Blood pressure (BP) is a major modifiable risk factor for CVD(Reference Collins, Peto and MacMahon1, Reference Lewington, Clarke and Qizilbash2). Hypertension is a self-amplifying condition, and diet is believed to play an important role in its genesis. Consequently, relatively small reductions (2–5 mmHg) in BP in the population may have a large impact on cardiovascular mortality(Reference Law, Frost and Wald3). BP rises with age, and it is positively associated with BMI, alcohol and salt intake, and is negatively associated with K intake(Reference Elliott, Stamler and Nichols4). Cross-sectional as well as prospective cohort studies indicate that higher fruit and vegetable consumption is associated with a lower BP, and risk of stroke and CHD(Reference He, Nowson and MacGregor5, Reference Hu and Willett6). Much attention has been focused on the antioxidant nutrients (vitamin C, β-carotene and tocopherol) provided by fruit and vegetables, but randomised controlled trials on dietary supplements of these nutrients have failed to show any favourable effect on BP or on risk of CVD(Reference Bjelakovic, Nikolova and Gluud7). Phytochemicals such as flavonoids present in fruit and vegetables may influence vascular function by the way of their antioxidant properties, but evidence for their beneficial effect on CVD from prospective cohort studies is inconclusive(Reference Lin, Rexrode and Hu8, Reference Mink, Scrafford and Barraj9). The high K content of fruit and vegetables is a plausible mechanism to explain why high intakes of fruit and vegetables are associated with a lower risk of CVD. In support of this is a meta-analysis of oral K supplementation which indicated reductions in systolic BP of 3 and 7 mmHg in normotensive and hypertensive subjects, respectively(Reference Whelton, He and Cutler10). Furthermore, a BP-lowering effect of potassium citrate, the major form of the mineral in fruit and vegetables, has also been reported(Reference He, Markandu and Coltart11, Reference Braschi and Naismith12).
The PREMIER study(Reference Appel, Champagne and Harsha13) conducted among free-living subjects in the USA indicated that intensive dietary advice, which included advocating the consumption of ten servings of fruit and vegetables a day and increased K excretion by 19 mmol/d, lowered systolic/diastolic BP by 5·7/3·2 mmHg. The average UK intake of fruit and vegetables is three portions daily, with slightly lower intakes in low income groups(Reference Nelson, Erens and Bales14, Reference Hoare, Henderson and Prentice15). A trial conducted in a general practice setting in the UK reported that advice to increase fruit and vegetables from three to five portions daily lowered systolic BP by 4 mmHg(Reference John, Ziebland and Yudkin16), but it was not designed to test BP as a primary outcome and this important observation requires confirmation.
The present study was designed to test the effects on BP and vascular function of increasing K intake from usual levels, either by increased intakes of fruit and vegetables or by supplementation, in subjects with high-normal or elevated BP, who were not receiving BP-lowering medication.
Subjects and methods
Subjects
Men and women (aged 22–65 years) were recruited in three cohorts between October 2004 and November 2005, through educational institutions in London; participants included staff, family members of students and students undergoing higher education. Advertisements and flyers offering BP checks were circulated to staff, students and parents. Inclusion criteria were a seated diastolic BP>80 and < 100 mmHg on two occasions. The first screening visit took place in the participant's workplace/school or in a clinic. Seated BP was measured, using an automated sphygmomanometer (Omron 70CP, Tokyo, Japan), according to British Hypertension Society guidelines(Reference Williams, Poulter and Brown17) following a 10 min rest. Participants with diastolic BP>80 mmHg attended a clinic for further BP measurements, to provide a fasting blood sample for haematology, liver function tests and lipid profile, and to have measurements of weight and height in order to confirm eligibility to the study. Exclusion criteria included were clinical history of myocardial infarction, diabetes mellitus, renal disease, gastrointestinal disease, pancreatitis, cholestatic liver disease or cancer; current use of systemic corticosteroids, androgens, phenytoin, erythromycin, thyroid hormones, lipid-lowering, BP-lowering or anticoagulant medication; cigarette smoking; history of substance abuse or alcoholism; alcohol intake exceeding a moderate intake (>24–36 g alcohol/d); pregnancy or having had a baby in the last year; allergy or intolerance to intervention foods; unwillingness to refrain from the use of dietary supplements; weight loss>3 kg in the preceding 2 months; BMI < 20 and>35 kg/m2; and BP and other risk factors that make them eligible for drug treatment of raised BP according to the British Hypertension Society guidelines(Reference Williams, Poulter and Brown17). Cross-sectional surveys indicate that in the UK about 30 and 50 % are hypertensive (seated systolic BP>140/90 mmHg measured using the Omron device) among those aged 45–54 and 54–64 years, respectively(Reference Eastwood18). However, these estimates are based on single occasion measures of BP, and it is well known that BP regresses towards the mean on repeated measures as demonstrated in the present study where the fall in seated systolic BP/diastolic BP between first and second screening visits was 7/3 mmHg. We chose to include diastolic BP>80 and < 100 mgHg as an indicator of early hypertension as it reflects increased systemic vascular resistance and because values above 75 mmHg are associated with increased risk of CHD and stroke(Reference Lewington, Clarke and Qizilbash2). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Research Ethics Committee of King's College London (reference 03/04-105). Written informed consent was obtained from all the subjects.
