Faba beans (Vicia faba L.), also commonly known as fava, horse and broad beans, are widely consumed in different parts of the world including Egypt, Sudan, The Netherlands, Spain, Saudi Arabia, India and China. Their seed coat colours range from white, buff (or beige), purple, green to red, with the buff-coloured beans being the most commonly accepted for human consumption. In 2008, the worldwide production of dry faba beans was approximately 4·1 million tonnes, and the export trade was valued at approximately US$291 million(1). Faba beans are grown in Australia as a break crop and exported to the Middle East and Asia(Reference Matthews and Marcellos2), with export trade valued at approximately US$58 million in 2008(1).
Pulses are well known to be an economical source of protein, carbohydrate and fibre, and are low in fat. Pulses are also incorporated in human diets for their additional nutritional benefits, especially their microconstituents including phenolic compounds, oligosaccharides(Reference Bouhnik, Flourie and D'Agay-Abensour3), enzyme inhibitors, phytosterols and saponins(Reference Mathers4, Reference Campos-Vega, Loarca-Piña and Oomah5). Intake of legumes is reported to potentially lower the risk of cancer(Reference Aune, De Stefani and Ronco6), CVD(Reference Anderson and Major7), hypertension and diabetes(Reference Ranilla, Kwon and Genevese8). Some of the microconstituents are currently marketed as functional foods and nutraceutical ingredients(Reference Ferguson9). Also, there have been many attempts to incorporate pulses into food products for enrichment of product quality and additional health benefits(Reference Gomez, Oliete and Rosell10, Reference Patterson, Maskus and Bassett11).
A wide range of methods are used to prepare faba beans including soaking, boiling and roasting. Heating was reported to result in significant decreases in polyphenols, enzyme inhibitors, phytic acid, some minerals and vitamins, but increase protein digestibility of faba beans(Reference Alonso, Aguirre and Marzo12, Reference Khalil and Mansour13). Interestingly, Acar et al. (Reference Acar, Gokmen and Pellegrini14) reported that roasting at 150°C for 60 min increased the antioxidant capacity of different types of pulses including black bean, borlotti bean, kidney bean, red soybean, yellow bean, giant lentils and chickpea, with an initial fall observed in the yellow and red soybeans after roasting for 10 min. Comparatively, in faba beans, the tannin content increased after roasting at 149°C/20 min and 177°C/18 min, but decreased after roasting at 204°C/14 min and 232°C/12 min(Reference Anderson, Idowu and Singh15).
Phenolic compounds are one of the microconstituents which have been gaining an increasing interest for their health-promoting properties, largely defined by their antioxidant activity. Previous research has reported different types of phenolic compounds found in faba beans, such as procyanidins(Reference Merghem, Jay and Brun16–Reference Borowska, Giczewska and Zadernowski18), catechins(Reference Arts, van de Putte and Hollman19), flavonols(Reference Hertog, Hollman and Katan20), isoflavones(Reference Kaufman, Duke and Brielmann21), phenolic acids(Reference Sosulski and Dabrowski22) and tannins(Reference Moneam23–Reference Karamac, Kosinska and Rybarczyk25), which are natural antioxidants(Reference Amarowicz, Troszynska and Bary Ko-Pikielna26). Phenolic compounds extracted from a variety of plant materials have been reported to have an ability to inhibit carbohydrate and lipid digestion, therefore preventing them from absorption. These could potentially lower the postprandial hyperglycaemic response and contribute towards weight maintenance(Reference Ikarashi, Takeda and Ito27, Reference Shimura, Tsuzuki and Kobayashi28).
Phenolic extracts of different types of beans were found to have antioxidant activities(Reference Amarowicz, Troszynska and Bary Ko-Pikielna26), protective effects against radical-induced DNA damage(Reference Madhujith, Amarowicz and Shahidi29), as well as antimutagenic(Reference de Mejía, Castaño-Tostado and Loarca-Piña30) and anticancer(Reference Itoh, Umekawa and Furuichi31) properties. Many have reported substantial amounts of phenolic compounds in raw(Reference Amarowicz, Karamac and Kmita-Glazewska32) and cooked faba beans(Reference Kalogeropoulos, Chiou and Ioannou33), but limited reports focus on the health benefits of faba bean phenolic compounds and also on the impact of food preparation heat processes on the retention and activities of phenolic compounds. The present study hence aims to investigate in vitro the potential health benefits of crude extracts from raw and cooked faba beans in the prevention of chronic diseases including hypertension, diabetes, obesity and different types of cancers. The results could support increased consumption of faba beans and the development of new food products using faba beans, enhancing the exploitation of the crop and providing better returns to growers.
Materials and methods
Plant materials
For the purpose of the study, three faba bean genotypes including cv. Nura (buff-coloured), cv. Rossa (red-coloured) and breeding line TF(Ic*As)*483/13 (white-coloured) were grown at the Wagga Wagga Agricultural Institute experimental field in NSW, Australia in 2008. Harvested beans were air-dried and then stored at − 20°C until analysis.
Dry roasting
Roasting was performed at 150°C using dry heat in an oven (Premium Laboratory Oven, Thermoline Scientific) for 1 h (approximately 50 g per batch in a single layer on a foil tray and agitated gently after 30 min for uniform heating). Roasted beans were cooled at room temperature and ground into flour using an IKA-Universalmühle M20 Grinder (Janke and Kunkel).
