Prebiotics are non-digestible feed ingredients selectively affecting the growth and activity of beneficial bacteria and therefore maintaining an optimal gut environment, resulting in beneficial effects for the host(Reference Gibson and Roberfroid1). They are presently considered as an efficient strategy for the maintenance of gut health in humans and animals. The fermentation of fibre-rich substrates in the intestines selectively stimulates the proliferation and metabolic activity of health-associated beneficial bacteria such as Bifidobacterium spp. and Lactobacillus spp.(Reference Metzler, Bauer and Mosenthin2) and prevents the colonisation by potential pathogens(Reference Van der Waaij, Berghuis-de Vries and Lekkerkerk-van der Wees3) at the end of the small intestine and in the hindgut. The saccharolytic fermentation of dietary fibres by the endogenous microbiota yields SCFA among which butyrate helps creating and maintaining gut health and ecology. Butyrate is of special interest as it is the main source of energy for colonocytes(Reference Knudsen, Serena and Canibe4, Reference Roediger5), as it can decrease intestinal inflammation and as it enhances the intestinal barrier function(Reference Peng, Li and Green6). Butyrate production mainly arises from the Clostridium clusters IV and XIVa via the butyryl-CoA:acetate-CoA transferase enzyme(Reference Louis, Duncan and McCrae7) which are therefore considered as health-associated bacteria(Reference Lopetuso, Scaldaferri and Petito8).
The weaning period is a critical transitory phase in humans and animals(Reference Pohl, Medland and Moeser9). It is characterised by a multifactorial stress, contributing to altered intestinal activities and health issues(Reference Dong and Pluske10–Reference Pluske, Turpin and Kim12). In pig production, the piglet’s intestines become more susceptible to the invasion of pathogens, resulting in post-weaning diarrhoea. In this context, more attention has been paid to the presence of dietary fibres in the diet of young piglets as a strategy to reduce post-weaning-associated disorders and thus the need for antibiotics. Inulin is currently acknowledged as an efficient prebiotic for young mammals(Reference Veereman13, Reference Samanta, Jayapal and Senani14). Some agricultural and industrial by-products such as chicory root and pulp contain significant amounts of inulin even after undergoing the extraction process(Reference Van Waes, Baert and Carlier15). Similarly, remaining pectin can be found in citrus peels(Reference Wang, Chen and Lü16), while rye bran is rich in arabinoxylans and soya-oligosaccharides are present in soya hulls(Reference Hata, Yamamoto and Nakajima17). The potential prebiotic function of such fibre-rich by-products, considered as more economic and sustainable than inulin, is therefore of great interest.
Until now, several in vitro gut models investigated the properties of prebiotics(Reference Ortega-González, Ocón and Romero-Calvo18–Reference Zenhom, Hyder and Heller20) or probiotics(Reference Dimitrov, Gotova and Chorbadjiyska21, Reference Mangell, Nejdfors and Wang22) on the intestinal epithelial cells. In addition to their well-documented prebiotic activities, these fibre-rich ingredients could exert an indirect effect, through microbiota shifts and/or the production of fermentation metabolites(Reference Cilla, Alegría and Barberá23), which at their turn may result in an effect on intestinal inflammation and gut integrity. Owing to the complexity of the intestinal environment, arising from complex interactions between the host, the microbiota and the fermentation end products, it is valuable to consider a holistic approach to study the immunomodulatory and anti-inflammatory properties of prebiotics that were submitted to a colonic fermentation(Reference Cilla, Alegría and Barberá23). Therefore, we should consider an approach combining an in vitro gut fermentation model with pig faeces(Reference Williams, Voigt and Verstegen24, Reference Bauer, Williams and Bosch25) with an in vitro intestinal cell culture experiment. Intestinal porcine epithelial cells (IPEC-J2) are one of the few cell lines that is not derived from tumour origin and can be polarised into monolayer epithelial cells(Reference Brosnahan and Brown26, Reference Pearce, Coia and Karl27). They have been used for prebiotic and probiotic investigations and may therefore provide insights into metabolites and microbiota interactions with the intestinal mucosa. Although the IPEC-J2 cell line is derived from the small intestine of young piglets, no colonic cell line from porcine origin is available. In this way, the in vitro technique used in the present study simulated the interplay between the ingredients, the microbiota and the metabolites representing the intestinal chyme and cultured IPEC-J2 mimicking the intestinal lumen in the small intestine. The interest of the complete fermentation model taking into account both bacteria and metabolites could arise from the production of potential cytoprotective bacterial metabolites such as SCFA, and especially butyrate, which have the ability to modulate gene expression(Reference Borowicki, Stein and Scharlau28, Reference Munjal, Glei and Pool-Zobel29) as well as the protective effect of the microbiota itself interacting directly with the intestinal epithelial cells.
In the present study, chicory root and pulp, citrus pulp, rye bran and soya hulls that had been previously selected amongst various sources of carbohydrate-rich by-products based on an in vitro fermentation model were further tested for their prebiotic activities. The objective of the present study was to assess if the different sources of feed by-products reached the same prebiotic potential as inulin, considered as a positive control, in terms of fermentation characteristics and to compare the immunomodulatory profiles of the fermentation supernatants (FS) on cultured IPEC-J2.
Methods
Dietary fibre source
Six feed ingredients were chosen to represent a wide range of dietary fibre sources with a potential for inclusion in the weaning piglet’s diet (Table 1).
