Osteoporosis is a common bone metabolic disease characterised by low bone mass and high fracture risk(1). Bone mineral density (BMD) decline increases the risk of fragility fractures, mainly of the spinal vertebrae, hip and radius(Reference Diez2). Hip and radial fractures are usually caused by falling, while vertebral fractures usually occur without external force(Reference Karinkanta, Piirtola and Sievanen3). Vertebral fractures may result in back pain, decreased body height and deformity(Reference Rao and Singrakhia4). Hip fractures are common at the intracapsular where the femoral neck (FN) is broken. Severe fractures can lead to prolonged bed rest, which increases mortality risk(Reference Piscitelli, Brandi and Nuti5). Hormonal changes in postmenopausal women lead to accelerated bone loss and osteoporosis(Reference Camacho, Petak and Binkley6), making them more vulnerable to osteoporosis and fragility fractures.
Ca, vitamin D and exercise are considered to be effective intervention methods to prevent bone loss, as mentioned in worldwide osteoporosis guidelines(Reference Camacho, Petak and Binkley6–Reference Lorenc, Gluszko and Franek11). Guidelines also suggest oestrogens(Reference Khan and Fortier12), ‘natural’ oestrogens (isoflavones)(Reference Camacho, Petak and Binkley6) and vitamin K(Reference Camacho, Petak and Binkley6) supplements for prevention of bone loss in postmenopausal women. Many therapeutic treatments for osteoporosis are provided by guidelines but cannot completely restore bone integrity. People of all ages should pay attention to osteoporosis prevention, especially postmenopausal women(Reference Curry, Krist and Owens13). The effects of Ca, vitamin D, vitamin K, oestrogen, isoflavone and exercise singly or in combination on BMD in postmenopausal women have not been investigated in a network so far. It is uncertain which preventive measures can better reduce bone loss and should be chosen under particular conditions, such as when having limited budget, resource, time or when one is not suitable for a specific intervention.
Network meta-analysis is a relatively new meta-analysis technique that compares the therapeutic effects of different interventions based on both direct and indirect comparisons(Reference Mills, Ioannidis and Thorlund14). A randomised controlled trial (RCT) design can evaluate the effects of an intervention(Reference Grossman and Mackenzie15). The aim of the present study is to conduct a network meta-analysis of the existing RCT to compare the BMD changes generated by different combinations of osteoporosis prevention interventions in postmenopausal women and to rank the interventions for practical applications.
Methods
Search strategy and study selection
The present study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement extension for network meta-analysis(Reference Hutton, Salanti and Caldwell16). We systematically searched MEDLINE, Embase and Cochrane Library from inception of each database to 24 February 2019. The keywords and MeSH terms used in the search strategy included Ca, vitamin D, vitamin K, oestrogen, isoflavone, exercise, postmenopausal, BMD and random. The full search strategies used in MEDLINE, Embase and Cochrane Library are provided in eMethod1 in the Supplement. Searches for Ca, vitamin D, vitamin K, oestrogen, isoflavone and exercise were conducted separately.
To make the present study both rigorous and manageable, six authors followed the same standard to conduct the literature review process in three independent pairs: X. Z. J. and W. H. W., W. Z. Z. and L. T. and S. Q. M. and S. Y. These three pairs of authors independently selected different possible interventions based on titles and abstracts (X. Z. J. and W. H. W.: Ca, vitamin K and exercise; W. Z. Z. and L. T.: vitamin D; S. Q. M. and S. Y.: oestrogen and isoflavone). All relevant systematic reviews and meta-analyses were reviewed to extract extra eligible trials. After removing duplicated trials from the databases and from systematic reviews and meta-analyses, the full texts of potentially relevant trials were reviewed by two authors independently (X. Z. J. and W. H. W.). Any disagreement between the two authors was resolved by consensus after discussion with a third investigator (C. Y.).
Inclusion and exclusion criteria
The inclusion criteria were as follows:
(1) Study design: RCT and quasi-RCT, which uses a quasi-random method (such as medical record number) for allocating participants to different interventions;
(2) Participants: postmenopausal women with natural or surgical menopause;
(3) Intervention: single or combined treatment with Ca, vitamin D, vitamin K, oestrogen, isoflavone and exercise;
(4) Comparison: no treatment, placebo for supplements or any intervention mentioned in (3);
(5) Outcome: absolute mean difference in BMD, measured by dual-energy X-ray absorptiometry(Reference Kanis and Gluer17);
(6) Time: study duration longer than 2 months.
Trials were excluded if:
(1) they were abstracts, letters, conference reports without full text, duplications or not published in English;
(2) the investigated postmenopausal women had any disease affecting bone metabolism, including musculoskeletal disease, renal failure, liver disorders, hyperparathyroidism, hyperthyroidism, diabetes mellitus, arthritis or cancer;
(3) the intervention included dietary restriction, health education or other drugs that may affect bone metabolism, including bisphosphonate, fluoride, tamoxifen, calcitonin, corticosteroids, progestin, androgen or placebos for these drugs.