Preparation of phenolic extracts
Extraction of phenolic compounds was carried out by dispersing the flour in aqueous acetone (acetone–water, 70:30, v/v)(Reference Merghem, Jay and Brun16) in a solid:solvent ratio of 1:10 and shaking for 2 h at room temperature. The supernatant was collected after centrifugation at 4000 g using an Eppendorf 5415D Centrifuge (Eppendorf-Netheler-Hinz) for 5 min at 5°C. A second extraction was performed on the residue and the extracts were pooled, concentrated under reduced pressure at 40°C using a rotary evaporator (Rotavapor R-205; Buchi) and then freeze-dried using a Christ-Alpha 1–4 freeze dryer (Biotech International). The extracts were stored at − 20°C until used. Distilled water was used to dissolve the dried extract and the reconstituted extracts were filtered through 0·45 μm Millipore filters (Millipore Australia Pty Ltd, Australia) before analysis. All extractions and measurements were performed at least in triplicate, except extractions from the raw beans which were performed in duplicate.
Total phenolic content, total flavonoid content and antioxidant capacity assays
The total phenolic content (TPC) assay was conducted according to Konczak et al. (Reference Konczak, Zabaras and Dunstan34). The total flavonoid content (TFC), diphenylpicrylhydrazyl (DPPH) radical scavenging capacity and Trolox equivalent antioxidant capacity (TEAC) assays were performed as described by Michalska et al. (Reference Michalska, Ceglinska and Amarowicz35). The oxygen radical absorbance capacity (ORAC) assay was carried out as described by Prior et al. (Reference Prior, Hoang and Gu36).
Preparation of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) free radical for post column derivatisation assay
The 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS)+ radical cation was prepared by dissolving ABTS (Sigma Aldrich) in deionised water (7 mm) and mixed with 2·45 mm of potassium persulphate overnight to allow complete reaction. The solution was diluted using distilled water to obtain an absorbance of 0·70 (sd 0·02) at 734 nm and filtered through a polypropylene membrane (0·45 μm)(Reference Ee, Agboola and Rehman37).
On-line post column derivatisation with HPLC
Analysis of the antioxidant activity was carried out on-line using ABTS cation radical. The HPLC system (ProStar model 410, Varian, Inc., Australia) consisted of a Phenomenex Luna 5U C18 column (100A pore size; 150 × 3 mm), preceded by a guard column (Phenomenex, 4 × 3 mm), a Varian 240I pump and a Varian 335 PDA Detector. The mobile phase A was water–acetic acid (99:1; v/v) and phase B was methanol–acetonitrile (50:50; v/v). An aliquot (8 μl) of the extract sample (50 mg/ml) dissolved in solvent A was injected and eluted in a gradient of 0–48 % phase B for 40 min at a flow-rate of 0·4 ml/min. UV spectra were recorded at 280 nm. Post column antioxidant activity on-line was determined on the HPLC eluent from the system which arrived at a ‘T’ piece and reacted with ABTS+ that was added at a flow rate of 0·4 ml/min. The absorbance of the reaction products was measured by a UV–Vis detector (Model 9050, Varian, Inc., Australia) at 414 nm.
Cell cultures
All cells lines were purchased from the American Type Culture Collection except the BL13 (human bladder transitional cell carcinoma) cells which were obtained from Dr D. Brookes(Reference Brookes, Zandvliet and Watt38). All cells were cultured at 37°C in a humidified 5 % CO2–95 % air atmosphere. BL13 cells were cultured in Roswell Park Memorial Institute medium (Invitrogen Corporation); AGS (gastric adenocarcinoma) in F-12K Ham's medium (Invitrogen Corporation); Hep G2 (hepatocellular) in Eagle's minimum essential medium (EMEM; Sigma-Aldrich); HT-29 (colorectal adenocarcinoma) in McCoy's 5a modified medium (Invitrogen Corporation); RAW264.7 (macrophage; Abelson murine leukaemia virus-induced tumour) in Dulbecco's modified Eagle's medium (Sigma-Aldrich); HL-60 (acute promyelocytic leukaemia) in Iscove's modified Dulbecco's medium (IMDM; Invitrogen Corporation) and CCD-18Co (colon normal) cells were cultured in EMEM. Each medium was supplemented with 100 μg/ml streptomycin and 1000 units/ml penicillin (Invitrogen Corporation) and 10 % fetal calf serum, with the exception of IMDM for HL-60 which required 20 % serum.
Cellular antioxidant activity assay
The assessment of cellular antioxidant activity (CAA) was determined according to Tan et al. (Reference Tan, Konczak and Ramzan39) and Wolfe & Liu(Reference Wolfe and Liu40). Briefly, 1 × 105/ml of Hep G2 cells were plated in a ninety-six-well microplate and incubated for 24 h. Next, the medium was removed and the cells were washed using PBS. The cells were then treated with different concentrations of faba bean extracts (10 μl in PBS), added to 80 μl of PBS, followed by the addition of 2′,7′-dichlorfluorescein-diacetate (Sigma-Aldrich) (10 μl) (250 μm in PBS), and incubated for 1 h. Subsequently, the cells were washed using PBS (100 μl) and added to 100 μl of 2,2′-azobis (2-amidinopropane) dihydrochloride in Hank's balanced salt solution (600 μm). The fluorescence was measured at 485 nm excitation and 538 nm emission wavelengths for every 5 min in 1 h. The final fluorescence values were corrected for the blank sample readings, and a time v. fluorescence graph was plotted. A quercetin standard was used to express the results as quercetin equivalents per g of dry weight of beans.