Table 1. Chemical composition (g/kg DM) of the ingredients and total constituent monosaccharide composition of the non-cellulosic polysaccharide fraction
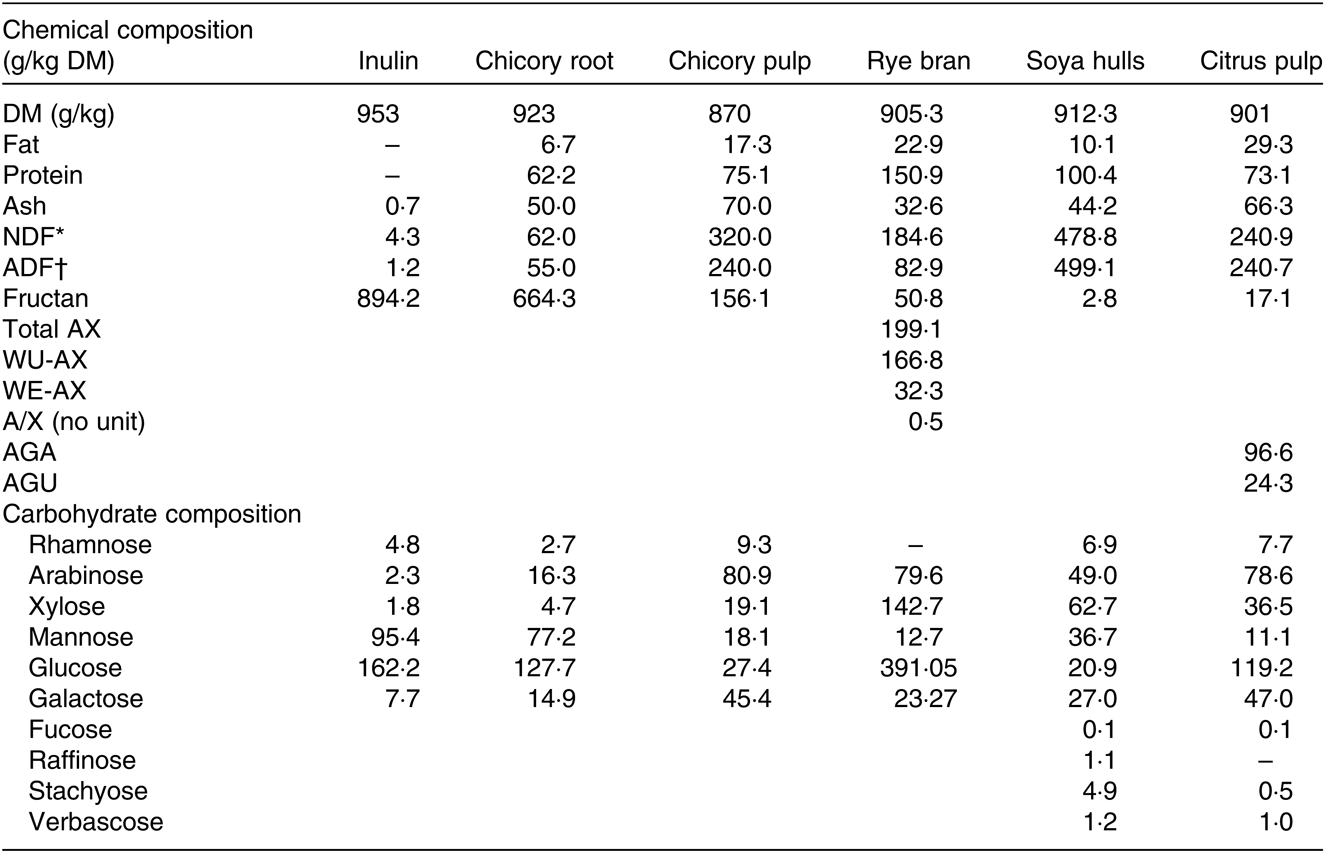
NDF, neutral-detergent fibre; ADF, acid-detergent fibre; AX, arabinoxylan; WU-AX, water-unextractable arabinoxylan; WE-AX, water-extractable arabinoxylan, AGA, galacturonic acid; AGU, glucuronic acid.
* Hemicellulose + cellulose + lignin.
† Cellulose + lignin.
Inulin (Fibruline Instant®), chicory root (Fibrofos 60®) and chicory pulp were provided by Cosucra Groupe Warcoing SA (Warcoing, Belgium). Citrus pulp (mix of albedo and flavedo), rye bran and soya hulls were obtained from a commercial supplier (Royal Agrifirm Group, Apeldoorn, The Netherlands). The ingredients were selected according to a previous screening of several sources of by-products differing in structural carbohydrates, based on their fermentation characteristics, that is, SCFA and microbiota profiles. An overview of the bacterial populations present in the FS of all tested ingredients is presented in online Supplementary Fig. S1 and was mainly used as selective criteria for the present study, along with the previous data(Reference Uerlings, Bindelle and Schroyen30).
Analysis of dietary fibre sources
The ingredients were analysed for organic matter (AOAC 923.03), DM (AOAC 967.03), crude protein (Foss Kjeltec Analyzer Unit 2300; CP = N × 6·25), fat content (Soxhlet method; AOAC 920.29) and neutral-detergent fibre (NDF) and acid-detergent fibre (ADF) (Foss Fibrecap system; Van Soest et al. (Reference Van Soest, Robertson and Lewis31)). Non-cellulosic total monosaccharide composition was determined according to the method of Englyst & Cummings (1984)(Reference Englyst and Cummings32) adapted by Aguedo et al. (2014)(Reference Aguedo, Fougnies and Dermience33). The uronic acids were detected by high-performance anion-exchange chromatography with pulsed amperometric detection(Reference Aguedo, Fougnies and Dermience33). The fructan amount was assessed by size-exclusion HPLC as previously described(Reference Aguedo, Fougnies and Dermience33). Total arabinoxylan content and water-extractable arabinoxylan content were measured according to the method of Gebruers et al. (Reference Gebruers, Courtin and Delcour34) using GC (Agilent Technologies).
In vitro digestion and batch fermentation of dietary fibre sources
The ingredients were studied using a modified three-step in vitro model of the pig’s gastro-intestinal tract(Reference Bindelle, Buldgen and Boudry35) combining an enzymatic hydrolysis and dialysis to a batch fermentation with faecal microbiota. The grinded ingredients underwent a pepsin-pancreatin hydrolysis adapted from the protocol of Boisen & Fernández(Reference Boisen and Fernández36) as described by Uerlings et al. (Reference Uerlings, Bindelle and Schroyen30) followed by a dialysis step according to Kalala et al. (Reference Kalala, Kambashi and Everaert37).
For the batch fermentation, a faecal inoculum was prepared from a buffer solution composed of salts and minerals devoid of reducing agent (pH 6·8; Menke & Steingass(Reference Menke and Steingass38)) and mixed frozen faeces (2·5 %, w/v) from piglets under anaerobic conditions (Invivo2, Led Techno) with three mucin microcosms (K1-carrier, AnoxKaldnes AB) per fermentation vial(Reference Tran, Boudry and Everaert39), with the hydrolysed ingredient or not (blank vials).
Williams et al. (Reference Williams, Voigt and Verstegen24) reported that faeces are a suitable and representative inoculum to mimic the in vivo gut fermentation which justifies the model chosen for the following research. Faeces were previously collected from pre-weaned 3 week-old-piglets (male and female) by faecal stimulation with sterile swabs. The sows and the piglets were housed in individual farrowing units, equipped with wood shavings as litter, and the piglets had a space with a heat lamp. There was no creep feed available to the piglets, and they were thus only consuming milk from their mother. All experimental procedures led on piglets (faeces collection) were in accordance with European and Belgian regulations concerning the care and use of animals for research purposes and were approved by the Animal Ethical Committee of Liège University, Belgium (protocol number: 1860).
Each ingredient was added in three vials for gas measurements, six vials for SCFA measurements at each time point, three vials for microbiota measurements at each time point and three vials for the cell culture assay. The different vials were placed into an agitating water bath at 39°C with 50 rpm agitation, and the fermentation broth was stored at −80°C.
Sampling times for SCFA and microbiota population measurements were based on the hindgut transit time in the large intestine of growing pigs(Reference Wilfart, Montagne and Simmins40). As substrate depletion is one of the limitations of the in vitro batch fermentation model, SCFA production and microbiota composition were assessed after 6, 12 and 24 h, with a limited decline in bacterial population (data not shown).
Fermentation kinetics profile of the in vitro batch fermentation
The released gas volumes were repeatedly recorded with a Tracker 200 manometer (Bailey & Mackey Ltd) with a needle of 0·6 × 25 mm at following time points: 2, 5, 8, 12, 16, 20, 24, 48 and 72 h according to the model of Groot et al. (Reference Groot, Cone and Williams41) (n 3 fermentation vials), and gas production recordings were fitted to the mathematical monophasic model:
with A (ml/g DM) as the maximum gas volume, G (ml/g DM) as the gas accumulation to time, B (h) as the time to half asymptote when G = A/2, RMAX as the maximum rate of gas production (ml/g DM × h) and TMAX as the time to reach RMAX.
