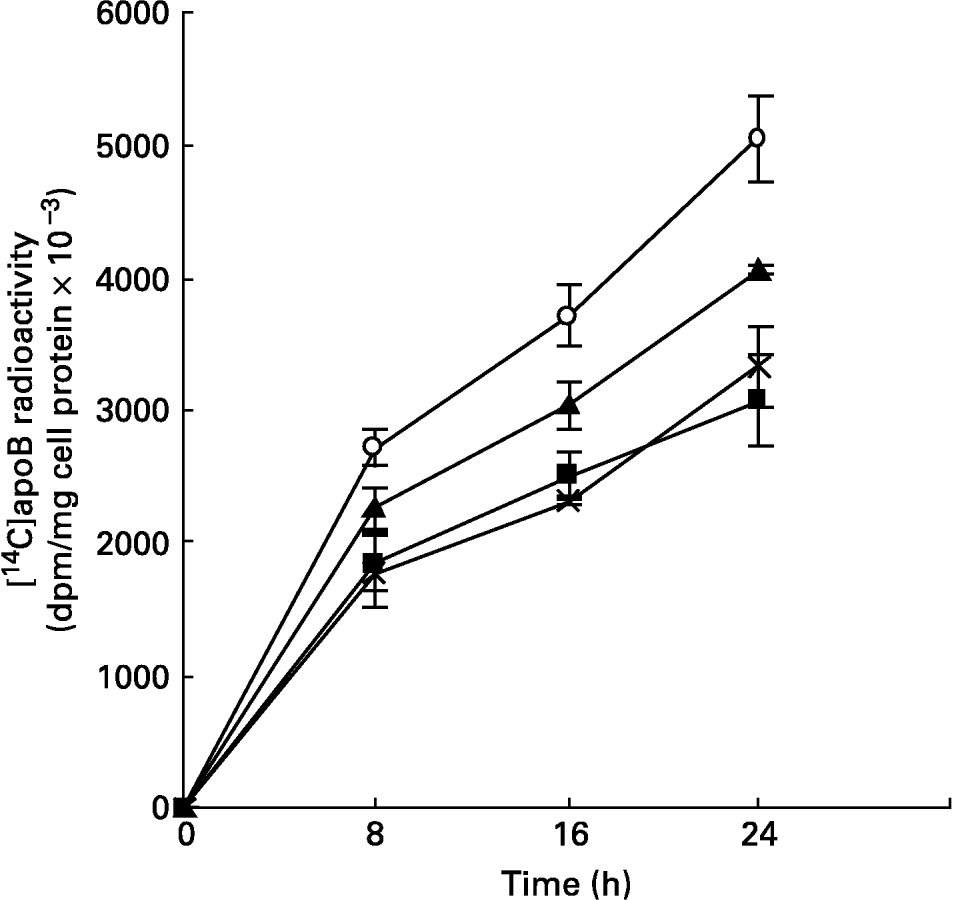
Fig. 2 Synthesis of [14C]apoB by hepatocytes isolated from rats fed different mucilages. Hepatocytes isolated from livers of rats fed control diet (○), galactomannan (■), arabinogalactan (▲) or glucomannan ( × ) were maintained in culture in media containing [14C]leucine (185 kBq/ml) for different time intervals. Medium was collected and analysed for [14C]apoB by immunoprecipitation. For details of procedures, see p. 1023. Values are means with standard deviations depicted by vertical bars (n 6). All mean values were significantly different from each other except between galactomannan and glucomannan: P < 0·05.
The cell layer-associated apoB radioactivity was also found to be decreased in cells derived from livers of rats fed galactomannan (P < 0·05), arabinogalactan (P < 0·05) and glucomannan (P < 0·05) compared to control rats (data not shown).
Lipids associated with secreted lipoproteins
A significant decrease in incorporation of [14C]acetate into lipids associated with secreted lipoprotein was observed in hepatocytes of rats fed galactomannan (P < 0·05), arabinogalactan (P < 0·05) and glucomannan (P < 0·05), when compared to control rats (Table 3). Among the different lipids associated with lipoproteins that secreted into the medium, [14C]acetate incorporated into triacylglycerols, cholesterol and phospholipids were found to be decreased significantly in all the diet groups (P < 0·05), when compared to control rats.