Experimental design and procedures
A randomised placebo-controlled cross-over Latin square design was used to compare three experimental treatments v. a control treatment. Participants were randomly allocated to the various treatment sequences by a computer so that a similar numbers of subjects were on each treatment at any one time. Participants underwent a 3-week run-in period on the control level of fruit and vegetable intake (providing 15 mmol K/d) before undertaking the allocated treatment sequence. This run-in period was to habituate the subjects to the dietary intervention, the ambulatory BP measurements and the vascular function measurements. Each treatment period lasted 6 weeks and was separated by a minimum of 5 weeks, where the subjects were allowed to revert to their usual diet (Fig. 1). The experimental treatments compared additional 20 or 40 mmol K/d provided as fruit and vegetables, or 40 mmol/d provided as potassium citrate capsules (two capsules taken four times a day). The control treatment involved the consumption of placebo capsules (two capsules taken four times a day) and an intake of fruit and vegetables similar to the average UK intake. Participants were blinded to the allocation of placebo and potassium citrate capsules, which were matched in size and colour. The potassium citrate capsules were a slow-release formulation manufactured for the project by Penn Pharmaceuticals Limited (Tredegar, Gwent, UK). Researchers undertaking vascular function measurements were blinded to the treatment allocation.

Fig. 1 Outline of the study design. BP, blood pressure.
For the run-in period, control treatment period and potassium citrate supplementation treatment period, the participants were instructed to consume an amount of fruit and vegetables providing 15 mmol K/d which is equivalent to the contribution made by these foods in the average UK diet(Reference Hoare, Henderson and Prentice15, Reference Gregory, Foster and Tyler19). To consume the additional 20 or 40 mmol K/d as fruit and vegetables, the participants were asked to consume a total of 7 and 11 units of K daily, respectively. Participants consumed their habitual intakes of fruit and vegetables during the washout periods.
Personalised dietary advice was provided as a unit system (1 unit = 5 mmol K), and the participants were provided with fruit and vegetables free of cost for the duration of the intervention. Sample menus and instructions on how to prepare the fruit and vegetables were provided so that they retained maximum nutritional value (i.e. cooking in minimum volume of water and avoiding exposure of cut vegetable to air, light or heat for long periods). Written advice, cards illustrating the portion sizes and recommended intakes of fruit and vegetables at each level of intake were provided. They were discouraged from consuming fruit in the form of syrup or pickled vegetables and those preserved in oil. Furthermore, in line with the then current UK Department of Health advice, they were advised that fruit juice (150 ml) only counted for one portion. Participants were advised to avoid using soya products throughout the study because of their high K content. Potatoes were not included as a portion of fruit and vegetables.