Fermentation products profile of supernatants from in vitro batch fermentation
Fermentation broths sampled after 6, 12 and 24 h of fermentation (n 6 fermentation vials) were analysed by isocratic HPLC using the Alliance System e2695 (Waters) with an Aminex HPx-87H column (BioRad) as described by Uerlings et al. ( Reference Uerlings, Bindelle and Schroyen30 ). A calibration curve with known concentrations of SCFA and lactate was used to quantify the amounts present in the samples.
Microbiota composition of supernatants from in vitro batch fermentation
Microbiota profile kinetics were measured after 6, 12 and 24 h of fermentation (n 3 fermentation vials). Genomic DNA from fermentation broth samples was extracted using the QIAamp DNA Stool Mini Kit (Qiagen) following the manufacturer’s instructions adapted by Uerlings et al. ( Reference Uerlings, Bindelle and Schroyen30 ). The DNA concentration and quality were, respectively, determined by Nanodrop (Thermofisher Scientific Nanodrop 2000) and agarose gel (1 %).
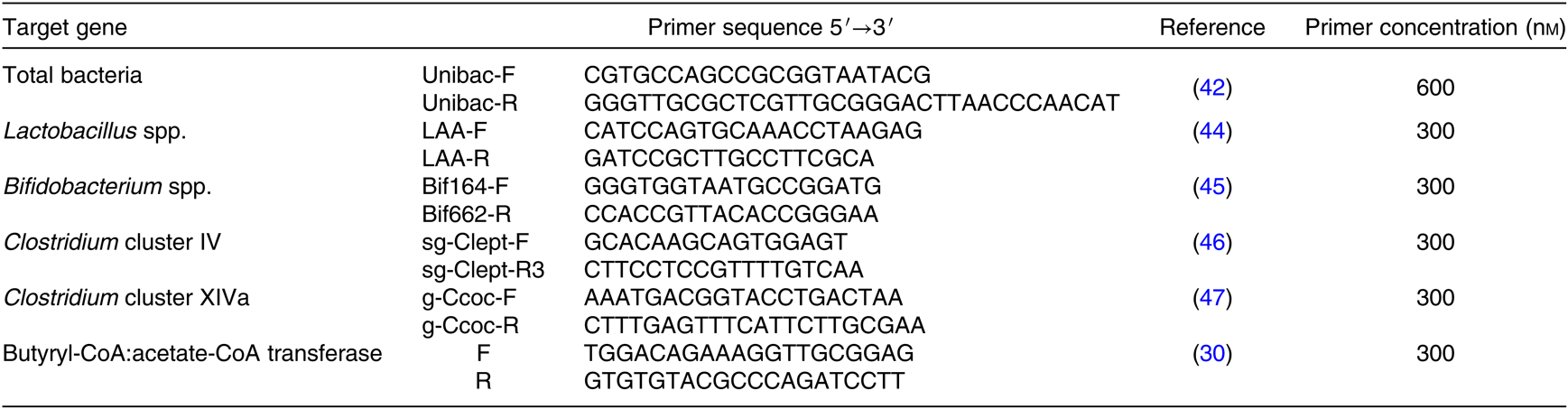
Quantitative PCR (qPCR) was performed on DNA samples to quantify Lactobacillus spp., Bifidobacterium spp., Clostridium clusters IV and XIVa, total bacteria as well as the butyryl-CoA:acetate-CoA transferase gene abundance. Real-time PCR analysis was conducted using the StepOne Plus (ThermoFisher Scientific) using SYBR Premix Ex Taq, Tli RNase H Plus (Takara Bio Inc. Ltd). The commercially manufactured gene specific primers are shown in Table 2 (Eurogentec). qPCR conditions were optimised to obtain primer efficiency values between 90 and 110 %. All runs were performed with the default protocol, with a pre-denaturation step (30 s, 95°C) followed by amplification for forty cycles with a denaturation step (5 s, 95°C), an annealing step (1 min, 60°C) and an extension step (30 s, 72°C). Primers specificity was verified through melting curves. Total bacteria(Reference Amit-Romach, Sklan and Uni42) was selected as a reference gene after verification of the stability for all experimental conditions. For each target gene, the relative gene abundance level was calculated by the 2−ΔΔct method(Reference Livak and Schmittgen43) using a pooled sample as an internal control.
Table 2. Nucleotide sequences of primers for the microbiota composition of fermentation supernatant
Fermentation supernatant preparation
The FS of the five ingredients and inulin after 12 h of fermentation (pooled FS coming from three different fermentation vials) were sterile-filtered with 0·22-µm ø pore diameter (Filter Service) for the cell proliferation assay (‘sterile-filtered FS’) and with 0·8-µm pore ø (‘complete FS’), to remove the matrix debris for the cell response assay by high-throughput qPCR.
Intestinal porcine epithelial cell line and culture conditions
The non-transformed porcine intestinal epithelial cell line (IPEC-J2), originally isolated from jejunal epithelia of a neonatal unsuckled piglet(Reference Schierack, Nordhoff and Pollmann48), was grown at 37°C in a humidified atmosphere of 5 % CO2 in complete Dulbecco’s modified Eagle’s medium (DMEM)/F-12, supplemented with 1 % penicillin–streptomycin, 5 % fetal bovine serum, 2 mm l-glutamine, 5 ng/ml epidermal growth factor, 5 μg/ml insulin, 5 μg/ml transferrin and 5 ng/ml Se (all from Sigma). Plain medium was added once every 2 d, and cells were passaged when they reached confluence.
Modulation of intestinal porcine epithelial cell viability by fermentation supernatant
Cell viability was used to determine the concentration of FS to be applied for the cell response assay of testing the impact of FS on gene expression in IPEC-J2. Cell proliferation was measured with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. IPEC-J2 cells between passages 15 and 20 were seeded in ninety-six-well flat bottomed plates at a density of 20 000 cells/100 μl (100 µl/well). Cells were allowed to adhere for 24 h until confluence was reached and were re-fed with experimental media without antibiotics before being treated with different concentrations of 0·22-µm ø sterile-filtered FS (50, 25, 10, 5, 2·5 %, v/v). After incubation with different concentrations of FS for 24 h, the culture medium was removed. Next, fresh antibiotic-free culture medium and 15 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Promega) were added to each well for another 4 h at 37°C prior to measurement of cell viability. The absorbance at 570 nm was determined in a micro-plate reader (VICTOR plate reader, PerkinElmer). There were six well-replicates per treatment. According to the cell viability test, a concentration of 10 % (v/v) was chosen to study the impact of FS on gene expression in IPEC-J2 cells.
Impact of fermentation supernatant on gene expression in intestinal porcine epithelial cells
IPEC-J2 cells between passages 15 and 20 were seeded in 24-well plates at a density of 2·5 × 105 cells/ml (1 ml/well). Prior to treatment, confluent monolayers of the IPEC-J2 cells were washed with plain medium without antibiotics. FS (0·8-µm ø filtered) was applied (10 %, v/v) for 24 h. For sham-stimulation, cells were maintained in the culture medium for 24 h. After washing with PBS, lysis buffer (RNeasy Mini Kit, Qiagen) supplemented with β-mercaptoethanol (Sigma) was added to the IPEC-J2 cells and lysates were collected and kept at −80°C until further processing. There were three well-replicates per treatment.
