CVD and breast cancer (BC) have a major impact on morbidity and mortality among women in the Western world(Reference Curado, Edwards and Shin1–Reference Loomba and Arora3). It is therefore important to identify modifiable risk factors that may prevent these diseases and improve their prognoses. A high intake of marine n-3 PUFA from fish is associated with a reduced risk of CVD(Reference Calder4) and possibly also of several cancer forms including BC(Reference Saadatian-Elahi, Norat and Goudable5, Reference Larsson, Kumlin and Ingelman-Sundberg6). Some of the proposed mechanisms behind the protective effect of marine n-3 PUFA are similar in both diseases, including production of eicosanoids with a more anti-inflammatory profile and effects on the properties of the cell membrane(Reference Jude, Roger and Martel7). Most clinical studies in CVD have been dominated by male participants, and the results and recommendations have been extrapolated to women, although coronary disease may be of a different character in women(Reference Elsaesser and Hamm8). The risk of CVD in women increases dramatically with menopause, probably due to a significant reduction in the synthesis of oestrogens(Reference Lee and Foody9). The incidence of BC also increases rapidly with age, but the increase slows down at menopause, indicating the importance of female hormones for the development of BC(Reference Key, Verkasalo and Banks2).
The influence of menopause on the uptake and on the subsequent turnover of marine n-3 PUFA in the body is largely unknown. Several studies have shown that DHA concentrations in different tissues are higher in women than in men, independent of diet(Reference Bakewell, Burdge and Calder10–Reference Giltay, Duschek and Katan13), and that treatment with oral contraceptives or oestrogens affects levels of DHA(Reference Childs, Romeu-Nadal and Burdge14–Reference Sumino, Ichikawa and Murakami18). Stable isotope studies have also shown that women have a much greater capacity to convert α-linolenic acid to DHA compared to men of similar age(Reference Burdge, Jones and Wootton19, Reference Burdge and Wootton20). All these studies indicate that the content of marine n-3 PUFA in tissues is, to some extent, under the influence of oestrogen levels in the body.
Furthermore, marine n-3 PUFA may reduce the activity of aromatase enzymes which convert androgens to oestrogen, thus leading to a potential oestrogen-lowering effect in the body(Reference Larsson, Kumlin and Ingelman-Sundberg6).
To study whether the incorporation of marine n-3 PUFA into cells (platelets) and adipose tissue was influenced by menopausal status, and whether the levels of oestrogens in the blood were influenced by supplementation with marine n-3 PUFA, we performed a randomised, parallel-group, double-blind, placebo-controlled trial.
Material and methods
Study population
During 2007, ninety-two Danish women were recruited primarily among employees at Aalborg Hospital and among women living in Northern Jutland in Denmark. Eligible women were aged between 18 and 70 years, healthy or with a chronic, stable and well-treated disease (i.e. hypertension, diabetes mellitus), and a menopausal status that was unambiguous. The menopausal status was defined as a spontaneous cessation of menses for 12 months or longer, and, in case of doubt, we also measured follicle-stimulating hormone and luteinising hormone. Hysterectomised women could be included if they were aged 60 years or older, or their follicle-stimulating hormone and luteinising hormone values were clearly postmenopausal. Criteria for exclusion were consumption of fish oil products, any form of hormones including oral contraceptives during the last 2 months before enrolment, known allergies to fish and anticipated compliance problems. Vaginal local oestrogen therapy was accepted. Half of the women were premenopausal and the rest postmenopausal.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki.
All participants gave written informed consent before participating in the trial. The study protocol was approved by the ethics committee of the Region of Northern Jutland and by the Danish Data Protection Agency. If any abnormal laboratory value was found, the participants were referred to their general practitioner for further investigation. The study was registered in ClinicalTrials.gov (identifier: NCT00901589).
Intervention
The participants were randomly assigned to receive 12 weeks' daily supplementation with either 2·2 g n-3 PUFA (38·5 % EPA, 25·9 % DHA and 6·0 % docosapentaenoic acid (DPA)) in four capsules, or four corresponding capsules of a control oil (thistle oil) containing 73·7 % linoleic (18 : 2n-6), 11·7 % oleic (18 : 1n-9), 6·8 % palmitic (16 : 0) and 2·4 % stearic (18 : 0) acids. The two types of capsules were identically coloured gelatine capsules provided and packed by Mezina A/S, Esbjerg, Denmark. Thistle oil was chosen due to a very limited content of n-3 PUFA, neutral taste and availability.