Data extraction and risk-of-bias assessment
Two authors (X. Z. J. and W. H. W.) extracted data from all eligible publications independently. Information including trial name, first author, year of publication, country, population, number of participants, average age, years since menopause (YSM), BMI, study duration, blinding, interventions and mean difference in BMD was extracted.
Two authors (X. Z. J. and W. H. W.) independently assessed the risk of bias with the Cochrane risk of bias assessment tool described in the Cochrane Handbook(Reference Higgins and Altman18), including the following seven categories: random-sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Each category was judged as low risk, unclear risk or high risk. Discrepancies in data extraction and risk-of-bias assessment were resolved through discussion.
Statistical analysis
To compare all interventions simultaneously, a Bayesian network meta-analysis using Markov chain Monte Carlo simulation was conducted(Reference Lu and Ades19) to incorporate both indirect and direct comparisons. Treatment effects were estimated by random-effects network meta-analysis(Reference DerSimonian and Laird20). The generalised linear models were conducted with a logit link function with four chains and 20 000 iterated simulations, and the initial 5000 iterations were discarded as burn-in.
Effect sizes were summarised as weighted mean differences and 95 % credible intervals (95 % CrI) presented in forest plots. Trials reporting mean difference in BMD without standard deviation or standard error were included in the analysis, with standard deviation or standard error imputed when feasible(Reference Higgins and Altman18,Reference Furukawa, Barbui and Cipriani21) . The correlation between BMD at baseline and the end of intervention was calculated for all studies with complete outcome reports. The mean correlation was used to estimate the standard deviation or standard error in studies without available standard deviation or standard error values(Reference Abrams, Gillies and Lambert22). If two or more groups received the same intervention with different dosages, these groups were combined into a single group.
The relative ranking of osteoporosis prevention interventions and BMD changes was presented as rank probabilities and surface under the cumulative ranking (SUCRA) probabilities. SUCRA, which ranges between 0 and 100 %, was calculated by cumulative ranking probability, which represents the likelihood of being the best intervention(Reference Salanti, Ades and Ioannidis23,Reference Rucker and Schwarzer24) . In the present study, a higher SUCRA score represented a better intervention and increased BMD.
Between-study heterogeneity was assessed using the I² statistic, which ranges from 0 to 100 %. Between-study heterogeneity was also assessed by τ, which is independent of the study size(Reference Turner, Davey and Clarke25). The assumption of transitivity across treatment comparisons was assessed by comparing the distribution of BMI, the potential effect modifier, across the different pairwise comparisons using box plots(Reference Jansen and Naci26). Another important prerequisite for effective results is the consistency of direct and indirect evidence from the same treatment comparison, so the node-splitting model was used to assess potential inconsistency(Reference Bucher, Guyatt and Griffith27,Reference Dias, Welton and Caldwell28) . Publication bias was assessed using funnel plots(Reference Chaimani, Higgins and Mavridis29). Sensitivity analyses were performed by repeating the meta-analysis using the minimum and maximum correlation values of mean differences in BMD, adjusting the mean differences in BMD according to intervention duration and excluding studies with single group sample size less than 15.
Network meta-analysis was conducted using R software (version 3.5.1) with the gemtc(Reference Neupane, Richer and Bonner30) and rjags packages, JAGS (Plummer M, version 4.3.0) and STATA (version 13)(Reference Shim, Yoon and Shin31).
Results
Study selection
A total of 15 041 studies were identified from the three electronic databases (Fig. 1), among which 346 systematic reviews or meta-analyses were considered to be relevant to the topic and received full-text review. Of all the extracted articles considered eligible, 266 were extracted from systematic reviews or meta-analyses and another 549 were identified after screening the titles and abstracts from the databases; 642 articles received full-text review after removing duplicates. Of these studies, a total of ninety RCT met the inclusion criteria.

Fig. 1. Flow diagram of literature search and study. RCT, randomised controlled trial; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry.
Study characteristics
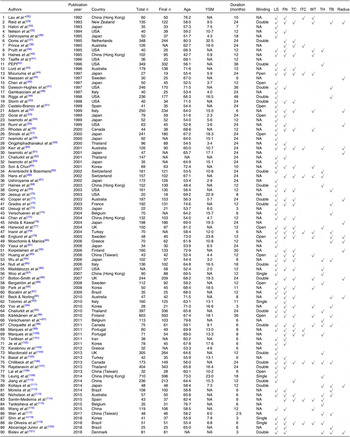
The characteristics of the RCT included are summarised in Tables 1 and 2. There were ninety RCT published between 1992 and 2018 that were included, and they had an average duration of 15·6 months. The present study included 10 777 participants with an average age of 62·7 years (range of average age, 42·7–82·4 years), an average YSM of 11·4 (range of average YSM, 0·9–32·5) and an average BMI of 25·4 kg/m2 (range of average BMI, 19·7–31·0 kg/m2). The population of three RCT were institutionalised women, and the remaining were non-institutionalised women.