Total RNA from IPEC-J2 cells treated with 0·8-µm ø FS was extracted using an RNeasy Mini kit (RNeasy Mini Kit), according to the manufacturer’s protocol. RNA concentration and quality were determined by Nanodrop (Thermofisher Scientific Nanodrop 2000, Thermo Fisher Scientific) and agarose gel (1 %), respectively. Extracted RNA was converted into cDNA by reverse transcription of 60 ng total RNA using a Reverse Transcription Master Mix (Fluidigm Corporation) and analysed by high-throughput qPCR as described by Stoy et al. (Reference Stoy, Heegaard and Sangild49).
Briefly, pre-amplification was performed according to the PreAmp MasterMix manufacturer’s instructions (Fluidigm Corporation) followed by an exonuclease I treatment (New England Biolabs).
Intron spanning primer pairs, yielding a PCR product lower than 150 bp, were designed using Primer-BLAST (NCBI) and were validated through agarose gel electrophoresis and through melting curves (Table 3). Pooled pre-amplified cDNA samples with 3-fold dilution series were used to obtain primer efficiency. Results are shown for those that had an appropriate primer efficiency, between 90 and 110 %. High-throughput qPCR was performed in 96 × 96 dynamic array integrated fluidic circuits (Fluidigm Corporation). After loading, the dynamic array was placed in a BioMark HD Real-Time PCR System (Fluidigm Corporation) and the following cycle parameters were used: 60 s at 95°C, followed by thirty cycles with denaturing for 5 s at 96°C, and annealing/elongation for 20 s at 60°C. Reactions were performed in six replicates (cDNA replicates). Non-template controls were included to reflect nonspecific amplification or sample contaminations.
Table 3. Primer sequences for the gene expression levels of intestinal porcine epithelial cells treated with fermentation supernatants

ACTB, actin beta; B2M, beta-2-microglobulin; ESPN, espin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HBMS; hydroxymethylbilane synthase; HPRT1, hypoxanthine phosphoribosyltransferase 1; PCNA, proliferating cell nuclear antigen; PPIA, peptidylprolyl isomerase A; RPL, ribosomal protein L; SDHA, succinate dehydrogenase complex, subunit A; TBP, TATA box binding protein; YWHAZ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta; AKT1, serine/threonine-protein kinase 1; MAPK14, mitogen-activated protein kinase 14; MyD88, myeloid differentiation primary response 88; NF-kBIα, NF-κB inhibitor alpha; NOD1, nucleotide-binding oligomerisation domain-containing protein 1; TLR, toll-like receptor; CCL5, chemokine ligand 5; COX2, cyclo-oxygenase 2; CXCL10, C-X-C motif chemokine 10; DEFβ, defensin beta; EGFR, epidermal growth factor receptor; IFN, interferon; ILRN1, IL-1 receptor antagonist; MCP1, monocyte chemoattractant protein 1; CASP3, caspase 3; CDH1, E-cadherin; MARVELD2, tricellulin; MUC1, mucin 1; TGFβ1, transforming growth factor beta 1; VIL1, villin 1; ZO-1, zonula occludens-1.
Quantification cycles (Cq) were acquired using the Fluidigm real-time PCR analysis software 3.0.2 (Fluidigm Corporation). The geometric mean of four reference genes (ribosomal protein L 13a (RPL13a), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylprolyl isomerase A (PPIA), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ)) was used to normalise samples. These genes were found to be stably expressed reference genes across 0·8-µm ø filtered supernatants (of inulin, chicory root, chicory pulp, rye bran, soya hulls and citrus pulp) using NormFinder( Reference Andersen, Jensen and Ørntoft50 ). For each target, the relative gene expression levels were calculated by the 2-ΔΔct method( Reference Livak and Schmittgen43 ). However, gene expression was different between the control treatment sham-treated cells and the 0·8-µm ø FS treatments for the eleven reference genes studied. Therefore, the different fermented ingredients were compared with inulin (considered as a positive control in this case).
Statistical analysis
Homogeneity between variances and normality among treatments was confirmed using, respectively, Bartlett’s and Ryan-Joiner’s tests. The experimental units for the fermentation parameters and for the immunomodulatory parameters were the fermentation vial and the cell culture wells, respectively. The experimental data concerning gas production and high-throughput qPCR data were subjected to GLM procedures, and the significantly different means were identified by post-hoc Tukey’s multiple range HSD test using SAS 9.4 software (SAS Institute). The procedure included one fixed criteria of classification (type of ingredient). For the high-throughput qPCR data, adjusted P-values were obtained using a false discovery rate correction with the linear method of Benjamini and Hochberg. The analyses of SCFA and microbial communities were performed similarly. However, the procedure included two fixed criteria of classification (type of ingredient and sampling time) as well as their interaction. For SCFA and microbiota profiles, when a significant interaction of a time effect was encountered, parameters were studied by one-way ANOVA per time point. Previous in vitro trials were used to validate the sample size of the present study based on similar variables such as microbiota and SCFA analyses arising from in vitro batch fermentations(Reference Tran, Boudry and Everaert39, Reference Tran, Boudry and Everaert51, Reference Boudry, Poelaert and Portetelle52) as well as IPEC-J2 investigations(Reference Marciňáková, Klingberg and Lauková53, Reference Lee and Kang54). P-values <0·05, <0·01 and <0·001 were considered as statistically significant, highly significant and very highly significant, respectively.
Results
Fermentation kinetics profile of the in vitro batch fermentation
Inulin and chicory root contained high amounts of fructans (89·4 and 66·4 %, respectively), whereas chicory pulp was characterised by a low fructan amount and high NDF and ADF levels (Table 1). Glucose was the most abundant building block of the non-cellulosic fraction followed by mannose for inulin and chicory root and by arabinose for chicory pulp (Table 1). With a lower fructan amounts (5·1 %), rye bran was characterised by elevated levels of arabinoxylans, of which the main water-unextractable arabinoxylans fraction is majoritarian and consequently displayed high levels of glucose, arabinose and xylose monosaccharides after hydrolysis. Soya hulls had the highest NDF and glucose monosaccharide levels. Citrus pulp displayed intermediate NDF levels, a high pectin content and was mainly composed of glucose and arabinose (Table 1).
According to the cumulative gas production, chicory root along with inulin, considered as a positive control, induced an extensive fermentation (greatest total gas production; A) in comparison with the other ingredients (P < 0·0001; Table 4). With the lowest half-time to asymptotic gas production (B), the highest rate of fermentation (RMAX) and the lower time to reach RMAX (TMAX), chicory root was the most rapidly fermented feed ingredient after inulin (Table 4). Chicory pulp and citrus pulp demonstrated intermediate fermentation kinetics. Rye bran, fermented in a slow (intermediate B and TMAX) and less extensive (low RMAX) manner, reached the lowest maximal gas production (A) (P < 0·0001; Table 4). Although an intermediate total gas production (A) was recorded for soya hulls (similar to the ones of chicory pulp, root and citrus pulp), this ingredient demonstrated the slowest rate of fermentation (RMAX; Table 4).
