Gastric cancer is one of the most common tumours in Europe, with an estimated 160 000 new cases per year and approximately 120 000 deaths as reported in 2006. Although there is a consistent downward trend in incidence and mortality, gastric cancer remains the second leading cause of death from cancer in the world(Reference Ferlay, Autier and Boniol1).
For patients with localised disease, surgical resection is the chosen treatment, while perioperative neoadjuvant or adjuvant chemotherapy is indicated in more advanced stages of gastric carcinoma(Reference Morabito, Carillio and Longo2).
The effects of gastro resection or gastrectomy and chemotherapy on the nutritional status of the patients are well known(Reference Vandebroek and Schrijvers3). Patients with gastric cancer are at very high risk of deterioration of their nutritional status during the period of oncological treatment because of iatrogenic causes in addition to the disease itself. Thus, malnutrition is very common in patients with gastric cancer. The incidence ranges from 65 to 85 % of the cases(Reference Stratton, Green and Elia4) depending on the methods used to evaluate nutritional status, disease stage and oncological treatments. Malnutrition in cancer patients was reported to affect tolerance to treatments, as well as survival and quality of life (QoL). Nutritional screening is important for most types of cancer(Reference Huhmann and Cunningham5) and it is crucial in gastric cancer patients to start a timely nutritional intervention.
The specific aim of the present study was to analyse the nutritional status of patients recently diagnosed with gastric cancer before starting any treatment. The nutritional status was evaluated by measuring anthropometric, biochemical, inflammatory and functional variables. We also applied the Nutritional Risk Screening 2002 and investigated whether there were correlations between nutritional risk score (NRS), site and stage of stomach cancer and QoL of the patients.
The present study is part of a more comprehensive programme for surgical and medical patients with gastric cancer, where the overall purpose of nutritional screening is to identify patients who will need nutrition support during the entire oncological treatment.
Patients and methods
Study design
The investigation was a one-centre open, prospective clinical study, approved by the National Cancer Institute Ethics Committee and performed in accordance with the guidelines laid down in the Declaration of Helsinki.
All gastric cancer patients consecutively admitted to surgical or medical wards between January 2008 and June 2009 were assessed by a single trained dietitian within 2 d after hospital admission. Only patients with proven diagnosis of gastric adenocarcinoma were included in the study, after signing an informed consent. Exclusion criteria were previous oncological treatment, presence of severe disease that interferes with nutritional status (cirrhosis, chronic obstructive lung disease, chronic renal or intestinal failure, stroke or other neurological disease), upper limb deformities and incapacity to perform the hand grip strength test.
Oncological characteristics
Tumour site was determined by endoscopic examination; tumour histology was analysed on a surgical or endoscopic specimen and was defined according to Lauren's classification(Reference Lauren6); the stage of the disease was estimated according to the International Union Against Cancer's tumour-node-metastasis system (2002) and the American Joint Committee on Cancer stage grouping.
Nutritional status
Nutritional status was evaluated using anthropometric, biochemical and functional indicators. The nutritional risk screening was performed according to the European Society for Clinical Nutrition and Metabolism recommendations(Reference Kondrup, Allison and Elia7), using the NRS 2002(Reference Kondrup, Rasmussen and Hamberg8), in which nutritional risk is evaluated considering both the nutritional status and the severity of the disease. Recent weight loss, BMI and food intake in the preceding week are the nutritional variables considered. One point is given for weight loss>5 % in 3 months or food intake between 50 and 75 %; two points are given if weight loss>5 % is reported in 2 months or food intake is between 25 and 50 % or BMI is between 18·5 and 20·5 with impaired general condition; three points are given in patients where weight loss>5 % is reported in 1 month or food intake is almost nil (i.e. < 25 %) or BMI is < 18·5 with impaired general condition. For all patients the nutritional status score is incremented by one point for the presence of cancer and an additional point was added for patients with age ≥ 70 years. Patients were classified as high risk when NRS ≥ 3 and as low risk for NRS < 3.
Biochemical variables were analysed using blood samples collected on the first day of hospitalisation and included total protein, albumin, lymphocytes and C-reactive protein (CRP).