Antiproliferation assay
The assessments of antiproliferation activity of the faba bean crude extracts were carried out on the adhesive cells, BL13, AGS, Hep G2 and HT-29 using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay, as outlined by Tan et al. (Reference Tan, Konczak and Ramzan39). PBS was used to dissolve the sample extracts.
Assessment of apoptosis and cytolysis by flow cytometry
Suspensions (4·5 ml) of HL-60 cells (2·5–5 × 105/ml) were treated with 225 μl (0·8 mg/ml) of extracts for 3, 12 and 24 h in 25 cm2 culture flasks in triplicate. Untreated cells were used as a control. For the dose–response evaluation, the cells were treated with three different extract concentrations (0·4, 0·8 and 1·6 mg/ml in PBS) for 24 h. Cells were stained with Annexin V-Alexa Fluor 488 annexin V/Dead cell apoptosis kit with Alexa Flour 488 annexin V and PI for flow cytometry (Invitrogen Corporation) according to the manufacturer's directions. After the set incubation time, the cells were harvested, washed with cold PBS and resuspended in Annexin-binding buffer. Following this, 100 μl of cells were stained by adding 5 μl of Annexin V and 1 μl of propidium iodine and incubated for 10 min at room temperature. Next, the cells were mixed with 400 μl of Annexin-binding buffer and immediately placed on ice. Analysis was performed by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software (TreeStar Inc.) to determine the extent of cell apoptosis and lysis. From 3000 to 10 000 events were acquired for each measurement and the cell populations were gated for analysis.
Cellular protection against H2O2
The cellular protection against H2O2 assay was carried out using RAW264.7 cells according to Tan et al. (Reference Tan, Konczak and Ramzan39), except that the concentration of H2O2 used was 40 mm.
Angiotensin-converting enzyme inhibition assay
The angiotensin-converting enzyme (ACE) inhibition assay was carried out as described in Shalaby et al. (Reference Shalaby, Zakora and Otte41) using furanacroloyl-Phe-Glu-Glu as substrates. Results were expressed as μg of captopril equivalents/g of dry weight of beans.
α-Glucosidase inhibition assay
The α-glucosidase inhibition was determined as described by Ikarashi et al. (Reference Ikarashi, Takeda and Ito27) using sucrose as substrates, with slight modifications. An α-glucosidase enzyme solution was prepared by dissolving 100 mg of intestinal acetone powders from rat (Sigma-Aldrich) in 1 ml of 0·1 m maleate buffer (pH 6) and homogenised using an ultrasonicator for 6 min on a 30 s rest cycle. The enzyme solution was centrifuged at 3000 g for 30 min and the supernatant was diluted to 1:2 (v/v) using the buffer solution. Sample solutions (20 μl) were mixed with 2 % sucrose (w/v) in maleate buffer (20 μl). The enzymatic reaction was initiated by adding enzyme solution (20 μl) to the mixture and incubated at 37°C for 60 min. The enzymatic reaction was terminated by heating at 100°C for 10 min. Sample mixture (20 μl) was then used to react with the colour reagent (Glucose CII-Test Wako, Wako Pure Chemical Industries) (3 ml) at 37°C for 5 min and the absorbance was measured at 505 nm. Negative controls were prepared as described by replacing the sample solution with the buffer solution, whereas control and sample blanks were prepared by replacing the enzyme and sucrose solutions with the buffer solutions. The relative α-glucosidase inhibition was calculated using the following formula:
where A S and A C were the absorbance of sample and negative control, and A SB and A CB were the absorbance of sample and control blanks.
Lipase inhibition assay
The lipase inhibitory activity was assayed as described by Shimura et al. (Reference Shimura, Tsuzuki and Kobayashi28) using 4-methylumbelliferyl oleate as the substrate, except that the porcine pancreatic lipase (Sigma type II) was prepared using a concentration of 0·085 g/ml. The relative lipase inhibition activity was calculated using the following formula:
where F S and F C were the values of samples and negative control measured fluorometrically at an emission wavelength of 450 nm and excitation of 320 nm by a Varian Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies), and F SB and F CB were the fluorescence readings of sample and control blanks.
Statistical analysis
The significant differences between mean values were calculated based on at least three independent evaluations (n 3) and the standard deviations were also calculated. Student's t test was conducted to assess differences between the samples at the level of P < 0·05. All half maximal inhibitory concentration (IC50) values were calculated from the corresponding dose inhibition curve according to their best-fit shapes based on at least four reaction points using Microsoft Excel (Microsoft Corp, USA). Statistical correlation analyses were performed using Graphpad Prism 5 (Graphpad Software). Results for correlation analysis were considered statistically significant when P < 0·05.