Table 4. Gas fermentation parameters (A, B, RMAX, TMAX) modelled according to Groot et al. (Reference Groot, Cone and Williams41) of feed ingredients in the presence of faecal inoculum of pre-weaned 3-week-old-piglets (n 3 fermentation vials)* (Mean values with their standard errors)
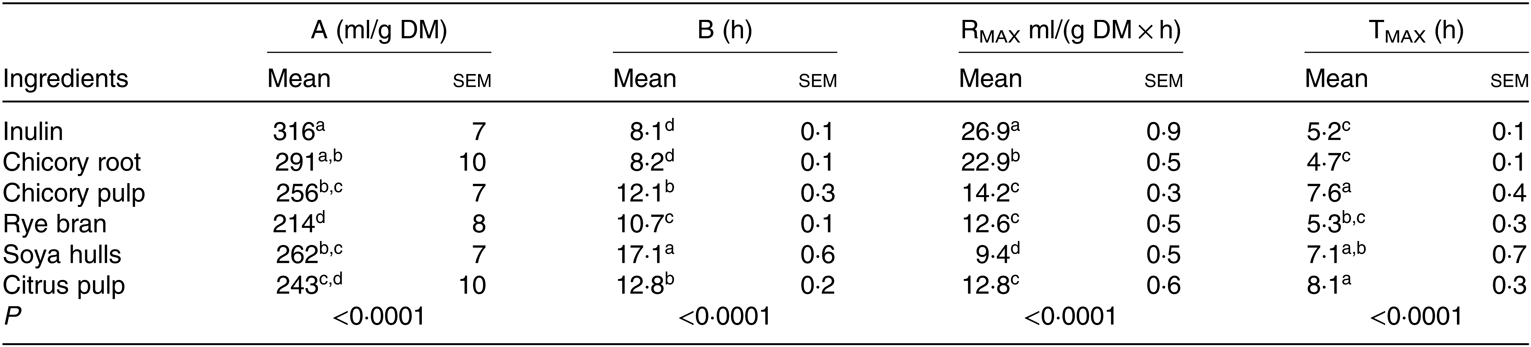
a,b,c,dMean values within a column with unlike superscript letters are significantly different (P < 0·05).
* A, total gas produced; B (h), time to half asymptote; RMAX, maximum rate of gas; TMAX, time at which RMAX is reached. Gas production values were recorded over 72 h using a manometer.
Fermentation products profile of supernatants from in vitro batch fermentation
The interaction between ingredients and the time of fermentation were significant for all the measured metabolites (Table 5). Acetate and butyrate molar ratios at 6 h were equal to zero for all ingredients, explaining the extremely high percentages of BCFA encountered (Table 5). Total SCFA amounts as well as propionate and BCFA ratios were similar at 6 h. Lactate levels encountered for the major part of the produced metabolites with inulin displaying the greatest amounts followed by chicory root (P < 0·0001). Inulin and soya hulls displayed higher amounts of total SCFA at 12 h. Fermentation of ingredients resulted in different (P < 0·001; Table 5) butyrate net production at this time point with inulin, chicory root and rye bran displaying the highest net molar ratio of butyrate (% of total SCFA), although the total SCFA production of rye bran was the smallest. Reflecting the SCFA profile at 12 h, inulin, chicory by-products and soya hulls displayed the highest SCFA amounts at 24 h of fermentation, while rye bran was the smallest producer of SCFA. Inulin displayed the highest butyrate ratio, followed by chicory root. Alternatively, chicory pulp and citrus pulp were demonstrating higher acetate molar ratios compared with inulin and were correspondingly among the lowest butyrate producers (P < 0·0001; Table 5).
Table 5. Fermentation product profile of the fermentation supernatant of the different ingredients after 6, 12 and 24 h of fermentation (n 6 fermentation vials)* (Mean values of six measurements with their standard errors)
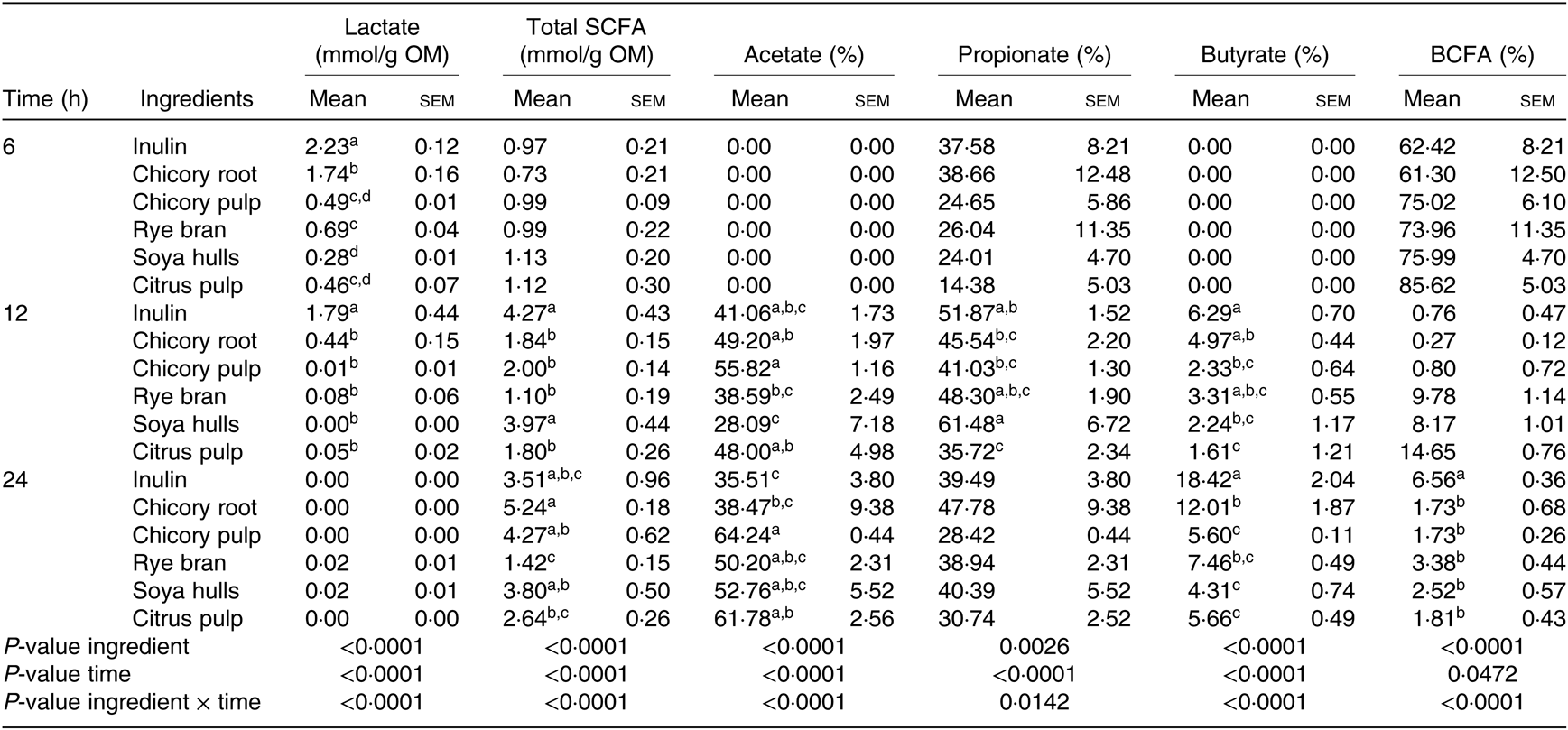
SCFA, total amount of SCFA (acetic + propionic + i-butyric + butyric + i-valeric + valeric; expressed as mmol/g organic matter); acetic, propionic and butyric acid proportions (expressed as % of SCFA); OM, organic matter; BCFA, branched chain fatty acid proportion (i-butyric + i-valeric + valeric scaled to SCFA, expressed as %).
a,b,c,dFor one sampling time, mean values within a column with unlike superscript letters are significantly different (P < 0·05).