Compliance to the dietary intervention
During the dietary treatments, the participants were contacted via telephone on a weekly basis to arrange fruit and vegetable delivery orders and to confirm that they were following the dietary instructions. Participants were requested to complete food record cards detailing the fruit and vegetables consumed during each treatment as well as to make 24 h urine collections (week 3 of run-in period and weeks 5–6 of each treatment period) to ascertain compliance to the treatment by measurement of urinary K excretion. A controlled feeding study(Reference Nichols, Berry and Mulla20) had established the reliability of the measurement of 24 h urinary K to indicate K intake; this showed that urinary K excretion increased by 30 mmol/d following a 40 mmol potassium citrate supplement, and by 14 and 29 mmol/d following the provision of an additional 20 and 40 mmol/d K as fruit and vegetables, indicating that approximately 75 % of the additional K is recovered in urine.
Study outcomes
The primary outcome was a change in ambulatory BP measured over 24 h using an A&D TM-2430 device (ScanMed, Moreton-in-Marsh, Gloucester, UK) which is graded A/A according to the current guidelines(Reference O'Brien, Beevers and Lip21). The device was set to record BP measures at 30 min intervals during the day and hourly during the night, and the participants maintained a record of the activities of the recording period. Measurements were made in the last week of each study period but were not made on the day before the collection of blood sample. On the last day of each study period, the participants fasted overnight before attending a clinic visit at St Thomas' Hospital, London, UK. Vascular function measurements were performed with the participant supine in a quiet, darkened and temperature-controlled room (23°C). Supine BP was measured in triplicate after 15 min rest followed by triplicate measurements of carotid to femoral pulse wave velocity and radial pulse wave analysis by applanation tonometry using the SphygomoCor systems (version 7.01; AtCor Medical, Sydney, NSW, Australia). Flow-mediated dilatation (FMD) of the brachial artery was performed on the right arm, according to the international recommendations(Reference Corretti, Anderson and Benjamin22). Endothelial independent dilation was determined in response to 25 μg glycerol trinitrate that was administered sublingually. Following these measurements, fasting blood samples were collected for the measurement of serum total cholesterol, HDL-cholesterol, TAG, glucose and insulin(Reference Berry, Miller and Sanders23), high-sensitivity C-reactive protein (CRP) (P.Z. Cormay, Lublin, Poland), soluble intracellular adhesion molecule (R&D Systems Europe, Abingdon, Oxon, UK), vitamin C(Reference Jauniaux, Cindrova-Davies and Johns24), carotenoids and tocopherols(Reference Thurnham, Smith and Flora25), 8-isoprostane F2α(Reference Hall, Sanders and Sanders26), folate and homocysteine(Reference Pufulete, Al-Ghnaniem and Leather27).
Urine was collected in containers without additives, and the urinary Na and K were determined using ion-selective electrodes, and creatinine was determined by the Jaffé reaction on ADVIA 1650 analysers (Bayer Diagnostics, Newbury, UK). Urinary 2,3-dinor-8-isoprostane F2α was determined using a time-resolved immunofluorescence assay(Reference Theobald, Goodall and Sattar28).
Statistical analysis
Sample size calculations were based on thirty-two subjects completing the study to detect a 4 mmHg change in systolic BP (based on 80 % power, P = 0·01) by ambulatory BP monitoring assuming a within-subject sd for systolic BP of 5 mmHg; this sample size gave power to detect changes in the main secondary outcomes of 1 % for FMD and 0·8 m/s for carotid to femoral pulse wave velocity. The project set out to recruit forty-eight participants in order to allow for a 30 % drop-out rate. Statistical analysis was conducted using SPSS/PC version 14.0 for Windows. The endpoint analysis was on an intention-to-treat basis and involved all forty-eight participants who completed the study. Data were analysed by repeated measures ANOVA of the four treatments with the value for the run-in period as a covariate. Where the overall F value from the ANOVA indicated a statistically significant between-treatment effect, post hoc comparisons were made between the various treatment levels, and the change from the control value is shown with 95 % CI corrected for multiple comparisons using Bonferroni's multiple comparison test. CRP was analysed using non-parametric tests. Correlations between variables were made on the average of five within-subject measures using Spearman's correlation.