Results and discussion
Total phenolic content, total flavonoid content and antioxidant capacities
The effects of roasting on TPC, TFC and antioxidant capacity of the extracts from three faba bean genotypes are presented in Table 1. In comparison to the raw sample, a higher extraction yield was obtained for the roasted samples (approximately 10 % higher). The difference between the genotypes with regards to the extraction yield was negligible. The level of TPC and TFC of Rossa was not significantly different from Nura in both of their raw and roasted beans, except that the roasted Rossa had slightly higher TFC than the roasted Nura. The TPC in the raw beans of coloured genotypes were about four times, while the TFC was about three times higher than TF(Ic*As)*483/13. After roasting, the TPC and TFC contents of Nura and Rossa were about two times higher than TF(Ic*As)*483/13. The white-coloured TF(Ic*As)*483/13 (termed ‘tannin free’), was developed for its low tannin content, thus explaining the low TPC and TFC detected in the present study.
Table 1 Extraction yield, total phenolic content, total flavonoid content, diphenylpicrylhydrazyl (DPPH) radical scavenging activity, Trolox equivalent antioxidant capacity (TEAC) and oxygen radical absorbance capacity (ORAC) of crude extracts from the raw and roasted faba bean genotypes
(Mean values and standard deviations of at least three independent measurements, n 3)
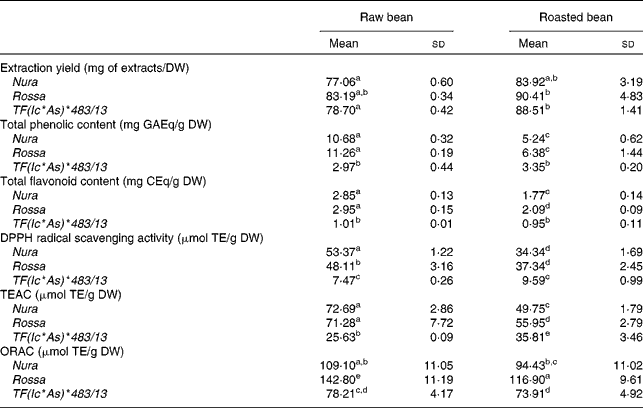
DW, dry weight; GAEq, gallic acid equivalents; CEq, catechin equivalents; TE, Trolox equivalents.
a,b,c,d,e Mean values with unlike superscript letters were significantly different in the respective assays (P < 0·05).
Roasting led to a 40–50 % decrease in the TPC, and a 30–40 % decrease in the TFC of both genotypes, Nura and Rossa. On the other hand, roasting only reduced the TPC and TFC of TF(Ic*As)*483/13 by 13 and 7 %, respectively. These results support earlier findings that heating applied through various cooking methods decreased the phenolic contents in different types of legumes(Reference Ranilla, Genovese and Lajolo42).
The antioxidant capacities of faba beans were evaluated using three assays: DPPH, TEAC and ORAC (Table 1). The antioxidant capacities of Nura and Rossa were comparable in all assays. However, the antioxidant capacity of TF(Ic*As)*483/13 was lower than the antioxidant capacities of the other two genotypes; about two to three times (ORAC and TEAC assays, respectively) and six times lower (DPPH assay). A significant decrease in the antioxidant capacity of Rossa and Nura occurred after roasting. However, roasting did not affect the antioxidant activity of TF(Ic*As)*483/13 significantly as tested by the DPPH and ORAC assays.
A high correlation was observed for the TPC with TFC (0·98, P < 0·001) and the antioxidant capacities, as evaluated using DPPH (0·93, P < 0·01), TEAC (0·96, P < 0·01) and ORAC (0·87, P < 0·05) assays. The HPLC-post column derivatisation profiles of crude extracts detectable at 280 nm from the raw beans of three genotypes (Fig. 1) showed that the phenolic compounds eluted in two separate regions, which can be arbitrarily classified as relatively polar (0–15 min) and less polar (15–40 min) regions. The HPLC-post column derivatisation results also showed that most of the antioxidant activities in Rossa and Nura were contributed by the less polar region. Distinct active peaks in the less polar region were observed in the HPLC chromatograms of Nura (Fig. 1(A)) and Rossa (Fig. 1(B)). Moreover, traces of anthocyanins were also detected (520 nm, data not presented) in the extract from Rossa, which may contribute to the antioxidant capacity. In contrary, the HPLC chromatogram of the extract from TF(Ic*As)*483/13 (Fig. 1(C)) lacked active compounds in the relatively less polar region. However, the three genotypes appeared to have similar HPLC-post column derivatisation profiles in the relatively polar region.
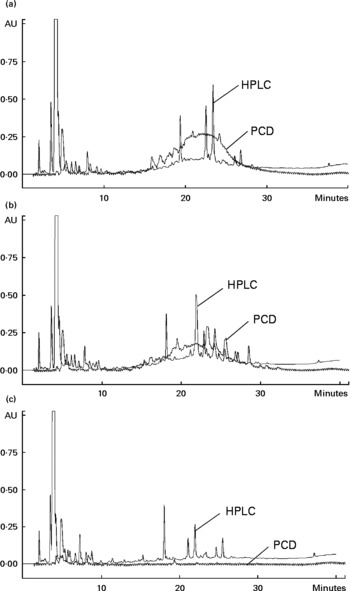
Fig. 1 Chromatograms of HPLC and on-line post column derivatisation (PCD) assay of crude extracts obtained from the raw faba bean genotypes: (A) Nura, (B) Rossa and (C) TF(Ic*As)*483/13.