Table 1. Description of included trials

YSM, years since menopause; LS, lumbar spine; FN, femoral neck; TC, trochanter; ITC, intertrochanter; WT, Ward’s triangle; TH, total hip; TB, total body; NA, not available; PEPI, Postmenopausal Estrogen/Progestin Interventions.
* Institutionalised women.
† Only have thigh bone mineral density.
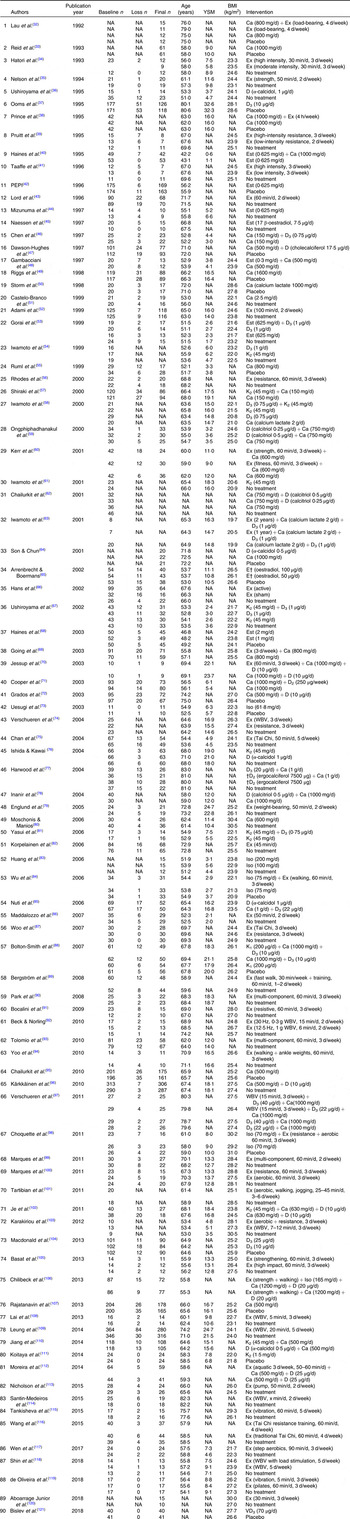
Table 2. Description of individual groups in included trials*
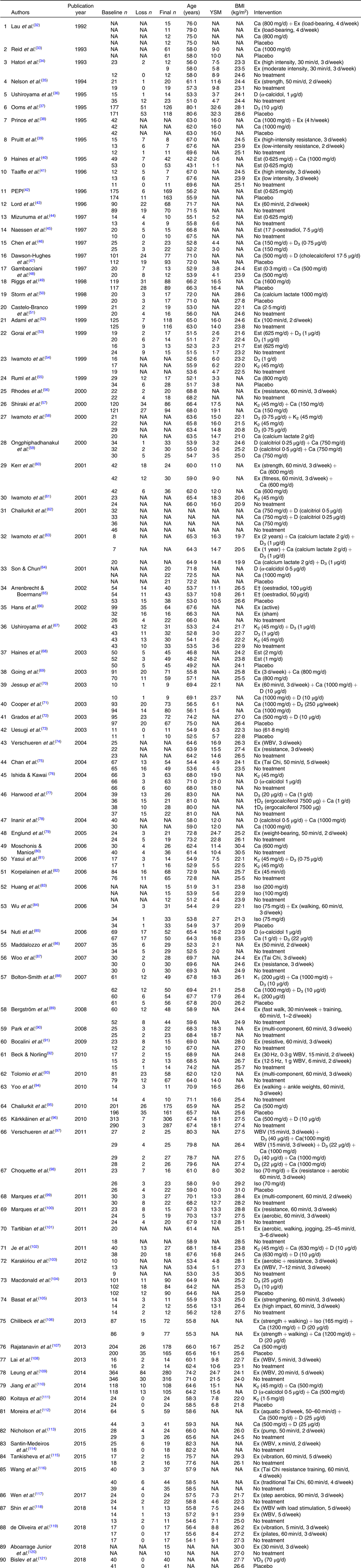
YSM, years since menopause; NA, not available; Ex, exercise; D, vitamin D; Est, oestrogen; PEPI, Postmenopausal Estrogen/Progestin Interventions; K, vitamin K; Iso, isoflavone; WBV, whole body vibration.
* Groups which did not meet the inclusion criteria are not shown.
† Injection.