Results
Fig. 2 shows the CONSORT diagram of the flow of subjects through the trial. Mean systolic/diastolic BP fell by 7·6 (sd 10·3)/3·1 (sd 6·0) mmHg between the first and second screening visits. Out of the fifty-seven participants who were randomised to treatment sequences, five withdrew during the run-in period, one withdrew during the first intervention for personal reasons, two were withdrawn from the study and placed on BP-lowering medication, and one withdrew from the study during the last intervention due to a brain tumour; data for the analysis were available for forty-eight subjects. The participants were in the upper tertile of the BP distribution of those screened. The mean seated BP (systolic/diastolic) of the fifty-seven participants at randomisation was 137/89 mmHg and of the forty-eight participants (twenty-five female, twenty-three male) who completed the study was 137/89 mmHg; twenty-nine participants were of European, nine were of Asian and ten were of African ethnic origin, and their details are shown in Table 1. Using the British Hypertension Society classification based on seated BP(Reference Williams, Poulter and Brown17), twenty-three participants had grade 1 hypertension, eighteen had high-normal BP, and seven had normal BP. Most of the participants (88 %) were overweight or obese (BMI>25 kg/m2); however, fasting plasma glucose concentrations were elevated (>5·5 mmol/l) in only two participants. Serum cholesterol and HDL-cholesterol concentrations were typical for the UK adult population; 71 % of the participants had a LDL-cholesterol concentration>3·0 mmol/l, and 8 % had low HDL-cholesterol concentrations ( < 0·9 mmol/l for males and < 1·1 mmol/l for females). Most of the participants (83 %) had waist measurements that were greater than 94 cm for males and 80 cm for females, and 29 % of the participants met the International Diabetes Federation definition of the metabolic syndrome(29).

Fig. 2 CONSORT diagram of the flow of subjects through the study. BP, blood pressure; DBP, diastolic BP.
Table 1 Details of participants completing the study
(Mean values and standard deviations)
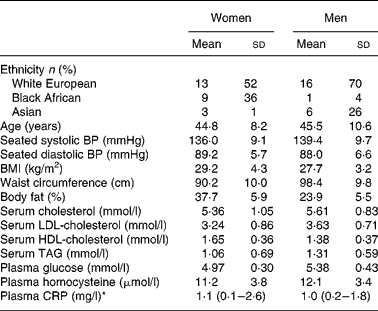
BP, blood pressure; CRP, C-reactive protein.
* Median with interquartile range.
Compliance to the intervention
Capsule counts indicated that 98·4 % (sd 11·4) of potassium citrate and 94·9 % (sd 14·5) of placebo capsules were taken. Mean intakes of K from fruit and vegetables calculated from the food record cards were close to those planned (Fig. 3). The mean intakes of fruit were 162 (sd 53), 165 (sd 79), 336 (sd 118), 402 (sd 190) g/d on control, 40 mmol potassium citrate supplement, 20 and 40 mmol K from fruit and vegetables, respectively; corresponding intakes of vegetables were 129 (sd 39), 128 (sd 45), 197 (sd 85) and 238 (sd 93) g/d, respectively. The fruit intake was mainly derived from bananas, apples, citrus fruits, pears, plums, peaches/nectarines, dried fruit and berries (mainly strawberries), and the vegetable intake was mainly derived from tomatoes, carrots, capsicum peppers, green vegetables (spinach, lettuce, cabbage and green beans) and mixed vegetables. Table 2 shows that urinary K excretion increased in line with the values obtained in the pilot study, and the increment on 40 mmol additional K from fruit and vegetables did not differ from that obtained following the 40 mmol potassium citrate supplement. The ratio of Na:K was significantly lower on the experimental treatments compared with the control treatment. Plasma carotenoids, tocopherols and Na excretion did not differ significantly between the treatments. No relationship was found between plasma vitamin C concentration and increasing fruit and vegetable intake, but this was not unexpected as the participants were non-smokers and habitual intakes of vitamin C are relatively high in the UK population even in low income groups(Reference Nelson, Erens and Bales14). Nor were changes in serum folate observed, but this is not surprising as the major source of fruit and vegetables consumed in the present study was not rich in folate.

Fig. 3 Self reported potassium (K) intake from fruit and vegetables (F&V) according to the dietary treatment (geometric mean with 95 % CI, P < 0·001 for both). Probability was estimated from repeated measures of ANOVA of the four treatments with run-in value as covariate. * Mean values were significantly different from control value (P < 0·01). † Mean values were significantly different from 40 mmol K value (P < 0·01). ‡ Mean values were significantly different from 20 mmol K value (P < 0·01).