Study design
The study was designed as a randomised, double-blind, placebo-controlled, parallel-group intervention trial. The randomisation process was computer-generated and conducted by an independent statistician at the Center for Cardiovascular Research at Aalborg Hospital. Participants, investigators and laboratory staff were blinded to dietary assignments during the entire study. Blood samples and adipose tissue biopsies were collected at baseline and after 12 weeks of intervention, in the morning after a minimum of 8 h fasting. Hormones fluctuate during the menstrual cycle and in order to reduce the effect of this natural fluctuation, the blood samples in the premenopausal group were taken early during the week the participants menstruated. Anthropometric measurements (weight, height, blood pressure and bioimpedance) were obtained before and after the interventions. Before and after the intervention period, the participants filled in a questionnaire that assessed their health status, intake of medicine, diet, exercise level and alcohol consumption.
Participants were requested to follow their usual diet throughout the study period. Compliance was examined by counting the remaining capsules and by fatty acid analyses.
Fatty acid analysis
Adipose tissue
Adipose tissue biopsies were obtained from the buttocks using an evacuated system consisting of S-Monovette needle, multiadaptor and evacuated blood tube (Sarstedt, D-51 588 Nümbrecht, Germany), as described by Beynen & Katan(Reference Beynen and Katan21). The fat was stored in vials filled with nitrogen at − 80°C to avoid oxidation until analysis, which was performed at the end of the study period. The biopsies were defrosted at room temperature, and a sample of approximately 2–4 mg was removed to a test-tube, and pre-warmed to 50°C for 10 min. The fat was dissolved in heptane at 50°C and the fatty acids were trans-esterified by 2 m KOH in methanol at 50°C for 2 min, according to IUPAC 2.301, chapter 5(22). The fatty acid composition was analysed by GC using a Varian 3900 GC with a CP-8400 autosampler (Varian, Middleburg, The Netherlands) equipped with a flame ionisation detector. Split injection mode, a CP-sil 88, 60 m × 0·25 mm inner diameter capillary column (Varian, Middleburg, The Netherlands), temperature programming from 90 to 210°C and constant flow were used. Helium was used as a carrier gas. Commercially available standards (Nu-chek-Prep, Inc., MN, USA) were used to recognise the individual fatty acids.
These approaches permitted quantification of fatty acid methyl esters with 12–24 carbon atoms and separation and quantisation of several trans fatty acids. The fatty acid content is presented as the percentage of total fatty acids. The interassay CV, calculated from repeated analyses of samples on different days, was 6·3 % for EPA, 4·3 % for DPA and 5·2 % for DHA.
Platelets
Platelets were separated from blood and anticoagulated with 1·6 mg/ml of K-EDTA. The tubes were left at ambient temperature for half an hour for sedimentation before centrifugation for 15 min at 180 g. Platelet-rich plasma was harvested and centrifuged for 5 min at 150 g to remove remaining leucocytes and erythrocytes. The platelet-rich plasma was transferred to a new plastic tube and centrifuged for 10 min at 1500 g to sediment the platelets. The platelet poor plasma was discharged, the cells were resuspended and washed twice in isotonic saline, centrifuged each time for 10 min at 1500 g. The precipitate was resuspended in isotonic saline and stored in the same way as adipose tissue until analysis. Fatty acids were extracted from platelets according to the method of van Kuijk et al. (Reference van Kuijk, Thomas and Stephens23). Briefly, the cell suspension was mixed with 1·0 ml dichloromethane and 1·0 ml methanol containing butylated hydroxytoluene 10 mg/100 ml; another 1·0 ml of dichloromethane and 500 μl H2O were added, mixed and centrifuged for 4 min at 1800 g. The lower organic phase was collected, and the extraction of the supernatant was repeated. The combined lipid phase was dried under nitrogen at 25°C.
The fatty acid composition was analysed in the same way as was used for adipose tissue. A similar platelet control sample analysed in different runs showed the following interassay CV: 0·9 % for EPA, 1·8 % for DPA and 2·6 % for DHA.
Analyses of oestrogens
Serum samples collected for hormonal analyses were frozen at − 20°C and after completion of the study, analysed at the Danish Serum Institute in Copenhagen, Denmark.
Quantification of oestrone, oestradiol and oestrone sulphate levels
Oestrone (E1), oestradiol (E2) and oestrone sulphate were measured using an in-house assay RIA. In brief, an internal standard was added to serum aliquots before extraction and fractionation on C2 solid phase extraction cartridges (Varian, Palo Alto, CA, USA). Oestrone sulphate was solvolysed and converted to E1 before RIA analysis, which was performed using in-house antibodies specific for either E1 or E2 17-β. The functional limits of detection were 40, 40 and 200 pm for E1, E2 and oestrone sulphate, respectively. The interassay CV were 14–18 % (E1), 12–16 % (E2) and 12–15 % (oestrone sulphate).