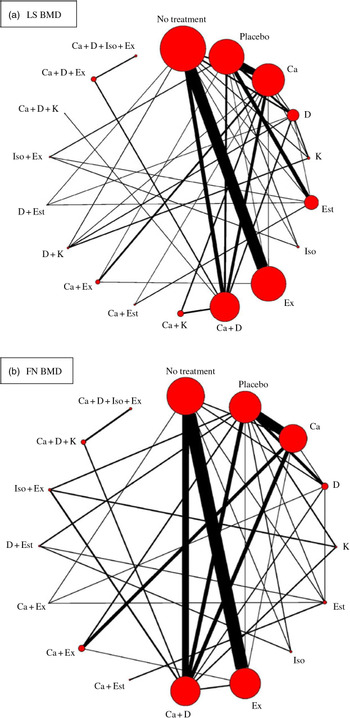
There were eighteen different intervention combination groups presented in the analysis: no treatment, placebo, Ca, vitamin D, vitamin K, oestrogen, isoflavone, exercise, Ca + vitamin D, Ca + vitamin K, Ca + oestrogen, Ca + exercise, vitamin D + vitamin K, vitamin D + oestrogen, isoflavone + exercise, Ca + vitamin D + vitamin K, Ca + vitamin D + exercise and Ca + vitamin D + isoflavone + exercise. The result of transitivity analysis conducted to assess the distribution of BMI across the different pairwise comparisons is shown in online Supplementary Fig. S1.
Among the ninety included RCT, seventy-four of them (n 8973, eighteen interventions) reported lumbar spine (LS) BMD, fifty-five (n 6707, sixteen interventions) reported FN BMD and 36, 11, 21, 25, 15 and 21 RCT reported trochanter, intertrochanter, Wald’s triangle, total hip, radius and total body BMD, respectively. Only the BMD values for LS and FN were included in the network meta-analysis because studies measuring the BMD of these two sites accounted for more than half the number of studies included and involved relatively complete intervention types (a total of eighteen different interventions were available in the present study).
Risk of bias
The risk of bias in the included RCT is shown in the Supplementary material (online Supplementary Table S1 and online Supplementary Fig. S2). Of the ninety RCT, the risk of bias was low for random-sequence generation in thirty-four RCT (37·8 %), allocation concealment in twenty RCT (22·2 %), blinding of participants and personnel in twenty-three RCT (25·6 %), blinding of outcome assessment in eighteen RCT (20·0 %), incomplete outcome data in thirty-six RCT (40·0 %) and other bias in eighty-eight RCT (97·8 %).
Publication bias
Funnel plots for publication bias in the network meta-analysis suggest no evidence of publication bias, but the fact that some studies were not in the 95 % CrI indicates the presence of heterogeneity (online Supplementary Fig. S3).
Lumbar spine
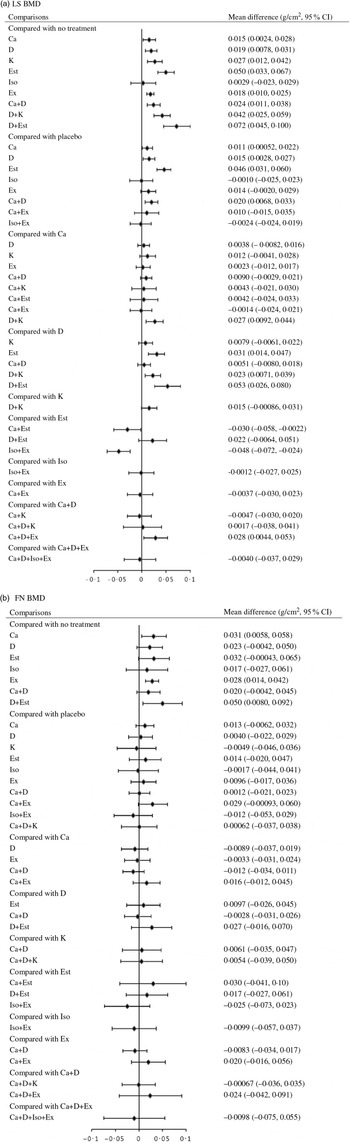