Table 2 Body weight, and plasma vitamin C, carotenoids, tocopherol concentrations, urinary potassium and sodium excretion in participants (female n 25, male n 23) according to additional intake of potassium (K) provided by increased fruit and vegetable intake or a potassium citrate supplement
(Mean values and 95 % confidence intervals)

* Probability estimated from repeated measures ANOVA of the four treatments with the run-in value as covariate.
† Geometric mean.
‡ P < 0·05 compared with control; Bonferroni's multiple comparison test.
Body weight remained stable throughout the study, and there were no reported side-effects in any of the treatment groups.
Primary and secondary outcomes
On an average thirty awake and eight asleep ambulatory BP readings were made in each 24 h period. Awake and asleep ambulatory systolic/diastolic BP following the 3-week run-in period were 138·5 (sd 14·4)/87 (sd 7·3) and 116 (sd 13·9)/71 (sd 7·3) mmHg, respectively, and were not statistically significantly different from 136·3 (sd 12·8)/85 (sd 8·9) and 116·3 (sd 15·3)/70 (sd 8·5) mmHg on the control treatment. Table 3 shows the changes in ambulatory BP, arterial stiffness and endothelial function compared with the control treatment which were not statistically significant. There was no evidence to indicate that higher intakes of K provided by either supplementation or intakes of fruit and vegetables resulted in lower BP, and that the changes were well below the 4 mm difference in systolic BP so that the study was powered to detect. These conclusions were not altered when the subjects with stage 1 hypertension were analysed separately. The mean changes and 95 % CI for FMD and carotid to femoral pulse wave velocity were well inside the size of effect that the study was powered to detect and they did not differ between the treatments.
Table 3 Ambulatory and supine clinic systolic and diastolic blood pressure (SBP, DBP in mmHg), carotid to femoral pulse wave velocity (PWVc-f), peripheral augmentation index (PAI), flow-mediated dilatation (FMD) and glycerol trinitrate (GTN) responses of the brachial artery in participants (male n 23, female n 25) during the control intervention, and changes from control following increased intakes of potassium (K) provided by increased fruit and vegetable intake or a potassium citrate supplement
(Mean values and standard deviations; mean values and 95 % confidence intervals)

* Adjusted for multiple comparisons using the Bonferroni method.
† Estimated from repeated measures ANOVA of the four treatments with run-in value as covariate.
Cardiovascular risk factors
There were no statistical significant treatment-related changes in serum total cholesterol, HDL-cholesterol, TAG, plasma homocysteine, insulin, glucose, CRP, plasma 8-isoprostane F2α concentrations and urinary 8-isoprostane F2α metabolite excretion (Table 4). In the female participants, mean serum intracellular adhesion molecule-1 was 2·14 mg/l (95 % CI 2·00, 2·30) on the treatment providing 40 mmol K as fruit and vegetables and was significantly greater (P < 0·05) than 2·53 mg/l (95 % CI 2·33, 2·75) on the control treatment, but no treatment-related differences in this analyte were noted between the male participants. Plasma 8-isoprostane F2α concentrations were correlated with total and LDL-cholesterol concentrations (ρ = 0·563 and 0·535; both P < 0·0001).
Table 4 Serum lipids, glucose, insulin, homocysteine, C-reactive protein (CRP), intracellular adhesion molecule (ICAM)-1, plasma 8-isoprostane F2α; concentrations and urinary 2,3-dinor 8-isoprostane F2α; excretion in participants (female n 25 and male n 23) according to additional intake of potassium (K) provided by increased fruit and vegetable intake or potassium citrate supplement
(Mean values and 95 % confidence intervals)

* Probability from repeated measures ANOVA of the four treatments with run-in value as covariate.
† Median value with interquartile range, probability from Friedman's test.
Discussion
The present study sets out to test the hypothesis that an increased intake of K (above the average UK intake) provided by raising fruit and vegetable intake lowers BP and improves vascular function in early hypertension. A 4 mm difference in systolic BP was selected as being of clinical significance and also because a previous report(Reference John, Ziebland and Yudkin16) indicated that this would be achieved by a single session of dietary advice to consume two additional portions of fruit and vegetables daily. In that study, which used a parallel design, the control group received no intervention which may have biased the trial. Our inability to demonstrate a BP-lowering effect is unlikely to be a consequence of poor compliance or measurement error.