Faba beans with coloured seed coat were reported to contain low and high molecular weight phenolic compounds such as flavanols and proanthocyanidins(Reference Borowska, Giczewska and Zadernowski18). The high molecular weight compounds were likely to appear at the relatively less polar region in the HPLC-post column derivatisation profiles (Fig. 1(A) and (B)) and contribute to the antioxidant capacity. High molecular weight phenolic compounds were reported previously to be very active hydrogen donors, and thereby radical quenchers(Reference Hagerman, Riedl and Jones43). The white-coloured TF(Ic*As)*483/13 contained noticeably lower TPC and TFC, and subsequently exhibited significantly lower antioxidant capacity. The HPLC chromatogram of extract from TF(Ic*As)*483/13 (Fig. 1(C)) showed a lack of active phenolic compounds at the less polar region, which might be the polymeric compounds. The present results confirm earlier findings by Madhujith et al. (Reference Madhujith, Amarowicz and Shahidi29), who also were not able to detect anthocyanidin and procyanidin in the white bean varieties.
Cellular protection by faba bean extracts
Cellular antioxidant activity assay
The CAA assay evaluates antioxidant activity at the cellular level. The final result of this assay depends on uptake, distribution and metabolism of the antioxidant compounds in a live cell. This information cannot be obtained in a reagent-based antioxidant testing. In comparison to animal models, the CAA is a cost-effective and fast way to obtain important information on the efficiency of antioxidants within a cell(Reference Wolfe and Liu40). Extracts from Nura and Rossa were evaluated in this study. No significant difference was found between the CAA of extracts obtained from both genotypes, regardless of heat treatment, as expressed in μmol quercetin equivalent/g dry weight of bean (Table 2). However, half maximal effective concentration (EC50) of the roasted Nura showed a tendency for a higher uptake of extracts than its raw beans. The HPLC chromatogram (Fig. 1) demonstrated compositional differences between the compounds detected at 280 nm in extracts from Nura and Rossa. The ‘hump’ at the less polar region dominated in extracts from both Nura and Rossa could be the polymeric compounds, while phenolic acids and flavonols were greater in the extract from Rossa (data not presented). A variety of phenolic compositions were likely to contribute to the different cell uptake rates and efficiency of protection against peroxyl radicals, within an hour. It can also be speculated that heat application during roasting of faba beans caused a partial oxidation of polymeric compounds which would affect the uptake and reflect on the antioxidant capacity within a cell. The CAA EC50 values of the faba beans are slightly lower than those of lentil (670 μg/ml), yellow pea (780 μg/ml) and green peas (1280 μg/ml) as reported in Xu & Chang(Reference Xu and Chang44) assayed using AGS cells.
Table 2 Cellular antioxidant activity of the crude extracts obtained from the raw and roasted faba bean genotypes, Nura and Rossa
(Mean values and standard deviations of three independent experiments, n 3)
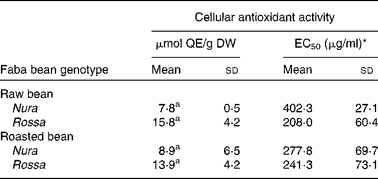
μmol QE/g DW, μmol quercetin equivalents per g of bean (dry weight basis); EC50, half maximal effective concentration.
a Mean values with same superscript letters were not significantly different.
* Concentration of the lyophilised extract in the culture medium (μg/ml) able to scavenge 50 % of free radicals effectively within a cell.
Cellular protection against H2O2
H2O2 is a reactive oxygen species which is present in live cells and is used in experimental models. In this experimental model, we evaluated the protective effect of the faba bean extracts against H2O2-induced apoptosis in RAW264.7 cells (Fig. 2). Extracts obtained from the raw faba bean genotypes, Nura and Rossa, were applied at concentrations of 0·1–0·4 mg/ml, and exhibited cellular protection against H2O2 in a dose-dependent manner (Fig. 2(A) and (C)). However, the protection diminished at concentrations higher than 0·4 mg/ml due to the commencement of antiproliferative effects, or possibly due to the pro-oxidative effects of phenolic compounds at high concentrations. The pro-oxidative effect might be caused by the interaction of the added phenolic compounds with undefined components from the culture media, resulting in the generation of H2O2(Reference Lapidot, Walker and Kanner45). The same tendency was observed for extracts obtained from the roasted Nura (Fig. 2(B)). On the other hand, the protective effects by extracts obtained from the roasted Nura and Rossa were observed at concentrations at 0·2 and 0·6 mg/ml, respectively (Fig. 2(B) and (D)). In comparison to other results on the protection of RAW264.7 cells from H2O2-induced injury, the faba bean extracts appeared less efficient than that of Kakadu plum extract(Reference Tan, Konczak and Ramzan39). However, the crude faba bean extracts were used for this study, whereas purified and concentrated Kakadu plum extracts (using XAD-7 resin column) were used. Chow et al. (Reference Chow, Shen and Huan46) found that 25 and 50 μm of quercetin (but not rutin and quercitrin) posed potent protection of RAW264.7 cells against H2O2-induced injury.