Network meta-analysis for the mean differences in LS BMD included seventy-four RCT (8973 participants) that used eighteen different types of interventions (Fig. 2(a)). The effects of each intervention are presented in Fig. 3(a). Ca (0·015 g/cm2, 95 % CrI 0·0024, 0·028 g/cm2), vitamin D (0·019 g/cm2, 95 % CrI 0·0078, 0·031 g/cm2), vitamin K (0·027 g/cm2, 95 % CrI 0·012, 0·42 g/cm2), oestrogen (0·050 g/cm2, 95 % CrI 0·033, 0·067 g/cm2), exercise (0·018 g/cm2, 95 % CrI 0·010, 0·025 g/cm2), Ca + vitamin D (0·024 g/cm2, 95 % CrI 0·011, 0·038 g/cm2), vitamin D + vitamin K (0·042 g/cm2, 95 % CrI 0·025, 0·059 g/cm2) and vitamin D + oestrogen (0·072 g/cm2, 95 % CrI 0·045, 0·100 g/cm2) were associated with significantly beneficial effects relative to no treatment. Ca (0·011 g/cm2, 95 % CrI 0·00052, 0·022 g/cm2), vitamin D (0·015 g/cm2, 95 % CrI 0·0028, 0·027 g/cm2), oestrogen (0·046 g/cm2, 95 % CrI 0·031, 0·060 g/cm2) Ca + vitamin D (0·020 g/cm2, 95 % CrI 0·0068, 0·033 g/cm2) were associated with beneficial effects compared with placebo. Vitamin D + vitamin K (0·027 g/cm2, 95 % CrI 0·0092, 0·044 g/cm2) was associated with positive effect with Ca. Oestrogen (0·031 g/cm2, 95 % CrI 0·014, 0·047 g/cm2), vitamin D + vitamin K (0·023 g/cm2, 95 % CrI 0·0071, 0·039 g/cm2) and vitamin D + oestrogen (0·053 g/cm2, 95 % CrI 0·026, 0·080 g/cm2) were associated with beneficial effect compared with vitamin D. Ca + vitamin D + exercise (0·028 g/cm2, 95 % CrI 0·0044, 0·053 g/cm2) had a beneficial effect compared with Ca + vitamin D. Ca + oestrogen (–0·030 g/cm2, 95 % CrI –0·058, –0·0022 g/cm2) and isoflavone + exercise (–0·048 g/cm2, 95 % CrI –0·072, –0·024 g/cm2) were related to negative effects relative to oestrogen.
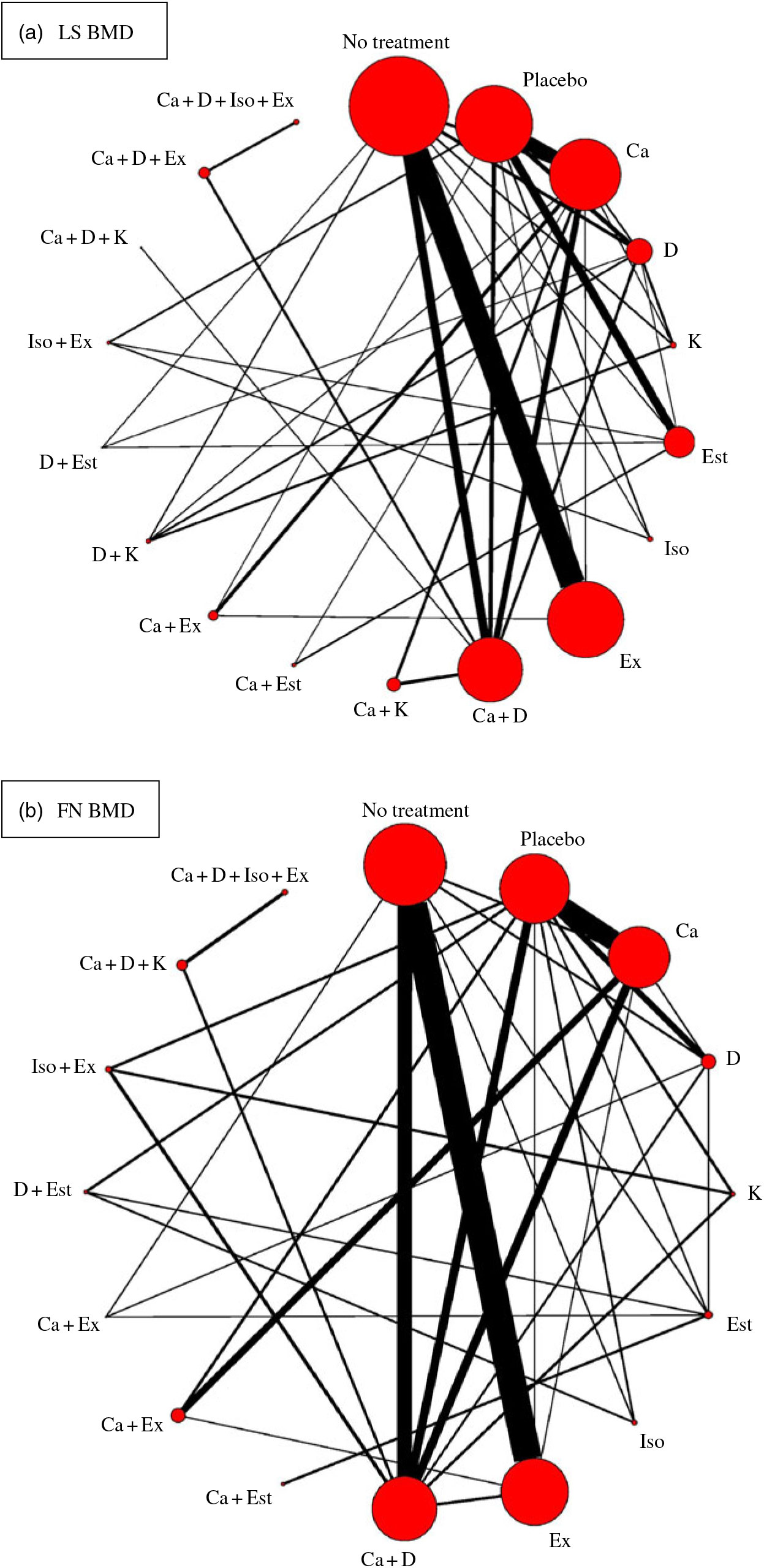
Fig. 2. Network plots for included studies with available direct comparisons for lumbar spine (LS) and femoral neck (FN) bone mineral density. Each node indicates an intervention and each line connecting two nodes indicates a direct comparison between two interventions. The size of the nodes and the thickness of the edges are weighted according to the number of participants evaluating each intervention and direct comparison, respectively. D, vitamin D; Est, oestrogen; Ex, exercise; K, vitamin K; Iso, isoflavone.