Functional assessment was performed using a Jamar hand grip dynamometer (Bolingbrook, IL, USA). Patients were asked to sit in a comfortable position with elbows on a table and to grip the dynamometer two times with their dominant hand, the second measurement was recorded. Grip strength measurements were compared to age and sex standard values(Reference Mathiowetz, Kashman and Volland9) and were expressed as percentage of these standard values. Grip strength measurements were considered in the normal range when ≥ 85 %, as recommended by Webb et al. (Reference Webb, Newman and Taylor10)
Quality of life
QoL was investigated using the official Italian translation of the self-administrated Functional Assessment of Anorexia/Cachexia Therapy(Reference Ribaudo, Cella and Hahn11), a QoL scoring system that focuses on specific nutritional issues (twelve items), in addition to physical and functional well-being (fourteen items) in cancer patients (see Table 1). All responses are graded from 0 to 4. Only questionnaires where the response rate was >85 % were considered. A standardised score was calculated and it ranged from 0 to 104, with 0 representing the worst and 104 representing the best QoL.
Table 1 Quality of life questionnaire*

* FAACT version 4; Elmhurst, IL, USA.
Statistical analysis
Based on literature findings(Reference Dewys, Begg and Lavin12–Reference Correia, Cravo and Marques-Vidal14), the proportion of malnourished patients (weight loss >10 % of usual weight) is reported ranging from about 20 to 50 %. We calculated that a sample size of 100 patients could produce a two-sided 95 % CI with a width ranging from 16·5 to 20·3 should the proportion be within the earlier hypothesised range. Association between NRS and oncological characteristics was analysed with univariable analysis using the χ2 test or Fisher's test, when appropriate, or with multivariable analysis. In the multivariable analysis we included all the oncological parameters as covariates in a binary logistic model; the model response variable was NRS ( ≥ 3, < 3) and the association between NRS and the covariates was tested by two-sided Wald tests. In the afore-mentioned analyses, the NRS adjustment for patients with age ≥ 70 years was not performed.
The association between the QoL score and NRS was studied using the Kruskal–Wallis or the Mann–Whitney test, when appropriate. To study an independent effect of nutritional status on QoL, we performed a multivariable general linear regression analysis, in which the response variable was QoL score and the covariates were NRS, CRP and tumour stage.
P values < 0·05 were considered statistically significant. The SAS statistical package (Cary, NC, USA)(15) and R software (Vienna, Austria)(16) were used for the statistical analysis.
Results
A total of 105 patients were screened from January 2008 to June 2009. Patients (n 2) were excluded because of previous oncological treatment, three patients were excluded because of the presence of associated severe diseases other than cancer. In all, 100 patients were included in the study (male/female 60:40, mean age 64 (sd 13·5) years). The nutritional parameters are shown in Table 2. Considering all patients together (column 1) mean BMI was at the higher limit of normal values, only sixteen patients presented a BMI below normal range. Weight loss was observed in a higher number of patients as compared with other nutritional variables. Actually thirty-five patients showed a weight loss ≥ 5 % of their usual weight in the preceding 3 months. Of these patients, seventeen had a weight loss >10 % (17 % of the overall sample; 95 % CI 10·2, 25·8). A total of twenty-nine patients reported a reduction of food intake in the previous week and among those, eight patients reported food intake that was < 25 % of requirement. Mean values of biochemical indicators and hand grip strength were all in the normal range.
Table 2 Nutritional characteristic overall and according to nutritional risk score (NRS)
(Mean values and standard deviations)

Pts, patients; HGS, hand grip strength; CRP, C-reactive protein.
a,b Mean values within a row with unlike superscript letters were significantly different between categories (P < 0·001).
* All participants have at least NRS score 1, since they have cancer.
† Nutritional assessment was within 2 d of admission and blood samples are from the first hospital day.
‡ Percentage of habitual weight.
§ Percentage of normal requirement.
∥ Percentage of standard values, as defined in the Methods section.
¶ Calculated excluding missing data (n 11).
According to NRS, thirty-six patients were malnourished or at risk for malnutrition. Nutritional characteristics according to NRS are also shown in Table 2 (columns 2 and 3). No difference was found in mean values of biochemical indicators or in the hand grip strength between the two groups. CRP mean value was normal in patients with NRS < 3 and it was slightly above the normal range in patients with NRS ≥ 3. This difference was not statistically significant; however, the percentage of patients with CRP>10 mg/l was significantly greater in patients with NRS ≥ 3 compared with patients with NRS < 3 (48 v. 22 %; χ2 test P = 0·023).
The majority of tumours were localised in the middle part of the stomach, i.e. fundus and body, while thirty-nine patients presented a tumour originating in the antrum. According to Lauren's classification, the most common histological type was the diffused form and 70 % of patients presented a poorly differentiated form. A total of twenty-nine and thirty patients were diagnosed with locally advanced tumours, classified as stage III and stage IV, respectively.