Quantification of non-sex hormone-binding globulin-bound oestradiol level
Non-sex hormone-binding globulin (SHBG)-bound E2 was measured as the percentage of total E2 levels by equilibrating serum samples with 3H-E2, precipitating the SHBG-bound 3H-E2 fraction with saturated ammonium sulphate and estimating the radioactivity in the supernatant by liquid scintillation counting (Beckman Coulter, Fullerton, CA, USA). In order to account for possible precipitation of albumin-bound 3H-E2, a separate set of sample aliquots was incubated with 5α-dihydrotestosterone to saturate SHBG-binding sites before ammonium sulphate precipitation. The percentage of non-SHBG-bound E2 was calculated as the sum of the percentage of radioactivity in the supernatant of non-5α-dihydrotestosterone-treated samples and the percentage of radioactivity in the precipitate of 5α-dihydrotestosterone-treated samples. The interassay CV was 5–14 %.
Additional analyses
Basic safety parameters and standard lipid analyses were performed according to the standard methods at the Department of Clinical Biochemistry at Aalborg Hospital.
Measurement of apo B was done on an Advia 1650 (Siemens Healthcare Diagnostics, Deerfield, IL, USA) at the Department of Clinical Biochemistry in Hjørring, Denmark.
Statistical analyses
The number of women needed for the trial was calculated from data on changes in the content of marine n-3 PUFA after supplementation with fish oil in women in a previous study(Reference Christensen, Christensen and Dyerberg24). The sd was estimated to be 0·3 %, and in order not to miss a true difference of 0·25 % between the two groups after supplementation with marine n-3 PUFA, we included twenty-two women in each group. The risk of type 1 error was set at 5 % and the risk of type 2 error was set at 20 %.
Comparison of baseline characteristics in the different groups was performed using a paired t test for continuous variables and a χ2 test for frequencies. Mann–Whitney two-sample rank-sum test was used for parameters with a non-normal distribution.
The change in marine n-3 PUFA from baseline to 12 weeks after intervention on the subject level was analysed by a two-way ANOVA, the factors being menopausal state and treatment group. Hence, a possible difference in treatment effect between menopausal groups could be assessed by analysing the statistical interaction between the factors. Furthermore, the treatment effect and the difference between menopausal groups can also be assessed. Adjustment for age was done by including age in a two-way analysis of co-variance. A P value below 0·05 was regarded as significant. All analyses were performed with Stata version 10.1 (Stata Corporation, College Station, TX, USA).
Results
A total of eighty-nine women completed the study. One woman was excluded due to pathological baseline laboratory parameters and two withdrew their consent. The compliance to the intervention was high, with few reported side effects, primarily gastrointestinal (fishy taste and eructation). There was no apparent change in dietary habits in any of the groups as judged from the questionnaires filled in before and after the intervention (data not shown).
The women were well-matched for baseline characteristics in both the pre- and the postmenopausal groups, respectively. Blood pressure, glucose, total cholesterol, HDL and LDL cholesterol, and apo B levels were significantly higher in postmenopausal women (Table 1).

Pre, premenopausal; post, postmenopausal; TC, total cholesterol; LDL, LDL cholesterol; HDL, HDL cholesterol.
* Comparison between fish oil and control groups in pre- and postmenopausal women, respectively, and comparison between pre- and postmenopausal women.
† BMI is presented as medians and quartile ranges.
At baseline, a significantly higher content of all marine n-3 PUFA was found in platelets (P < 0·05) and adipose tissue (P < 0·001) in the postmenopausal women compared with the premenopausal, except for the content of EPA in platelets (P = 0·05; Tables 2 and 3).
Table 2 Platelet fatty acid composition of the four arms*
(Mean values and standard deviations or median values and quartile ranges)

DPA, docosapentaenoic acid.
* Comparison between baseline and 12 weeks of intervention. The fatty acids are reported as % of total fatty acids.
† The difference in content before and after intervention is compared in pre- and postmenopausal women, respectively.
Table 3 Adipose tissue fatty acid composition of the four arms*
(Mean values and standard deviations or median values and quartile ranges)

DPA, docosapentaenoic acid.
* Comparison between baseline and 12 weeks of intervention. The fatty acids are reported as % of total fatty acids.
† The difference before and after intervention is compared in pre- and postmenopausal women, respectively.