* Values after different fermentation times with different feed ingredients were corrected for fermentation products formed in the vials without ingredient and with mucin carriers added and are thus solely the result of fibre degradation (net values).
Microbiota composition of supernatants from in vitro batch fermentation
Broth from fermented citrus pulp displayed the highest levels of Clostridium cluster IV at 6 h compared with inulin, while the other ingredients demonstrated intermediate values (P < 0·001; Fig. 1(A)). At 24 h, citrus pulp as well as chicory pulp and soya hulls induced the greatest abundance in Clostridium cluster IV. Citrus pulp exhibited the highest levels of Clostridium cluster XIVa, whereas inulin displayed the lowest levels at 24 h (P < 0·001; Fig. 1(B)). Butyryl-CoA:acetate-CoA transferase gene abundance was significantly higher in soya hulls compared with the other ingredients at 6, 12 and 24 h except for rye bran which reached the same levels as soya hulls after 12 h (P < 0·001; Fig. 1(C)). Lactobacillus spp. gene abundance greatly differed with the time of fermentation (Fig. 1(D)). Although several FS such as rye bran, chicory pulp, soya hulls and citrus pulp demonstrated the highest abundance of Lactobacillus spp. at 6 and 12 h, in contrast with inulin and chicory root, no difference was perceived between ingredients after 24 h of fermentation (P < 0·001; Fig. 1(D)). The greatest abundance of Bifidobacterium spp. was observed in soya hulls at 6 h and in rye bran fermented broths at 12 and 24 h of fermentation (Fig. 1(E)).

Fig. 1. Microbiota composition of supernatants after 6, 12 and 24 h of fermentation. (A) Clostridium cluster IV; (B) Clostridium cluster XIVa; (C) butyryl-CoA:acetate-CoA transferase; (D) Lactobacillus spp.; (E) Bifidobacterium spp. Values are means (n 3 fermentation vials) with their standard errors per bacterial group. a,b,c,dFor one sampling time, mean values with unlike letters are significantly different (P < 0·05). Total bacteria was selected as the reference and was stable across treatment. Inulin at 6 h was considered as control and was set at a value of 1·000. a.u., Arbitrary unit. ![]() , 6 h;
, 6 h; ![]() , 12 h;
, 12 h; ![]() , 24 h.
, 24 h.
Modulation of intestinal porcine epithelial cell viability by fermentation supernatant
In order to choose the most appropriate concentration of FS for the IPEC-J2 model, a cell viability assay was conducted (Fig. 2). Sterile-filtered FS collected after 12 h of fermentation was not toxic for IPEC-J2 at a concentration <25 % (v/v) for most ingredients, with a reduction of the cell viability approximately 50 % at the cited concentration (EC50). A concentration of 10 % (v/v) led to a reduction of approximately 30 % of cell viability for all ingredients and the fermentation blank (Fig. 2). According to these results and the literature, 10 % (v/v) was chosen as a concentration for the immunomodulatory model.
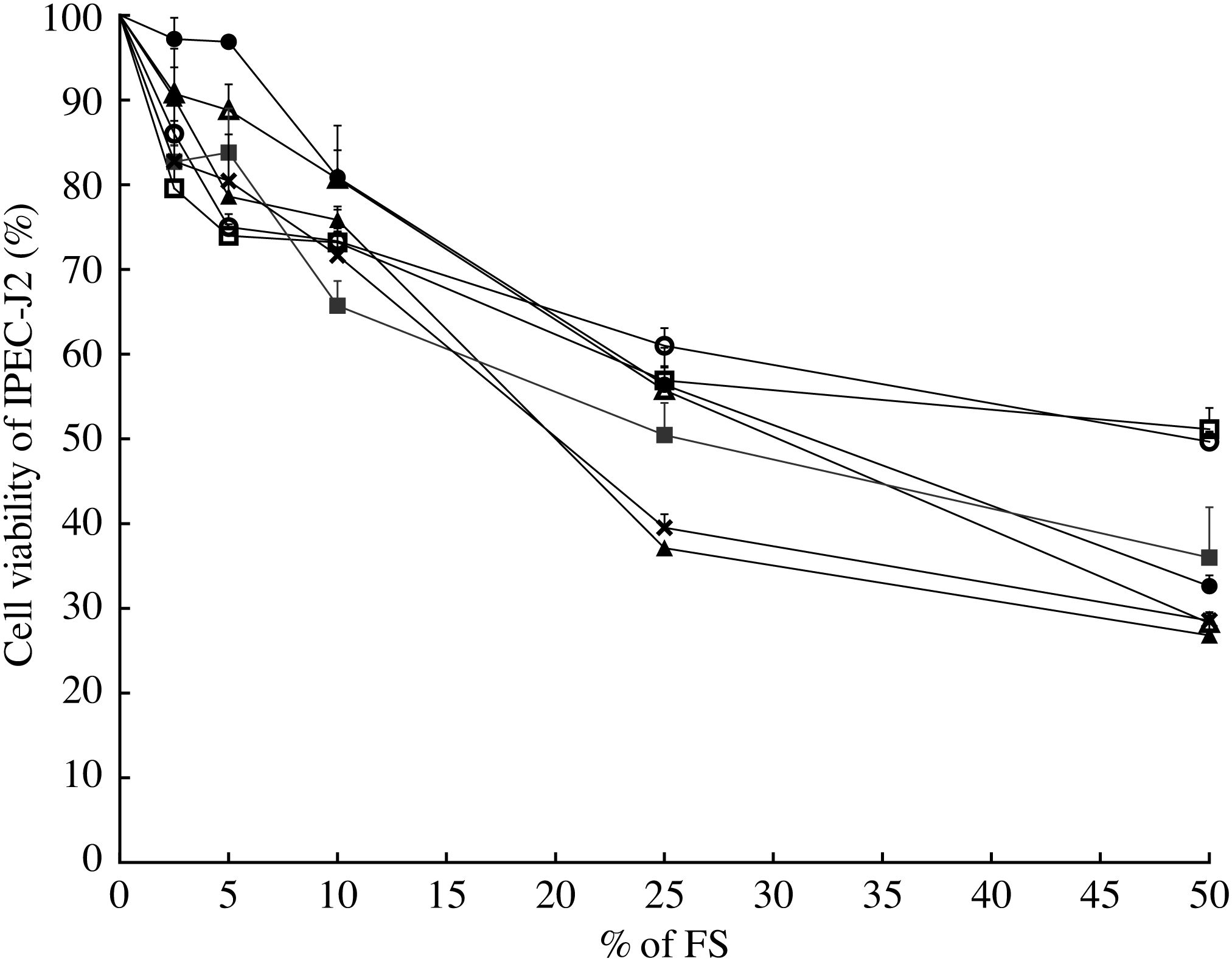
Fig. 2. Modulation of intestinal porcine epithelial cell (IPEC-J2) viability by fermentation supernatant (FS) collected after 12 h. Values are means of six well-measurements with their standard errors. ![]() , Inulin;
, Inulin; ![]() , chicory root;
, chicory root; ![]() , chicory pulp;
, chicory pulp; ![]() , rye bran;
, rye bran; ![]() , soya hulls;
, soya hulls; ![]() , citrus pulp;
, citrus pulp; ![]() , fermentation blank.
, fermentation blank.