Fig. 3. Effect size for change in bone mineral density (BMD) using forest plots. LS, lumbar spine; D, vitamin D; Est, oestrogen; Ex, exercise; K, vitamin K; Iso, isoflavone; FN, femoral neck.
Within the network, there are thirty-seven intervention pairs for which both direct and indirect comparisons are available. Only the comparison between Ca and placebo (P = 0·037) and that between Ca + vitamin D and Ca (P = 0·031) showed significant evidence of inconsistency (online Supplementary Fig. S4).
The overall network heterogeneity τ was 0·021, and I² was 95·94. The heterogeneity of each comparison is shown in online Supplementary Table S2.
Femoral neck
Network meta-analysis for the mean differences in FN BMD included fifty-five RCT (n 6707) with sixteen different types of interventions (Fig. 2(b)). The effects of each intervention are presented in Fig. 3(b). Ca (0·031 g/cm2, 95 % CrI 0·0058, 0·058 g/cm2), exercise (0·028 g/cm2, 95 % CrI 0·014, 0·042 g/cm2) and vitamin D + oestrogen (0·050 g/cm2, 95 % CrI 0·0080, 0·092 g/cm2) were associated with significant beneficial intervention effects relative to no treatment.
Within the network, both direct and indirect comparisons are available for thirty-two intervention pairs. None of them showed significant evidence of inconsistency (online Supplementary Fig. S5).
The overall heterogeneity τ was 0·019 and I² was 96·59 in this network. The heterogeneity of each comparison is shown in online Supplementary Table S3.
Ranking probability
As shown in Table 3, the SUCRA values demonstrated that vitamin D + oestrogen had the highest SUCRA values for change of BMD in the LS (97·29 %), followed by Ca + vitamin D and exercise (86·86 %) and oestrogen (85·70 %). Ca + exercise had the highest SUCRA values for change of BMD in the FN (79·71 %), followed by Ca + oestrogen (79·38 %) and vitamin D + oestrogen (78·33 %). As for single interventions, oestrogen might be the best intervention to improve BMD in the LS (85·70 %) and Ca for FN (60·58 %). Most intervention combinations had higher SUCRA values than single interventions. The details of cumulative rank probabilities are supplied in the Supplementary material (online Supplementary Tables S4 and S5).
Table 3. Intervention rankings using surface under the cumulative ranking (SUCRA) values
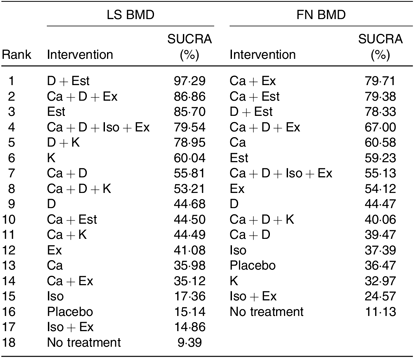
LS, lumbar spine; BMD, bone mineral density; FN, femoral neck; D, vitamin D; Est, oestrogen; Ex, exercise; Iso, isoflavone; K, vitamin K.