Regarding the association between nutritional risk and oncological characteristics, univariable analysis showed a significant result only for tumour stage (P < 0·001), but not for tumour site or grade. Compared with low-risk patients, high-risk patients had a greater percentage of stage IV tumours (53·3 v. 20·0 %; P < 0·001). In multivariable analysis, the association between NRS and tumour stage was also significant (P = 0·005).
QoL questionnaires were evaluable in eighty-seven patients. QoL score values tended to be inversely associated with NRS as median values of QoL score (interquartile range) were 84·6 (80·0–89·9) for NRS = 1 (thirty-seven patients), 80·0 (74·0–85·5) for NRS = 2 (twenty-one patients), 79·0 (68·4–80·6) for NRS = 3 (thirteen patients), 49·2 (40·3–62·0) for NRS = 4 (eight patients) and 59·6 (48·2–76·2) for NRS = 5 (eight patients) (Kruskal–Wallis test P < 0·001).
This negative association between QoL score and NRS was confirmed as significant when stratifying patients based on low (NRS < 3) or high nutritional risk (NRS ≥ 3). The median values of QoL score (interquartile range) were 83·0 (77·5–88·4) for NRS < 3 (fifty-eight patients) and 68·3 (49·2–78·0) for NRS ≥ 3 (twenty-nine patients) (Fig. 1; Mann–Whitney test P < 0·001).
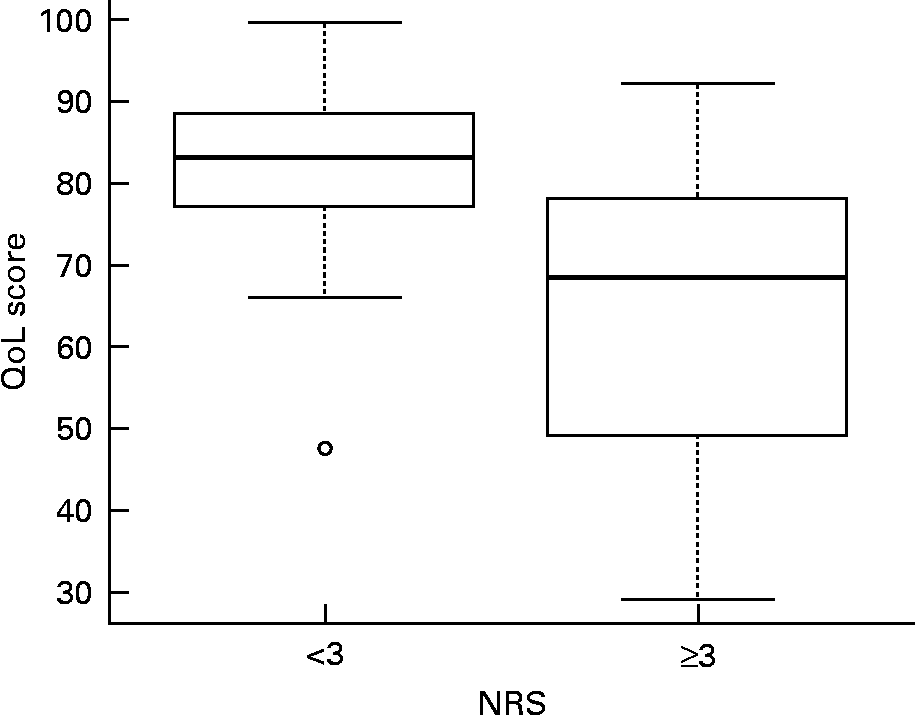
Fig. 1 Quality of life (QoL) score v. nutritional risk score (NRS). Each ‘box-plot’ shows some descriptive statistics of QoL score, i.e. (from bottom to top line): 1st quartile, median (bold line), 3rd quartile and maximum value. The circle represents one extreme value. Patients with NRS ≥ 3 (twenty-nine patients) presented lower QoL score values as compared with patients with NRS < 3 (fifty-eight patients).
In the multivariable general linear regression analysis, NRS was demonstrated as an independent predictor of QoL score (F test P = 0·0002), whereas CRP and tumour stage were not significantly associated with QoL (P = 0·393 and 0·086, respectively).
Discussion
Malnutrition has been recognised as an important prognostic factor in cancer patients since 1980, when Dewys et al. (Reference Dewys, Begg and Lavin12) reported a shorter survival in malnourished compared with well-nourished patients, with this being particularly true for patients with gastric cancer undergoing chemotherapy.