Table 3 Synthesis of VLDL by hepatocytes: incorporation of [14C]acetate into lipids (dpm/mg cell protein×10−3)* (Mean values and standard deviations for six rats per group)
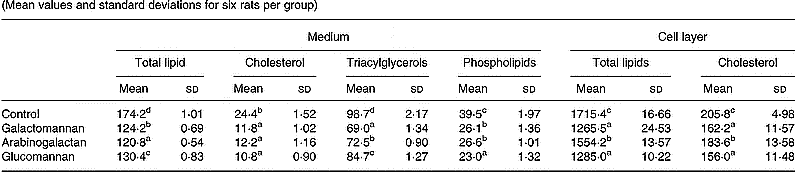
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Hepatocytes were isolated from livers of rats of all groups and maintained in culture in the presence of [14C]acetate (185 kBq/ml) for 8 h. Lipids associated with VLDL that secreted into the medium and that associated with cells were extracted and individual lipids were separated by TLC and radioactivity associated with each fraction was determined. For details of procedures, see p. 1023.
A significant decrease in the incorporation of [14C]acetate into total lipids and cholesterol associated with hepatocytes of rats fed mucilage diet, compared to control, was also observed. Among the different mucilage groups glucomannan and galactomannan showed more of an effect followed by arabinogalactan (P < 0·05).
Pulse chase analysis of apoB
The rate of secretion of apoB-containing lipoproteins was studied by pulse labelling followed by chase analysis and the results are shown in Fig. 3. The [35S]apoB radioactivity that secreted into the medium was quantitated. The rate of secretion of [35S]apoB was significantly low in hepatocytes from mucilage-fed rats. Among the mucilage-fed rats, the lowest rate of secretion was shown by hepatocytes from glucomannan-fed animals and this was in the order glucomannan < galactomannan < arabinogalactan < control.

Fig. 3 Pulse chase analysis of the secretion of apoB-containing lipoproteins. Hepatocytes were isolated from livers of rats fed control diet (○), galactomannan (■), arabinogalactan (▲) or glucomannan ( × ) for 8 weeks. The isolated hepatocytes were maintained in culture in the presence of [35S]methionine (740 kBq/ml) for 3 h, removed from the medium, fresh non-radioactive medium was added and secretion into the medium of [35S]apoB was chased at different time intervals. For details of procedures, see p. 1023. Values are means with standard deviations depicted by vertical bars (n 6). All mean values were significantly different from each other: P < 0·05.
Viscosity and water-holding capacity of mucilages
In order to examine whether the hypolipidaemic effect of different mucilages is related to their physical characteristics, two important physical properties that may be physiologically relevant, namely viscosity and capacity to hold water, were determined and the results are given in Fig. 4. The relative viscosity of all the three mucilages increased with increase in concentration of the solution; 0·5 % of the solution of the mucilages showed relative viscosity in the order galactomannan of fenugreek>glucomannan of Dioscorea>arabinogalactan of Colocasia. Similarly, the mucilages varied in their capacity to hold water and the water-holding capacity was in the order galactomannan>glucomannan>arabinogalactan.

Fig. 4 Relative viscosity (A) and water-holding capacity (B) of mucilages. (A), Aqueous solutions of different mucilages (◆, galactomannan; ●, arabinogalactan; ▲, glucomannan) of varying concentrations were prepared and their relative viscosity with respect to water was determined using an Ostwald's viscometer. (B), Each mucilage (0·5 g;, galactomannan;, arabinogalactan; ■, glucomannan) was suspended in water for 16 h and the amount of water held was determined. For details of procedures, see p. 1022. Values are means of triplicate experiments with standard deviations depicted by vertical bars.