Fatty acid results
Tables 2 and 3 show the differences in fatty acid composition in platelets and in adipose tissue before and after 12 weeks of intervention. The control oil had no effect on the content of marine n-3 PUFA in the pre- or postmenopausal women. In the fish oil groups, there were significant increases in total marine n-3 PUFA, EPA, DPA and DHA in both groups, in platelets as well as in adipose tissue. The increase in marine n-3 PUFA was larger in platelets than in adipose tissue, and in both compartments the largest relative increase was seen in EPA content. Significant changes in the content in platelets of several fatty acids including arachidonic acid were observed in both pre- and postmenopausal women treated with fish oil.
We found no effect of menopausal status on the increase of marine n-3 PUFA, either in platelets or in adipose tissue, (Table 4), and there was no effect of age on the increase.
Table 4 The mean increase in marine n-3 PUFA in platelets and in adipose tissue after 12 weeks of intervention with fish oil*
(Mean values and standard deviations)
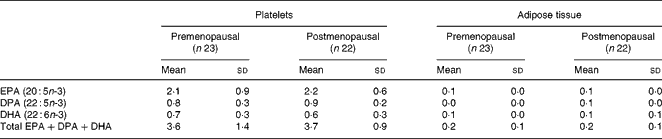
DPA, docosapentaenoic acid.
* The increase is reported as % of total fatty acids.
Hormone analyses
Table 5 shows that all levels of oestrogens were significantly lower in postmenopausal than in premenopausal women. In postmenopausal women, fish oil and control oil had no detectable effect on oestrogen levels. However, levels of both E2 (P < 0·04) and E1 (P < 0·02) increased significantly in premenopausal women in the fish oil group compared with the control group.
Table 5 Oestrogen concentrations before and after intervention in pre- and postnopausal women
(Median values and quartile ranges)

NonSHBG, non-sex hormone-binding globulin-bound oestradiol.
* The differences in oestrogen concentrations before and after the intervention are compared in the two groups of women.
Discussion
The present study aimed to evaluate whether menopause affected the incorporation of marine n-3 PUFA into platelets and adipose tissue. We also examined whether supplementation with marine n-3 PUFA had any effect on the concentration of circulating oestrogens in women.
This trial of 12 weeks of supplementation with marine n-3 PUFA demonstrated that pre- and postmenopausal women are equally efficient at incorporating marine n-3 PUFA into platelets and adipose tissue. We found significantly higher baseline levels of marine n-3 PUFA in platelets and adipose tissue in postmenopausal women compared with premenopausal women, which is in accordance with some other studies(Reference Schafer, Overvad and Thorling25, Reference Tavendale, Lee and Smith26).
In contrast to the present study, a previous intervention study showed that 3 months of fish oil supplementation (2·4 g of EPA+DHA/d) resulted in a significantly higher increase of DHA and EPA in plasma fatty acids in older women than in younger women(Reference Meydani, Natiello and Goldin27). However, the study was small (n 25) and did not include a placebo group or control for diet during the intervention period. In a placebo-controlled intervention study with EPA (supplementation with 1·35, 2·7, or 4·05 g/d) to ninety-three men, there was a significantly larger incorporation of EPA into plasma and mononuclear cell phospholipids in older men compared with younger men, consistent with higher baseline EPA content in the older men(Reference Rees, Miles and Banerjee28). A limitation to that study was that there was no record of dietary habits.
Other studies indicate that fish intake may be higher in older people than in younger(Reference Crowe, Skeaff and Green11, Reference Bolton-Smith, Woodward and Tavendale29), but even after adjusting for this difference, older people seem to have a higher content of marine n-3 PUFA in adipose tissue(Reference Bolton-Smith, Woodward and Tavendale29). The explanation for the age-related effect is unknown, but one plausible explanation might be a change of body composition with age, and a decrease in energy expenditure, resulting in a lower proportion of supplemented marine n-3 PUFA being oxidised, and a higher proportion being incorporated into tissues(Reference Rees, Miles and Banerjee28). Another plausible explanation might be reductions in desaturase activity by age(Reference Ford and Tavendale30).
In addition to the age-related effect, several studies indicate that hormones have an influence on the content of marine n-3 PUFA in the body(Reference Childs, Romeu-Nadal and Burdge14). DHA concentrations are higher in women than in men in several tissues, independent of diet(Reference Bakewell, Burdge and Calder10–Reference Giltay, Duschek and Katan13), and treatment with oral contraceptives or oestrogens may affect levels of DHA(Reference Childs, Romeu-Nadal and Burdge14–Reference Sumino, Ichikawa and Murakami18). The difference in DHA concentrations between women and men is probably related to a higher conversion of α-linolenic acid to EPA and DHA in women, as shown in stable isotope studies. These studies also indicates the regulatory effects of oestrogens(Reference Burdge31). Treatment with anastrozole (an aromatase inhibitor), on the other hand, results in lower E2 and lower DHA in plasma(Reference Giltay, Gooren and Toorians12). However, the present study showed that the incorporation of marine n-3 PUFA from a dietary supplement into platelets and adipose tissue was independent of menopausal status, and therefore possibly independent of oestrogen concentrations.