Sensitivity analysis
The minimum and maximum correlation values between BMD at baseline and the end of the intervention used to impute missing sd of BMD change were subject to a sensitivity analysis (online Supplementary Tables S6–S9). The findings were similar to those of the primary analysis. Another sensitivity analysis was conducted using the mean difference of BMD change after 15 months of intervention, which was the average duration of intervention in the included studies (online Supplementary Tables S6–S9). For LS BMD, the ranking of exercise was higher, from 12th to 5th, Ca + vitamin D and exercise appeared to be the highest rank and the rankings of higher ranked interventions remained stable. For FN BMD, the ranking of exercise was also higher, from 8th to 4th. Other findings were similar to those of the primary analysis. The last sensitivity analysis was conducted by excluding studies with group sample size less than 15. Higher ranked interventions remained ranking high.
Discussion
To our knowledge, this network meta-analysis is the first to compare the effects of various osteoporosis prevention methods on BMD in postmenopausal women, including Ca, vitamin D, vitamin K, oestrogen, isoflavone, exercise and their combinations. In this network meta-analysis, direct and indirect evidence from ninety RCT including 10 777 postmenopausal women was combined to compare the effect size of each intervention on BMD in both the LS and the FN. The results showed that compared with placebo or no treatment, many interventions can prevent bone loss. In addition, different single or combined interventions may have different impacts on different sites. However, some of the interventions had limited participants or involved limited studies, which may exaggerate or reduce the effect size of those interventions.
Ca and vitamin D supplements have long been considered as ways to prevent osteoporosis, and their effectiveness is consistent with our findings. Ca and vitamin D combined with exercise have beneficial effects on BMD in both the LS and the FN. It was found that the effect of Ca alone on FN BMD is greater than that of LS, which may be due to the different sensitivity of different sites to Ca supplementation, but the exact mechanism needs to be investigated further. Low bone density can not only cause fractures but also lead to bone pain and body metamorphosis(Reference Rao and Singrakhia4). This is the reason that BMD was chosen as the primary outcome in our study, although there has been some controversy about whether Ca and vitamin D effectively improve BMD and fracture rates(Reference Abrahamsen122) and Ca and vitamin D supplements may not prevent women from fracture(Reference Zhao, Zeng and Wang123). Fracture prevention requires all-round efforts, including improving BMD, maintaining muscle strength, maintaining a sense of balance and creating a safe home(Reference Johansson, Kanis and Oden124). Increasing BMD is important, but it is not the only component of fracture prevention.
Vitamin K plays an important role in the γ-carboxylation of osteocalcin, allowing osteocalcin to bind Ca and thus rendering it functional(Reference Hamidi, Gajic-Veljanoski and Cheung125). The effect size of vitamin K on BMD was different between the LS and the FN. Vitamin K ranked 6th among the eighteen interventions for LS but 14th among the sixteen interventions for FN, indicating that vitamin K supplementation can increase LS BMD but not FN BMD. This result is consistent with Fang’s meta-analysis that assessed the effects of vitamin K on BMD(Reference Fang, Hu and Tao126). Another meta-analysis showed that vitamin K2 can improve vertebral BMD in postmenopausal women with osteoporosis, while it did not have any effect in postmenopausal women without osteoporosis(Reference Huang, Wan and Lu127). The present study also showed that vitamin K2 might have a higher adverse reaction rate than control treatment. Considering the adverse reactions and different effects on postmenopausal women with and without osteoporosis, vitamin K should be carefully chosen for osteoporosis prevention.
Oestrogen is mainly generated by the ovaries in premenopausal women. Functional decline of the ovaries after menopause reduces oestrogen secretion. Oestrogen acts on osteoblasts and osteoclasts, thus affecting bone metabolism(Reference Zallone128). In this network meta-analysis, oestrogen + vitamin D was demonstrated to be the most effective way to improve LS BMD, and oestrogen + Ca was the most effective way to improve FN BMD. Oestrogen alone can be effective as well, a similar result to those of previous studies(Reference Fitzpatrick129). There were no interventions of oestrogen + Ca or vitamin D in our study, so the effects of these combinations remain unknown. If such studies are conducted in the future, this analysis can be updated. A previous meta-analysis showed that hormone replacement therapy (including oestrogen and progesterone) has a consistent, favourable, and large effect on bone density at all sites(Reference Wells, Tugwell and Shea130). However, considering the possible side effects of oestrogen and the limitations of access to oestrogen(Reference Lebech131), it should be taken under the guidance of a physician.