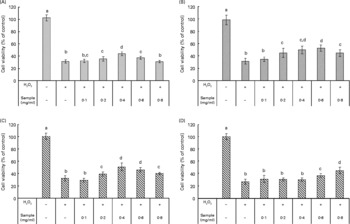
Fig. 2 Cellular protection against H2O2 (40 mm) on RAW264.7 cells by crude extracts obtained from the (A) raw and (B) roasted Nura; (C) raw and (D) roasted Rossa. Values are means of four replicates, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
Effects of faba bean extracts on proliferation and apoptosis of cancer cells
The effect of extracts obtained from the raw and roasted faba bean genotypes, Nura and Rossa, on the proliferation of different types of cancer cells including AGS, HT-29, BL13, Hep G2 and one non-transformed cell line, CCD-18Co, is presented in Table 3. The crude faba bean extracts, applied at a concentration range of 0·2–2·0 mg/ml, exhibited a dose-dependent suppression of all of the tested human cancer cell proliferations, while exhibiting negligible proliferation effect on the non-transformed human colon CCD-18Co cells. Extracts from the raw Nura suppressed some proliferation of non-transformed colon cells, CCD-18Co, while the raw Rossa did not (Fig. 3(A)). Extracts from raw beans of both genotypes effectively suppressed proliferation of the human colon cancer cells, HT-29 (Fig. 3(B)). On the other hand, extracts from the roasted Nura and Rossa did not affect the proliferation of the non-transformed CCD-18Co cells (Fig. 4(A)), but effectively suppressed the proliferation of the human colon cancer cells, HT-29 (Fig. 4(B)).
Table 3 Effects of faba bean crude extracts on the proliferation of human cancer cells: AGS, BL13, Hep G2 and non-transformed cells: CCD-18 Co
(Mean values and standard deviations)
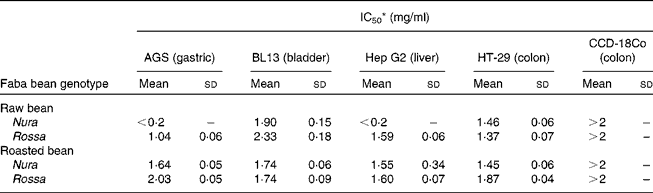
IC50, half maximal inhibitory concentration.
* Results were presented as concentration (mg/ml) of crude extracts in the culture medium needed to achieve suppression of cell growth by 50 % (IC50). Sample concentration ranged from 0·2 to 2·0 mg/ml. The results were obtained via nonlinear regression and based on at least four replicates.

Fig. 3 The effect of crude extracts obtained from the raw faba bean genotypes, Nura (![]() ) and Rossa (
) and Rossa (![]() ) on proliferation of (A) non-transformed human colon cells, CCD-18Co and (B) human colon cancer cells, HT-29. Values are means of at least three independent experiments with four replicates, with standard deviations represented by vertical bars, n 4.
) on proliferation of (A) non-transformed human colon cells, CCD-18Co and (B) human colon cancer cells, HT-29. Values are means of at least three independent experiments with four replicates, with standard deviations represented by vertical bars, n 4.

Fig. 4 The effect of crude extracts obtained from the roasted faba bean genotypes, Nura (![]() ) and Rossa (
) and Rossa (![]() ) on proliferation of (A) non-transformed human colon cells, CCD-18Co and (B) human colon cancer cells, HT-29. Values are means of at least three independent experiments with four replicates, with standard deviations represented by vertical bars, n 4.
) on proliferation of (A) non-transformed human colon cells, CCD-18Co and (B) human colon cancer cells, HT-29. Values are means of at least three independent experiments with four replicates, with standard deviations represented by vertical bars, n 4.
Heating appeared to cause decreases in phenolic contents and antioxidant capacities, which were in line with some but not all of the antiproliferation results. This suggests that different types of phenolic compounds in the faba bean extracts might exert a diverse degree of activities on specific target sites of cells. The IC50 value represents the concentration required to inhibit 50 % of cell proliferation, and therefore, a lower IC50 indicates a greater antiproliferation ability. The IC50 values of extract from the raw Nura ( < 0·2 mg/ml) and Rossa (1·04 mg/ml) were lower than green pea (3·25 mg/ml), chickpea (3·23 mg/ml) and lentil (1·27 mg/ml) as tested on AGS cells(Reference Xu and Chang44). Seeram et al. (Reference Seeram, Adams and Hardy47) found that different cancer cell lines had diverse levels of sensitivity to phenolic compounds extracted from cranberries using different cell viability testing assays. Extracts from the raw Nura posed exceptionally high antiproliferative effects on AGS and Hep G2 cells in comparison to the raw Rossa. The reason is not known and the results are under further investigation. However, in support of our findings, the IC50 values of six types of berry extracts tested on six different human tumour cell lines were all reported to be less than 0·2 mg/ml(Reference Seeram, Adams and Zhang48).