Marine n-3 PUFA have an inhibitory effect on cyclo-oxygenase-2 and might act as a potential aromatase P450 inhibitor, resulting in a lower production of endogenous oestrogens(Reference Horia and Watkins32). In addition, the production of PGE3, an eicosanoid product from the metabolism of EPA, has a less activating effect on the aromatase enzyme than the arachidonic acid-derived PGE2, and hence may result in a decrease in oestrogen concentrations(Reference Larsson, Kumlin and Ingelman-Sundberg6). The present study, however, could not support these mechanisms. In fact, contrary to our expectation, we found a significant increase in E2 and E1 in premenopausal women supplemented with fish oil and no effect in postmenopausal women. The mechanisms behind this are unknown and whether there is an absolute increase, an increase in bioavailable oestrogens or a decrease in the metabolism of oestrogens, or all three, remain to be settled. We analysed non-SHBG-bound E2 because there may be a good correlation between non-SHBG E2 and BC risk(Reference Greendale, Palla and Ursin33) and because the analysis is more readily available than the measurement of free E2. Supplementation with fish oil had, however, no effect on this parameter. Supplementary analyses of free E2 and SHBG might have provided more information.
There was a significant decrease in the content of arachidonic acid in platelets in both pre- and postmenopausal women given fish oil, suggesting a reduced formation of proaggregatory thromboxane A2 after stimulation which may partially explain the beneficial effect of marine n-3 PUFA in CVD(Reference Harizi, Corcuff and Gualde34).
The strengths of the present study were the study design and a reliable confirmation of the high compliance of participants and the measurements of marine n-3 fatty acids in both platelets and adipose tissue. There were few withdrawals, and the use of a questionnaire also helped to evaluate potential changes in dietary habits during the intervention period.
The limitations were that the intervention period of 12 weeks may have been too short to detect a difference in the fatty acid content in adipose tissue in the two groups(Reference Katan, Deslypere and van Birgelen35). The composition of fatty acids in platelets and adipose tissue reflects the dietary intake of PUFA, the former over weeks and the latter over months/years(Reference Katan, Deslypere and van Birgelen35–Reference Arterburn, Hall and Oken37). Also, our questionnaire was not designed to evaluate the absolute amounts of daily fish intake, and hence we could not adjust for the actual amount of fish eaten. To our knowledge, the study is the first to evaluate whether marine n-3 PUFA have an effect on circulating oestrogens in the human blood. Unfortunately, some of the hormone measurements in the postmenopausal group were below detection limit for the method of analysis and absolute values could not be obtained. The results must therefore be interpreted with caution. Endogenous hormone levels are known risk factors for BC, but standardised methods for routine measurement need to be developed and evaluated(Reference Cummings, Tice and Bauer38). The aromatase enzyme is particularly expressed in breast adipose tissue and in breast tumour tissue, and a more conclusive assay might have been to evaluate the aromatase activity or measure oestrogen levels in breast adipose tissue before and after fish oil supplementation.
In conclusion, this trial demonstrated that pre- and postmenopausal women are equally efficient at incorporating marine n-3 PUFA into platelets and adipose tissue. We also found that marine n-3 PUFA may increase the levels of oestrogens in premenopausal women, thereby potentially affecting BC and CVD risk. Further studies should explore the association between marine n-3 PUFA and female sex hormones and its clinical relevance.
Acknowledgements
We would like to thank the staff at the Lipid Research Clinic at Aalborg Hospital for their invaluable and competent assistance during the entire study process. We also thank Mezina A/S, Esbjerg, Denmark, for their kind contribution of oil capsules. All authors have contributed to the drafting of the paper. J. H. C., E. B. S. and P. M. W. designed the study; I. V. A. and P. M. W. performed the data collection and P. M. W. performed the data analysis with statistical assistance from Claus Dethlefsen. No conflicts of interest exist. The present research was made possible through financial support from the Danish Cancer Society, the Ebba and Aksel Schoelins Foundation, the Frits, Georg and Marie Cecilie Gluds Foundation (Faculty of Health Sciences, University of Aarhus), the Heinrich Kopps Foundation, the Erichsen Family Memorial Foundation and the Research Initiative of Aarhus University Hospital.