Impact of fermentation supernatant on gene expression in intestinal porcine epithelial cells
High-throughput qPCR was performed with 0·8-µm ø filtered FS containing both metabolites and bacteria (‘complete supernatant’) which is representative of the in vitro gastro-intestinal model used in this research. Data revealed that none of the eleven reference genes studied was stable between the sham-treated cells and the cells receiving the FS (data not shown). Therefore, the fermented feed ingredients were compared with the acknowledged prebiotic control, that is, inulin to assess the immunomodulatory effect of their FS (Fig. 3(A) to (E)), while comparisons between all ingredients are displayed in online Supplementary Table S1.
Fig. 3. Impact of fermentation supernatant (FS) 10 % (v/v) collected after 12 h on gene expression in intestinal porcine epithelial cells (IPEC-J2). (A) Chicory root; (B) chicory pulp; (C) rye bran; (D) soya hulls; (E) citrus pulp. Values are means of triplicate well-measurements with their standard errors of the mean. Gene expression was not stable between the control treatment and the 0·8-µm ø FS treatments for eleven reference genes studied; hence, the different fermented ingredients were compared with inulin. Figures display the % of difference of the different genes for one ingredient in comparison with inulin, considered as 100 %. Significantly different from inulin FS: *, **, *** for false discovery rate corrected-P <0·5, <0·01 and <0·0001, respectively. The geometric mean of ribosomal protein L 13a (RPL13a), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylprolyl isomerase A (PPIA) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) was used to normalise samples. AKT1, serine/threonine-protein kinase 1; MAPK14, mitogen-activated protein kinase 14; MyD88, myeloid differentiation primary response 88; NF-kBIα, NF-κB inhibitor alpha; NOD1, nucleotide-binding oligomerisation domain-containing protein 1; TLR, toll-like receptor; CCL5, chemokine ligand 5; COX2, cyclo-oxygenase 2; CXCL10, C-X-C motif chemokine 10; DEFβ, defensin beta; EGFR, epidermal growth factor receptor; IFN, interferon; ILRN1, IL-1 receptor antagonist; MCP1, monocyte chemoattractant protein 1; CASP3, caspase 3; CDH1, E-cadherin; MARVELD2, tricellulin; MUC1, mucin 1; TGFβ1, transforming growth factor beta 1; VIL1, villin 1; ZO-1, zonula occludens-1.
Beta-2-microglobulin (B2M), espin (ESPN), hydroxymethylbilane synthase (HBMS), interferon beta (IFNβ), IL1β and proliferating cell nuclear antigen (PCNA) genes showed low expressions in IPEC-J2 cells. Chemokine ligand 5 (CCL5) and monocyte chemoattractant protein 1 (MCP1) primers’ efficiencies did not range between 90 and 110 %, and their results were excluded from the study.
The mRNA levels of all target genes were similar between chicory root and inulin except for AKT1, mitogen-activated protein kinase 14 (MAPK14), myeloid differentiation primary response 88 (MyD88), claudin-1 and MARVELD2 gene expressions which were significantly higher in chicory root (Fig. 3(A)). Chicory pulp displayed higher adherens (CDH1, i.e. e-cadherin) and tight junction gene expression levels (occludin, claudin-4 and MARVELD2, i.e. tricellulin) in comparison with inulin (P < 0·01; Fig. 3(B)). Mucin 1 mRNA (MUC1) and caspase 3 (CASP3) levels were also higher for chicory pulp than for inulin, whereas epidermial growth factor receptor (EGFR) levels were down-regulated for chicory pulp. Considering inflammatory pathways, serine/threonine-protein kinase 1 (AKT1), cyclo-oxygenase 2 (COX2), NF-κB inhibitor alpha (NF-kBIα), nucleotide-binding oligomerisation domain-containing protein 1 (NOD1), toll-like receptor 4 (TLR4) and TNFα gene expression levels were significantly lower in chicory pulp compared with inulin, whereas the opposite was observed for the genes defensin beta 1 (DEFβ1), NF-kB1, IL18, IL-1 receptor antagonist (ILRN1) and PPARγ. Rye bran exhibited significantly lower vilin 1 (VIL1), claudin-3 and EGFR compared with inulin and higher gene expression levels of IL8 (Fig. 3(C)). MyD88 mRNA levels were significantly up-regulated in soya hulls compared with inulin, while TNFα and claudin-3 were down-regulated (P < 0·05; Fig. 3(D)). NOD1 gene expression levels were significantly up-regulated in citrus pulp compared with inulin, while TLR4 and claudin-1 were down-regulated (Fig. 3(E)).
Discussion
The first aim of the present research was to determine if fibre-rich agricultural and industrial by-products reach the same prebiotic potential as inulin in terms of gas and SCFA productions and microbiota profiles. Inulin and chicory root exhibited fast and intense fermentation patterns with the highest cumulative gas productions (A) and rate of fermentation (RMAX) and the lowest time to half asymptote (B) and time to reach RMAX (TMAX). Chicory root, with a higher ratio of soluble to insoluble polysaccharides and the soluble fraction being mainly composed of fructans, is highly fermentable and therefore is more likely to be fermented at the end of the small intestine( Reference Jha, Fouhse and Tiwari55 ). Chicory pulp displayed slower fermentation kinetics which is related to its high insoluble fibre content (high NDF and ADF levels) and its low amount in fructans, mainly composed of polysaccharides with high polymerisation degree( Reference Uerlings, Bindelle and Schroyen30 ). This supports the hypothesis that the rapidity and extensiveness of the fermentation are modulated by the fructan content of the ingredient which was already demonstrated by Shim et al. ( Reference Shim, Verdonk and Pellikaan56 ) and Pellikaan et al. ( Reference Pellikaan, Verdonk and Shim57 ) and previous experiments( Reference Uerlings, Bindelle and Schroyen30 ).
Similarly to chicory pulp, citrus pulp demonstrated mid-range values for gas kinetics (intermediate values for A, B and RMAX) due to a mixture of soluble (highly methylated pectin) and insoluble fibres (cellulose) with the soluble fraction being fermented rapidly, while the insoluble one is extensively fermented following the disappearance of the soluble fibres( Reference Tamaki, Konishi and Tako58 ). Rye bran is composed of considerable amounts of fructan, although lower to the chicory by-products. Nevertheless, the complexity of the carbohydrate fraction( Reference Van Craeyveld, Swennen and Dornez59 ) rich in insoluble fibres, in particular water-unextractable arabinoxylans, presumably accounts for the intermediate rate of fermentation (RMAX) and the low final gas production (A) for this ingredient in comparison with the fructan-based ingredients. The same effect can be observed with soya hulls characterised by high concentrations of cellulose (high ADF) which is known to ferment to a lower extent. These ingredients are more likely to be fermented in the hindgut.