In order to understand the mechanism behind the suppression of cancer cell proliferation, an investigation to identify apoptotic and necrotic cells within the populations treated by the faba bean extracts was carried out. Apoptosis is a natural cell death process which cancer cells evade. Induction of apoptosis in cancer cells is the preferred way to remove them from the human body, which is an approach used in chemotherapy treatments(Reference Fulda and Debatin49). Food compounds that are able to induce apoptosis of cancer cells might contribute to cancer prevention.
The flow cytometric analysis revealed that exposure of HL-60 (human promyelocytic leukaemia cells) to crude extracts obtained from the raw and roasted faba bean genotypes, Nura and Rossa, induced cell apoptosis (Fig. 5). The percentage of apoptotic cells increased with greater extract concentrations (Fig. 5(A)). After the first 3 h of incubation, early apoptotic events were detected. The number of apoptotic cells increased over the treatment time (Fig. 5(B)), with the greatest percentage of apoptotic cells induced by both, the raw and roasted faba bean extracts, over 24 h. In addition, the percentage of necrotic cells remained very low. This result suggests that the suppression of cancer cell proliferation was due to induction of apoptosis by the applied faba bean extracts.

Fig. 5 Apoptosis of human leukaemia cells, HL-60, induced by crude extracts obtained from the raw and roasted faba beans as determined by flow cytometric analysis based on (A) different dose–response and (B) time course (extract concentration: 0·8 mg/mL). Values are means of three independent flow cytometric analysis for 3000–10 000 cells in each population and presented as a percentage of each cell population, with standard deviations represented by vertical bars, n 3. ![]() , Necrotic cells;
, Necrotic cells; ![]() , late apoptotic cells;
, late apoptotic cells; ![]() , early apoptotic cells;
, early apoptotic cells; ![]() , viable cells.
, viable cells.
Inhibition of angiotensin-converting enzyme, α-glucosidase and lipase
ACE is a key blood pressure regulator which is responsible for vasoconstriction that leads to an increase in blood pressure. Inhibition of ACE activity can potentially prevent ACE from elevating blood pressure, reducing the incidence of hypertension. The enzymes, α-glucosidase and lipase are important enzymes in the digestive tract and are responsible for sugar and lipid digestion, respectively. Inhibition of α-glucosidase activity could potentially reduce starch digestion and sugar absorption, therefore contributing to a lower postprandial hyperglycaemic response, whereas the inhibition of lipase activity could reduce fat uptake contributing to weight maintenance.
The raw and roasted faba bean extracts inhibited the activity of all the investigated enzymes. Condensed tannins (proanthocyanidins) in faba beans are prone to forming complexes with proteins(Reference Naczk, Amarowicz and Zadernowski50). Therefore, the observed inhibition of the various enzymes investigated in the present study is probably due to the formation of proanthocyanidin–enzyme complexes(Reference Zadernowski, Borowska and Naczk51).
Extracts from Nura exhibited the greatest ACE inhibition activity in both of the raw and roasted beans, followed by extracts from Rossa and TF(Ic*As)*483/13 (Fig. 6). Unfortunately, roasting reduced ACE inhibition activity in the faba beans significantly, except for TF(Ic*As)*483/13.

Fig. 6 Angiotensin-converting enzyme inhibition by crude extracts obtained from the raw (![]() ) and roasted (
) and roasted (![]() ) faba bean genotypes. Results were expressed as μg of captopril equivalents/g of dry weight of beans. Values are means, with standard deviations represented by vertical bars, n 3. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
) faba bean genotypes. Results were expressed as μg of captopril equivalents/g of dry weight of beans. Values are means, with standard deviations represented by vertical bars, n 3. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
Roasting was found to decrease the level of α-glucosidase inhibitory activity of all the investigated genotypes (Table 4). Among extracts obtained from both of the raw and roasted beans, Rossa exhibited the highest α-glucosidase inhibition activity, followed by Nura and TF(Ic*As)*483/13. Similar decreases of α-glucosidase inhibitory activities after thermal processing in most of the coloured bean genotypes were also reported by Ranilla et al. (Reference Ranilla, Kwon and Genevese8).
Table 4 Effects of roasting on the α-glucosidase inhibitory activity of faba bean crude extracts
(Mean values and standard deviations, n 3)
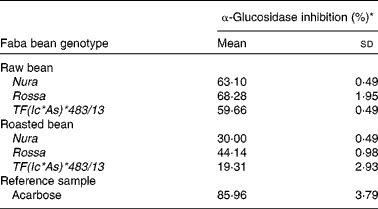
* Relative α-glucosidase inhibition activities of crude extracts from the raw and roasted faba bean genotypes. Results were based on the averages of three determinations. The concentrations of the samples (including the reference sample) used were set at 2 mg/ml.
Extracts from Rossa exhibited the strongest lipase inhibitory activity followed by Nura and TF(Ic*As)*483/13 (Table 5). In contrary to the results of roasting effect on the other types of enzymes, heating was found to cause an increase in lipase inhibitory activity in all faba bean genotypes. Similar to the present results, Zadernowski et al. (Reference Zadernowski, Borowska and Naczk51) also reported lipase inhibition activity in both faba bean and pea varieties.