Most K supplementation studies have used a parallel design, and as the common standard deviation is about 12 mmHg for systolic BP, a sample size of about 140 subjects per group is required to detect a 4 mm change in BP at P = 0·05 and 80 % power. The use of a cross-over design provides greater statistical power to detect changes in BP than a parallel design because there is a strong correlation between repeat measurements within the same subject. However, the weakness of cross-over studies is that they can be confounded by carry-over effects and seasonal variations. This can be avoided by the use of a washout period between the treatments and the allocation of treatment sequences using a Latin square design to balance out seasonal variations between the treatments as observed in the present study. The use of a matched placebo capsule in the reference treatment is also important as any intervention may have an effect. Post hoc power analysis of the results of present study showed that it had 80 % power to detect a 3 mmHg change in systolic BP at P = 0·05 indicating that it is unlikely that a change of this magnitude was missed due to insufficient sample size. Increased K intake through increased fruit and vegetable consumption might lower systolic BP by 2 mmHg, which would be of significance to the population attributable risk of CVD. However, a parallel designed study with 564 participants in each arm or a cross-over study with 106 participants would be required to prove this.
The present study provides no evidence for the beneficial effects on endothelial function, measured in the fasting state by changes in FMD or on indices(Reference Griendling and FitzGerald30) of lipid peroxidation (plasma or urinary 2,3-dinor-8-isoprostane F2α). However, a novel observation was the relatively strong correlation between plasma 8-isoprostane concentration and total and LDL-cholesterol concentrations. The lower soluble intracellular adhesion molecule-1 in women on the highest level of fruit and vegetable intake requires confirmation as this difference could have arisen by chance. It is possible that transient changes in endothelial function in the postprandial period may occur following the consumption of flavonoid-rich fruit and vegetables. In contrast to our findings, He et al. (Reference He, Marciniak and Carney31) reported improvements in FMD with values of 5·8 % (sd 4·4) on 64 mmol potassium chloride/d, 4·6 % (sd 2·9) on 64 mmol potassium bicarbonate/d compared with 3·1 % (sd 2·5) on placebo in forty-two mildly hypertensive participants in a cross-over study. However, measurement of FMD is subject to substantial operator error and the influence of cigarette smoking and passive smoking. In the present study, the participants were non-smokers, and values for FMD were higher. An improvement in endothelial function as measured by the changes in forearm blood flow was reported in a parallel designed randomised controlled trial in 117 hypertensive participants(Reference McCall, McGartland and McKinley32), which included cigarette smokers, when they changed from a diet providing a very low intake of fruit and vegetables (one portion a day) to diets providing higher intakes (three or five portions). We were also unable to confirm an effect of K on arterial stiffness(Reference He, Marciniak and Carney31). However, it is to be noted that there were changes in arterial stiffness that the study ranged from 0·5 to 0·8 m/s and as we estimated that anything less than 0·8 m/s could be a type 2 error. He et al. also reported improved left ventricular filling function with K supplementation. It is possible that higher intakes of K than that were used in the present study have a favourable effect of arterial stiffness and other indices of cardiovascular function. However, to provide an additional 64 mmol K would require consuming about 1 kg of fruit and vegetables daily.
Our failure to detect a statistically significant reduction in BP-lowering effect with increased K intake is in agreement with another UK cross-over trial by He et al. (Reference He, Marciniak and Carney31) who used even higher intakes of K. These findings are in agreement with a Cochrane systematic review that concluded that there was no statistically significant effect of K on BP in patients with hypertension(Reference Dickinson, Nicolson and Campbell33). However, this review did note significant heterogeneity between trials. K might only lower BP when changing from very low intakes to higher intakes. In support of this is the observation that the fall in BP in the Dietary Approaches to Stop Hypertension (DASH)-2 study(Reference Sacks, Svetkey and Vollmer34) with increased K excretion occurred from a much lower value (41 mmol/d) than in our study or that of He et al. (Reference He, Marciniak and Carney31). Variability in the response to K might also depend upon the activity of the renin–angiontensin system, which declines with age and shows some marked variations between individuals. Low renin activity is more prevalent among people of African ethnic origin(Reference He, Marciniak and Visagie35), and large changes have been reported in some African K supplementation studies(Reference Dickinson, Nicolson and Campbell33). The activity of the renin–angiotensin system was not evaluated in the present study but should be considered in future studies.