Despite these early observations, only few studies analysed the nutritional status at the beginning of the oncological treatment and focused mostly on surgical patients.
A weight loss >10 % of usual weight is considered an indicator of severe malnutrition and was reported in 33 % out of 317 patients affected by advanced gastric adenocarcinoma in the study by Dewys et al. (Reference Dewys, Begg and Lavin12). In smaller and heterogeneous groups of gastric cancer patients, significant weight loss was reported ranging from 21·6 to 50 %(Reference Farreras, Artigas and Cardona13, Reference Correia, Cravo and Marques-Vidal14).
In our analysis, severe weight loss ( ≥ 10 % of usual weight) was recorded in only 17 % of the patients and this percentage was similar to the one recently published by Pacelli et al. (Reference Pacelli, Bossola and Rosa17) in patients undergoing surgery. Furthermore, data showed normal values of albumin, suggesting that simple reduction of food intake rather than a wasting syndrome was the major cause of weight loss in the initial clinical oncological history of patients with gastric cancer, although approximately one third of patients had an initial increase of CRP values.
The main reason for evaluating the nutritional status in our cohort was to identify patients that would need to be supported with nutritional therapy during oncological treatment. As a tool to stratify patients in different groups, we applied the NRS 2002, which is based on the analysis of 128 randomised clinical studies and it is meant to identify patients who will probably benefit from nutritional support. NRS combines degrees of undernutrition with degrees of severity of disease. The criteria of exclusion that we adopted allowed us to give to all patients the same score for the severity of disease, i.e. one point for the oncological pathology. NRS has been largely used to predict surgical complication and to monitor nutritional status after curative gastric surgery(Reference Schiesser, Müller and Kirchhoff18, Reference Ryu and Kim19). The association between nutritional risk and clinical outcome has also been demonstrated in a large cohort of patients including different types of cancer(Reference Sorensen, Kondrup and Prokopowicz20). However, the relation between NRS and cancer stage has not yet been investigated. The present study, although considering a relatively small sample, evaluated a very homogeneous cohort of patients with a recent diagnosis of gastric carcinoma.
The analysis performed in the present study highlighted the correlation between patients with gastric tumours and NRS. We found a significant correlation between disease stage and NRS (P = 0·005 at multivariable analysis) while no correlation was observed between NRS and tumour site.
Furthermore, 60 % of patients with NRS 4 or 5 were candidates for chemotherapy to reduce the tumour burden. Owing to a high degree of malnutrition, these patients were reported to suffer from a higher rate of side effects that limit the completion of the scheduled therapies(Reference Andreyev, Norman and Oates21). We, therefore, strongly suggest that these patients should undergo adequate nutritional support during oncological treatments.
The relationship between nutritional status and QoL in cancer patients has been well described by Marín Caro et al. (Reference Marín Caro, Laviano and Pichard22). The presence of the tumoural mass and the side effects of the oncological treatments have an impact on several parameters, such as food intake, absorption and metabolic alterations, which alter the nutritional status and interfere with the QoL. Among patients with different types of tumours and therapeutic interventions Ravasco et al. (Reference Ravasco, Monteiro-Grillo and Vidal23) reported that patients with stomach and oesophagus cancer had the worst QoL as assessed with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire. In patients that had total gastrectomy because of stomach cancer Tian & Chen(Reference Tian and Chen24) demonstrated that there was a statistical correlation between the daily nutrition intake and QoL, suggesting that even after surgical treatment the nutritional status deteriorates and influences negatively the QoL. The interesting results in the present study show that the relationship between NRS and QoL starts very early in patients affected by gastric cancer, even before starting any oncological treatment and it is independent from oncological characteristics, as demonstrated by the multivariable general linear regression analysis.
Acknowledgements
The authors report no conflict of interest. All the authors made significant contributions and specific responsibilities were as follows: C. G. study design, interpretation of results and drafting the manuscript; S. C. assessment of patients, data collection and analysis; A. S. review of oncological data; V. M. recruitment of patients; R. M. statistical analysis and drafting the manuscript. This research received no specific grant from any funding agency in the public or commercial sector. V. M. was partially supported by the Italian Association for Cancer Research. We wish to thank all patients included in the study, Anna Armonti, Franca Filincieri, Carmen Maiorana and Lorena Riva, for their help in data collection and Fabio Stossi (University of Illinois at Urbana–Champaign) for the assistance in English revision.