Table 5 Half maximal inhibitory concentration (IC50) and relative lipase inhibitory activity of crude extracts from the raw and roasted faba bean genotypes
(Mean values and standard deviations, n 3)
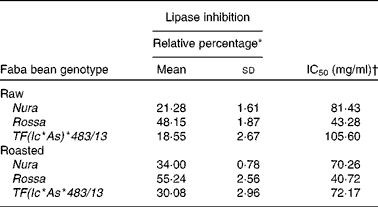
* Relative lipase inhibitory activity of crude extracts from the raw and roasted faba bean genotypes. Results were based on an average of three determinations. The sample concentrations were set at 40 mg/ml.
† The final concentration of faba bean crude extracts required to achieve the inhibition of enzymatic activity by 50 % under assay condition.
The Pearson's correlation disclosed that ACE results were positively correlated with the TPC (0·88), TFC (0·83), DPPH radical scavenging activity (0·81) and TEAC (0·81) results (P < 0·05), but not significantly correlated with the ORAC results (0·55, P = 0·26). The data suggest that ACE inhibition of faba bean extracts might be caused by the phenolic compounds. In contrary, the results of α-glucosidase and lipase inhibitory activities did not correlate with the results of other assays. Zhang et al. (Reference Zhang, Li and Hogan52) also found no correlation between the TPC and α-glucosidase inhibitory activity among the extracts of seven raspberry cultivars. This suggests that TPC were not directly related to the α-glucosidase and lipase inhibition activities in faba beans. Ranilla et al. (Reference Ranilla, Kwon and Genevese8) reported positive and negative correlations of the TPC with the α-glucosidase and ACE inhibitions as being 0·24 and − 0·42, respectively, in the different dry bean cultivars. On the other hand, Silva Pinto et al. (Reference Silva Pinto, Kwon and Apostolidis53) and Mai et al. (Reference Mai, Thu and Tien54) who tested Brazilian strawberries and Vietnamese edible plants found positive relationships between the TPC and the α-glucosidase inhibitory activity, respectively. This suggests that the relationship between phenolic compounds and α-glucosidase activity depends on the phenolic compositions and subsequently could be plant-specific.
In fact, the solvent used in this study possibly extracted constituents other than phenolic compounds in faba beans, such as trypsin inhibitors(Reference Helsper, Kolodziej and Hoogendijk55), oligosaccharides(Reference Lattanzio, Bianco and Miccolis56), vicine and convicine(Reference Brown and Roberts57), lipase(Reference Dundas, Herderson and Eskin58), saponins(Reference Amarowicz, Yoshiki and Pegg59) and particularly phytate which has been reported to have the ability to bind with proteins(Reference Sharma and Sehgal60), and these microconstituents might affect the results of enzyme inhibition assays. The strong bioactivities of faba bean could be a result of synergistic interaction between those constituents.
The phenolic extracts of three faba bean genotypes exhibited significant differences in activities as evaluated using a range of in vitro assays. Therefore, the present study suggests a possible role for plant breeders to select genotypes for specific health functionality. Faba bean flour or fractionated components can be potentially incorporated into new food products as ingredients to impart desirable health benefits. It would be useful to determine the actual faba bean (or ingredient) intake required to have significant desirable health-promoting effects. However, the actual bioavailability of the phenolic compounds (especially proanthocyanidins) to humans remains unclear(Reference Deprez, Mila and Huneau61, Reference Monach, Williamson and Morand62). Therefore, further research is required to (1) identify these native compounds and their metabolic products and (2) examine their effects on the human digestive system (e.g. metabolised by the colonic microflora) through human clinical studies, before drawing a final conclusion on the faba bean polyphenols in relation to human health.
Conclusions
The crude phenolic extracts obtained from the raw and roasted faba beans evaluated in the present study exhibited potential health-benefiting properties, including potent antioxidant activities (based on both reagent- and cell-based assays), chemopreventative effects (through induction of cancer cell apoptosis) and protection against reactive oxygen species, H2O2. In addition, these extracts showed inhibitory effect on ACE, α-glucosidase and lipase as studied using in vitro methods. Faba bean extracts suppressed proliferation of different types of cancer cells in a dose-dependent manner, but posed negligible effect on the CCD-18Co (colon normal) cells particularly after roasting. According to the reagent-based assays, roasting decreased the antioxidant activities of faba bean extracts; however, the roasting effect on cellular and enzymatic assays varied. Overall, Nura (buff-coloured) and Rossa (red-coloured) exhibited comparable functional properties, while TF(Ic*As)*483/13 (white-coloured) contained the lowest level of all tested functional properties. The present study encourages a wider utilisation of faba bean in human diets for its potential health benefits due to their known microconstituents, such as phenolics.
Acknowledgements
Funding for the present study was provided by Food Futures Flagship, CSIRO Food and Nutritional Sciences; Grains Research & Development Corporation (GRDC Research Code GRS166) and EH Graham Centre for Agricultural Innovation (an alliance between Charles Sturt University and NSW Department of Primary Industries). The support received from the Charles Sturt University Postgraduate Writing-Up Award Scheme is gratefully acknowledged. The authors thank Jeffrey Paull, Peter Matthews, Eric Armstrong and Gerard O'Connor for providing samples, and Dr Michelle Bull for acquiring sample data using the flow cytometer. The order of authors reflects their relative contributions. The authors declare no conflict of interest.