After 24 h of fermentation, chicory root and pulp produced significant amounts of total SCFA, like inulin, due to their amount in fructo-oligosaccharides. The high butyric acid content induced by the fermentation of fructan-rich ingredients, that is, chicory root and inulin, was already reported in porcine studies( Reference Loh, Eberhard and Brunner60 ) and is confirmed in the present study. Total SCFA and butyrate amounts are highly correlated not only with an extensive fermentation but also with the fructan content( Reference Uerlings, Bindelle and Schroyen30 ), which is shown by similar results in terms of gas production, total SCFA and butyrate ratios for inulin and chicory root. Similarly, no remarkable difference was reported in terms of microbiota populations between the two ingredients. Surprisingly, the bifidogenic capacities of fructo-oligosaccharides reported in the literature, in humans( Reference Roberfroid, Van Loo and Gibson61 , Reference Howard, Gordon and Pace62 ), are not apparent in our results. The consumption of fructan-based ingredients provides acetic acid to butyrate-producing bacteria used as a co-ingredient to produce butyrate which corroborates with the low acetate and high butyrate levels in inulin and chicory root at 24 h. Contradictory, inulin and chicory root displayed the lowest level of Lactobacillus spp. and the highest levels of lactate after 6 and 12 h of fermentation. This can be explained by the fact that the mucin carriers, acting as adhesive sites for Lactobacillus spp., are more representative of the genus abundance than the fermentation broth( Reference Tran, Boudry and Everaert39 ).
Acetate productions for chicory pulp, citrus pulp and soya hulls were higher compared with the ratio of inulin, probably due to the high concentrations in cellulose, and the moderate soluble fibre and hemicelluloses contents. Remarkably, citrus pulp showed the highest butyrate producing capacity, based upon the Clostridium cluster IV (along with chicory pulp and soya hulls) and Clostridium cluster XIVa results, while chicory root and inulin were having low relative levels of these two clusters.
Our study highlighted that rye bran induced the greatest stimulation of Bifidobacterium spp. at 12 and 24 h and the second greatest amount of butyryl-CoA:acetate-CoA transferase after 24 h. Fermentation of arabinoxylan was associated with a proliferation of Bifidobacterium spp., in several trials with human feces( Reference Arrigoni, Jörger and Kollöffel63 – Reference Hughes, Shewry and Li65 ) which is in line with our results.
The second aim of the research was to compare the immunomodulatory profiles of the five ingredients FS compared with inulin FS on cultured IPEC-J2. The findings in the present study showed that chicory pulp was able to increase gene expression levels of tight and adherens junctions thereby enhancing the barrier function of intestinal epithelium with higher CDH1, occludin, claudin-4 and tricellulin levels in comparison with inulin. Inulin FS had been shown to increase TEER in human cell models, therefore reinforcing gut barrier tightness( Reference Allsopp, Possemiers and Campbell66 , Reference Pham, Seifert and Richard67 ) which is in line with our findings. The high-throughput qPCR study supported the idea that complete FS may modulate the inflammatory state of the intestinal epithelial layer. Our results indicate that chicory pulp FS exerts anti-inflammatory effects on IPEC-J2 that mainly depend on the TLR as well as the NOD signalling pathways, both related to bacterial pattern recognition and ligation( Reference McGuire and Arthur68 , Reference Caruso, Warner and Inohara69 ). In our study, chicory pulp triggered the expression of PPARγ and inhibited pro-inflammatory cytokines such as TNFα. PPARγ was reported to inhibit the production of inflammatory cytokines in different cell types by interfering with TLR-dependent signalling pathway( Reference Zenhom, Hyder and Heller20 , Reference Jiang, Ting and Seed70 , Reference Ricote, Li and Willson71 ), which is in agreement with the present study. Furthermore, chicory pulp might have increased the transcription activity of pro-apoptotic targets via the PI3K-AKT pathway, seen by the decrease in AKT1 gene expression level, leading to the inhibition of pro-survival target genes( Reference Luo, Kamata and Karin72 ) and an up-regulated caspase-3 mRNA levels, a marker of advanced apoptosis( Reference Zhang, Rao and Guo73 ). As intermediate levels of butyrate were found during the fermentation of chicory pulp in comparison with inulin, it seems that a direct effect of the ingredient or a synergistic effect of the ingredients with fermentation metabolites and/or microbiota is responsible for the reinforcement in barrier function and the anti-inflammatory effect. Besides, bacterial modulation was found for this ingredient with a remarkable increase in Clostridium clusters IV and XIVa bacteria in comparison with inulin which might also affect the epithelial barrier tightness and inflammatory response( Reference Lopetuso, Scaldaferri and Petito8 ).
The gene expression levels of all tight and adherens junctions target genes were similar between chicory root and inulin except for claudin-1 and tricellulin mRNA levels which were significantly higher in chicory root. This is in line with Pham et al. ( Reference Pham, Seifert and Richard67 ) who found that inulin and dried chicory root FS played a protective role in reversing the gut permeability, probably due to butyrate, in HT29-MTX and HT29 cell models. Chicory root showed a significant induction of genes involved in the MAPK signalling pathway (AKT1, MAPK14, NF-kB and MyD88) typically resulting in up-regulation of different pro-inflammatory cytokines( Reference Chi, Barry and Roth74 , Reference Hommes, Peppelenbosch and van Deventer75 ), although no up-regulation of pro-inflammatory cytokines (IL6, IL8 and IL18) was induced by the chicory root supernatant. It might be that the signalling pathway did not reach the end-target cytokines after 24 h of FS exposure on the IPEC-J2. Rye bran, citrus pulp and soya hulls showed no additional immune-related activities in comparison with inulin in IPEC-J2. The increased Bifidobacterium spp. levels due to the fermentation of rye bran or the proliferation in butyrogenic species arising from the fermentation of soya hulls and citrus pulp did not induce differential gene expressions in IPEC-J2.
One limitation of our study was the use of supernatants collected from a batch fermentation model with substrate depletion, pH reduction and the accumulation of metabolites as major drawbacks of the in vitro technique. Moreover, the different FS could not be compared with the sham-treated cells, due to the instability of the reference genes between treatments. This implies that the addition of FS highly impacted the cell regulatory function which remains one limitation of our model.
Seen the positive effects of chicory by-products on gene expression related to gut barrier, industrial and agricultural by-products such as chicory root and pulp may be an interesting ingredient to be further tested in the feed of piglets in the weaning period, to modulate intestinal fermentation and consequently gut immunity and the mucosal barrier integrity. Then, in vivo trials should further evaluate the dosage of inclusion in the diet( Reference Le Sciellour, Labussière and Zemb76 ).
In conclusion, chicory root reached the same prebiotic potential as inulin in terms of fermentation kinetics and metabolites production, while soya hulls, rye bran and citrus pulp positively modulated health-promoting microbiota populations. We have also assessed that chicory pulp complete FS promoted the intestinal barrier integrity as can be seen by the up-regulated expression of tight and adherens junction genes in comparison with inulin. Chicory pulp seemed to induce different immunomodulatory pathways such as anti-inflammatory and pro-apoptotic regulations.
Acknowledgements
The expertise about culturing IPEC-J2 was acquired in the laboratory of Animal Biology from the National Research and Development Institute for Biology and Animal Nutrition (IBNA, Romania) under the supervision of Ionelia Taranu and Gina C. Pistol with the support of Wallonia-Brussels International. The IPEC-J2 cell line was a generous gift from the laboratory of Dr Ravallec at IUT « A » Génie Biologique, Polytech’ Lille, France.
The Research Foundation for Industry and Agriculture (FRIA-FNRS) funded this research as a grant attributed to J. U., consisting in PhD financing (ID33848511) and had no role in the design, analysis or writing of this article.
J. U., M. S., J. B., E. W., S. T., G. B. and N. E. designed the research question and study; J. U., A. B., C. C., A. R. and E. A. S. conducted research; J. U. analysed data. All authors read and approved the final manuscript.
There were no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519002873