Salt intake may also influence the BP-lowering effect of K as indicated in the meta-analysis by Whelton et al. (Reference Whelton, He and Cutler10) where the BP-lowering effect was only statistically significant when urinary Na excretion was>165 mmol/d. Salt intakes have fallen in the UK, and the level of urinary Na excretion was well below 165 mmol/d threshold in our study and that of He et al. (Reference He, Marciniak and Carney31). Braschi & Naismith(Reference Braschi and Naismith12) reported falls in seated BP of systolic/diastolic BP of 6·7/4·3 mmHg with a 30 mmol potassium citrate and of 5·2/4·3 mmHg with 30 mmol potassium chloride in predominantly normotensive subjects. However, baseline urinary K and Na excretion were broadly similar to the present study. A major limitation of that study was the use of Hg sphygmomanometers, which is subject to observer bias. The failure of the present study to confirm this finding is puzzling especially as we used the same potassium citrate supplement and placebo capsules. In both our study and that of He et al. (Reference He, Marciniak and Carney31), the participants were overweight/obese (mean BMI 29 kg/m2), whereas in the study of Braschi & Naismith, the mean BMI was below 25 kg/m2 and the subjects were younger (mean age 35 years). It is possible that overweight/obesity abrogates the BP-lowering effect of K.
The DASH study is widely cited as it demonstrates that an increased intake of fruit and vegetables lowers BP. However, in the original DASH study, the increased consumption of fruit and vegetables resulted in a fall in 24 h ambulatory systolic/diastolic BP of 3·1/2·0 compared with 5·6/3 mmHg on the DASH combination diet. The DASH combination diet provided ten portions of fruit and vegetables/d, supplying an additional approximately 45 mmol K/d(Reference Appel, Moore and Obarzanek36, Reference Conlin, Chow and Miller37). It is also noteworthy that the DASH combination diet involved several other changes in the diet including an increased intake of wholegrain cereal, modification of fat intake, less red meat and fewer sugar-sweetened beverages. Furthermore, in the DASH studies, participants had all food provided for them on a daily basis. Adherence to the DASH diet was accompanied by several other physiological changes notably a reduction in body weight, changes in blood lipids and insulin sensitivity which were not observed in the present study.
In conclusion, the present results do not provide support for dietary advice to increase K intake, above the levels habitually consumed in the UK, for subjects with early hypertension.
Acknowledgements
We thank Tracy Dew and Roy Sherwood (King's College Hospital, London, UK) for the CRP, intracellular adhesion molecule-1, folate and homocysteine assays, Peter Lumb (St Thomas' Hospital, London, UK) for the lipid and insulin assays, Frank Kelly (King's College London) for the vitamin C assays, Dr Duncan Talbot (Unilever Corporate Research, Bedford, UK) for the urinary isoprostane assays, Dr Benyu Jiang and Karen McNeil (Cardiovascular Sciences Division, King's College London) for the vascular function measurements, Liz Tydeman and Robbie Gray (Nutritional Sciences King's College London) for technical help and the carotenoid and vitamin E assays, and Jo Nicholson for pilot work. T. A. B. S. was responsible for study design, data analysis and is guarantor. S. E. B. was responsible for day-to-day running of study and participated in data analysis. U. Z. M. assisted with day-to-day running of study and participated in laboratory and data analysis. P. J. C. was involved in the study design, supervised vascular measurements and participated in data analysis. All the authors contributed to writing the paper and approved the final draft. Financial support was received from the Food Standards Agency (UK) and the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London. The funders played no part in the study design, the collection or analysis of data, the writing of the report or the decision to submit for publication. All the authors are independent of the funders. The authors (S. E. B., U. Z. M., P. J. C. and T. A. B. S.) report no financial or personal conflicts of interest. Financial support was received from the Food Standards Agency (UK) and the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London. This clinical trial was registered with ISRCTN50011192.