Discussion
Comparison of the hypolipidaemic effect of mucilages isolated from fenugreek seeds, tubers of Colocasia esculenta and Dioscorea esculenta, which are chemically distinct, showed that the lipid-lowering effect involving decrease in the production of VLDL by the liver varied with the nature of the soluble fibre. The fenugreek seed mucilage is a galactomannan, the mucilages isolated from tubers of Colocasia and Dioscorea are arabinogalactan and glucomannan, respectively. Results presented earlier showed that the three mucilages, namely galactomannan, arabinogalactan and glucomannan, decrease lipid levels in both serum and tissues. Although serum total cholesterol was decreased significantly by galactomannan and glucomannan, all the mucilages increased the HDL/VLDL+LDL ratio and decreased serum triacylglycerols. Liver cholesterol, triacylglycerols and aortic triacylglycerols were decreased significantly by all the three mucilages, but significant decrease in aortic cholesterol was shown by galactomannan and glucomannan and not by arabinogalactan. Generally, dietary soluble or viscous fibres have hypocholesterolaemic effect (Anderson, Reference Anderson1990; Glore et al. Reference Glore, Van Treeck, Knehaus and Guild1994). For example soluble fibres such as phyllium, oat bran, guar and pectin decrease serum total cholesterol and LDL cholesterol without affecting serum triacylglycerols. The present results showed that the lipid-lowering effect varied with the nature of the mucilage and was in the order glucomannan>galactomannan>arabinogalactan. Mannan-rich mucilage such as glucomannan showed more serum cholesterol- and triacylglycerol-lowering effect while the least effect was produced by mannan-free arabinogalactan. Although to a lesser extent, arabinogalactan also decreased serum lipids. But in a randomized controlled trial in normal human subjects, intake of arabinogalactan did not produce any significant effect on serum lipids (Marett & Slavin, Reference Marett and Slavin2004). Comparison of the effects of three different galactomannans of varying galactose content reported previously (Evans et al. Reference Evans, Hood, Oakenfull and Sidhu1992) showed that fenugreek galactomannan with a galactose to mannose ratio of 1:1 had a more cholesterol-lowering effect when compared to locust-bean gum galactomannan which contained a lower amount of galactose. But the level of galactomannan intake in the present study was much less. Further, the experimental animals were normocholesterolaemic. However, studies using cholesterol-fed animals also showed that galactomannan produced a significant hypocholesterolaemic effect (data not shown). Analysis of serum GOT, GPT, total protein and albumin:globulin ratio did not show any significant difference among control and mucilage-fed animals, indicating that the liver function of these animals has not been affected.
Different mechanisms have been suggested to explain the hypolipidaemic action of dietary fibres which include the decreased reabsorption of bile acids, interfering with lipid metabolism by SCFA produced in the colon by microbial fermentation. Results presented earlier indicate that the mucilages reduce VLDL and LDL lipids and apoB associated with these plasma lipoproteins. The synthesis and secretion of apoB by hepatocytes from livers of mucilage-fed animals was found to be decreased significantly by these three mucilages, indicating a decreased synthesis and secretion of VLDL particles. Decrease in [14C]acetate incorporation into total lipids and triacylglycerols associated with lipoproteins secreted into the medium also suggest a decreased production of VLDL by liver cells. This was further confirmed by pulse chase analysis which showed a decrease in the rate of secretion of apoB by hepatocytes from livers of mucilage-fed rats. Of the different mucilages, glucomannan produced the greatest decrease in the rate of production of VLDL and arabinogalactan produced the least. Liver is the major site for the synthesis of VLDL and the rate of production of VLDL by primary cultures of hepatocytes is a reflection of the nutritional status of the animal with respect to hepatic production of VLDL. Our previous studies showed that during early stages in culture, primary cultures of rat hepatocytes produce VLDL as the principal apoB-containing lipoprotein (Kumar et al. Reference Kumar, Abraham, Kumar, Sudhakaran and Kurup1994). The present results thus indicate that glucomannan reduced the synthesis and secretion of VLDL by hepatocytes. Assembly and secretion of VLDL by hepatocytes is a complex process involving the synthesis of apoB, synthesis of lipids, their assembly and secretion. Availability of lipids, particularly endogenously produced triacylglycerols and nacently formed cholesterol/ester, is a critical factor in the assembly and secretion of VLDL (Davis, Reference Davis1999; Olofsson et al. Reference Olofsson, Asp and Boren1999). Although it is not clear how glucomannan and other non-digestible mucilages produce effects in liver, it is possible that the fermented products of these mucilages, primarily the SCFA, may affect hepatic synthesis of VLDL. The present in vitro studies using isolated hepatocytes in culture showed that butyrate and propionate reduced synthesis and secretion of apoB as VLDL (data not shown). The fermentation of dietary fibre in the intestine by intestinal microflora produces SCFA. Although we have not analysed the nature of the fermentation products in our experiments, Pylkas et al. (Reference Pylkas, Juneja and Slavin2005) compared SCFA production by different dietary fibres using an in vitro fermentation model and found that SCFA production varied among the fibre from different sources. Hara et al. (Reference Hara, Haga, Aoyama and Kiriyama1999) reported that SCFA suppress cholesterol synthesis in rat liver and intestine. Butyrate has been shown to impair lipid transport by inhibiting microsomal triacylglycerol transfer protein in Caco-2 cells and a reduction in triacylglycerol-rich lipoprotein output (Marcil et al. Reference Marcil, Delvin, Grovel and Levy2003). The present results of the incorporation studies showed a decrease in the synthesis of total lipids and cholesterol associated with hepatocytes from mucilage-fed rats. Thus decrease in production of VLDL by liver is clearly one of the mechanisms by which these mucilages lower lipid levels in serum and tissues.