Isoflavone is a compound that has oestrogen-like activity in plants, and it exerts a weak oestrogenic effect by binding to the oestrogen receptor(Reference Setchell and Lydeking-Olsen132). It is still unknown whether its mechanism of action on bone turnover is the same as that of oestrogen(Reference Rickard, Monroe and Ruesink133). Isoflavone (not soya protein or foods containing isoflavone) was found to have a very limited effect on BMD in both the LS and the FN in the present study. Many studies, even meta-analyses, have shown inconsistent results about the role of isoflavone on BMD. In Taku’s meta-analysis, soya isoflavone extract supplements were found to have no effects on FN, total hip or trochanter BMD in menopausal women, and they concluded that it can only increase LS BMD(Reference Taku, Melby and Takebayashi134). Ricci’s meta-analysis reported that isoflavone mixtures cannot decrease bone loss in perimenopausal and postmenopausal western women(Reference Ricci, Cipriani and Chiaffarino135). Another two meta-analyses showed that lower doses were not effective at increasing BMD, while intake of more than 80–90 mg/d tended to have a beneficial effect(Reference Ma, Qin and Wang136,Reference Liu, Ho and Su137) . The effect of isoflavone on BMD is limited, but one study demonstrated that isoflavone may be safer than hormonal therapy for prevention of bone loss in postmenopausal women(Reference Xu, Qi and Deng138).
Exercise was shown to improve BMD to a certain extent in our study. The benefits of exercise lie not only in increasing BMD but also in improving muscle strength to prevent falling. Many meta-analyses have been conducted on different kinds of exercise. Kelley’s studies reported that aerobic exercise had a moderately positive effect on BMD in both the LS and the FN(Reference Kelley139,Reference Kelley140) , while resistance exercise did not maintain or improve BMD in either the LS or the FN(Reference Kelley and Kelley141). Most studies have suggested that combined exercise interventions effectively preserve postmenopausal women’s BMD(Reference Zhao, Zhang and Zhang142). Some meta-analyses have also suggested that exercise did not improve BMD in the FN(Reference Kelley and Kelley143). The studies may have had different results because of the different exercise protocols they used. In our study, exercise + Ca and vitamin D effectively prevented BMD loss. Exercise, as an intervention that can contribute to many other chronic non-communicable diseases in older people(Reference Penedo and Dahn144), is worthy of wide promotion.
Although there was high statistical heterogeneity indicated by I² in this network, it may be due to the large sample size in the study. The τ, which is independent of the study sample size, indicated low between-study heterogeneity. What is more, a node-splitting model was used to assess the potential inconsistency. Three other sensitivity analyses were conducted, which produced stable, consistent results. BMI, as a potential effect modifier, is generally thought to have a positive correlation with BMD(Reference Kumar, Sharma and Mittal145). However, study also indicated that BMI was not a determinant of BMD in postmenopausal women in an Asian population. What is more, mean differences in BMD were used to minimise the impact of baseline BMI in our study.
Limitations
The present study has several limitations. First, we did not conduct subgroup analyses of women with different YSM, BMI or osteoporosis status to define the best intervention methods for women with varying YSM, BMI and BMD. These information were not available from all included studies. Moreover, each type of intervention was combined into a single category, which makes it impossible to distinguish between high and low dosages or between slightly different forms of intervention (e.g. vitamin D2 v. D3, aerobic v. resistant exercise). The purpose of our research was to compare different kinds of interventions. Further studies should explore the effect sizes of different dosages and interventions in a network meta-analysis.
Second, we only included studies that employed oestrogen intervention and excluded studies that employed progesterone or androgens (such as hormone replacement therapy and tibolone), because it is unknown whether the effects of oestrogen on BMD will change if combined with progesterone or androgens. However, one study demonstrated that the effect size on BMD does not differ between tibolone and any oestrogen compound(Reference Doren, Nilsson and Johnell146). Progesterone can prevent endometrial hyperplasia during long-term oestradiol replacement(Reference Moyer, de Lignieres and Driguez147). If oestrogen is used to prevent postmenopausal osteoporosis, physicians’ guidance is necessary according to individual circumstances to decide the dosage and use of progesterone and androgens.
Third, the gemtc package is currently the most suitable package for analysing our study’s data. However, because of the limitations of the package, not all results of the comparisons between each pair of interventions were shown in the network forest plot, such as Ca + oestrogen compared with no treatment or placebo. Thus, mean differences were used to define if there was an effect or not in our study because some 95 % CrI of the effect sizes were not available.
Conclusion
The present study demonstrated that many interventions were valuable for improving BMD in the LS and FN of postmenopausal women. It confirmed the need for postmenopausal women to improve BMD through preventive measures such as nutrients or oestrogen. It also confirmed that different single or combined preventions can affect BMD at different sites in different orders. This reveals to medical and health workers and postmenopausal women which methods can be selected preferentially to prevent bone loss.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (71603167, 71673187 and 71603166) and the Shanghai Key Discipline Construction Project in Public Health (15GWZK1002). The National Natural Science Foundation of China and the Shanghai Key Discipline Construction Project in Public Health had no role in the design, analysis or writing of this article.
Z. X., H. W., Y. S., Q. S., L. T., Z. W. and Y. C. designed and conducted the study. Z. X. and H. W. analysed the data. All authors participated in the interpretation of data. Z. X. drafted the manuscript. All authors helped to revise the manuscript and accept this version for publication. Y. C. is the supervisor.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519002290