An increase in the excretion of cholesterol and bile acids through faeces may be another reason for the hypolipidaemic effect of dietary fibres. It has been reported that feeding psyllium increased faecal bile acid and sterol excretion in rats (Buhman et al. Reference Buhman, Furumoto, Donkin and Story1998). De Schrijver et al. (Reference De Schrijver, Fremaut and Verheyen1992) reported that with oat bran intake, there was an inverse linear relationship between plasma cholesterol concentration and faecal excretion of bile acids. The present results showed that there was significant increase in neutral sterol and bile acids excretion in rats fed these different mucilages but there was no difference in the amount of bile acids and neutral sterols excreted by animals receiving the different mucilages. Thus increase in excretion of neutral sterols and bile acids may be another reason for the hypolipidaemic effect of these mucilages, however, this may not explain the variation in the extent of lipid lowering shown by different mucilages.
It has been suggested that the hypocholesterolaemic effect of soluble fibres relates to their gel-forming properties (Anderson, Reference Anderson, Kritchevsky and Bonfield1995). Soluble fibres like gum acacia, which does not form a viscous solution in water, appear to have minimal hypolipidaemic effects (Haskell et al. Reference Haskell, Spiller, Jensen, Ellis and Gates1992). Guar gum showed a significant cholesterol-lowering effect whereas hydrolysed guar gum did not show any significant cholesterol-lowering effect in man (Anderson et al. Reference Anderson, Fischer and Talbott1993). Viscosity and water-holding capacity of the mucilage depends on their structure and chemical composition. The present data indicate that galactomannan and glucomannan have relatively higher viscosity and water-holding capacity than arabinogalactan. Although there is no direct relation between the hypolipidaemic effect and the viscosity and water-holding capacity of the different mucilages tested here, it appears that the hypocholesterolaemic effect of these soluble fibres is related to their viscosity and water-holding capacity particularly because arabinogalactan, which showed the minimum viscosity and water-holding capacity, unlike other mucilages had the minimum plasma lipid-lowering effect. Similar studies from other laboratories have suggested that a moderate increase in viscosity of intestinal content is effective in reducing plasma cholesterol (Schneeman, Reference Schneeman1999).
These gel-forming fibres have been suggested to contribute to hypolipidaemic action by decreasing the absorption of cholesterol or fatty acids and decreasing reabsorption of biliary cholesterol and bile acids in the gastrointestinal tract. As these fibres are free from any bioactive substances such as saponins, tocotrienols or carotenoids, it is unlikely that the hypocholesterolaemic effect is produced by any non-polysaccharide materials.
In conclusion, mucilages isolated from different sources differ in their physical and chemical nature. These mucilages differ in their potential to lower lipid levels in rats. A greater lipid-lowering effect was shown by mannans like glucomannan and galactomannan than arabinogalactan. The hypolipidaemic effect of these mucilages appears to be due to a decrease in the synthesis and secretion of VLDL by hepatocytes. A decrease in production of VLDL can be due to a decrease in the synthesis of apoB as well as lipids associated with VLDL. Differences in the gelling property and viscosity which depend on the chemical nature of the mucilage may affect cholesterol and fatty acid absorption from the intestine and reduction in the availability of lipids can affect the assembly and secretion of VLDL by liver cells. Further, fermentation products like butyrate and propionate decrease VLDL production by liver cells. Thus the present results suggest that differences in the chemical nature of these mucilages contribute significantly to the differences in their physiological effects.
The present results are also relevant in human nutrition and health, particularly because mannan-rich soluble fibres are more effective in reducing lipid levels. There are a number of plant-based food materials rich in mannan-containing soluble fibres. The materials selected in the present study as sources of soluble fibres, such as fenugreek and tubers, form part of the diet for different populations. For example, fenugreek is used as a spice whereas Dioscorea is used as a vegetable. As elevated blood lipids are one of the major risk factors in the development of chronic conditions like atherosclerosis, diet-based lipid lowering is a useful approach. Further controlled studies on hypercholesterolaemic human subjects are required to establish whether such fibres can promote reverse cholesterol transport and regression of atheromatous lesions.
Acknowledgement
P. T. Boban was supported by a research fellowship from the Council of Scientific and Industrial Research, New Delhi.









