Hepatocellular cancer (HCC) is the most frequent primary liver tumour and the second most lethal among all human neoplasms( Reference Ferlay, Soerjomataram and Dikshit 1 ). Approximately 80 % of HCC occur in developing countries of Asia and Africa. However, incidence of HCC and mortality from this malignancy have been increasing in the USA and parts of Europe (including Italy, until the mid-1990s)( Reference Bosetti, Levi and Boffetta 2 – Reference El-Serag 4 ). Inflammation is the body’s response to any kind of tissue injury or insult in the presence of inflammatory stimulants such as cytokines( Reference Keibel, Singh and Sharma 5 , Reference Pan, Lai and Dushenkov 6 ). Chronic inflammation – which is characterised by the continuous presence of inflammatory cytokines in circulation and in the tissues – is known to play a key role in the development of various cancers, including HCC( Reference Zhao and Lawless 7 ) where chronically inflamed liver parenchyma represents a precancerous milieu in which 70–90 % of the HCC arise( Reference Schutte, Bornschein and Malfertheiner 8 ). The key causes of HCC are hepatitis B and C infection, which lead to chronic liver inflammation. Other important risk factors for HCC include smoking, alcohol, obesity and diabetes( Reference Bosetti, Turati and La Vecchia 9 , Reference Chuang, La Vecchia and Boffetta 10 ).
Besides hepatitis B and C, there is growing evidence that specific dietary components influence both inflammation( Reference de Mello, Schwab and Kolehmainen 11 – Reference Shivappa, Steck and Hurley 14 ) and HCC( Reference Kuper, Tzonou and Lagiou 15 – Reference Bravi, Bosetti and Tavani 19 ). Research on the role of diet in inflammation suggests that diet represents a complex set of exposures that often interact and whose cumulative effect modifies both inflammatory responses and health outcomes. A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet( Reference Shivappa, Steck and Hurley 20 ). A pro-inflammatory diet is rich in consumption of food items rich in SFA, carbohydrate and protein and low in the consumption of PUFA, flavonoids and other dietary components. The DII has been validated with various inflammatory markers, including C-reactive protein (CRP)( Reference Shivappa, Steck and Hurley 21 ) and IL-6( Reference Shivappa, Hebert and Rietzschel 22 , Reference Wood, Shivappa and Berthon 23 ). Additionally, the DII has been shown to be associated with glucose intolerance and dyslipidaemia components of the metabolic syndrome( Reference Alkerwi, Shivappa and Crichton 24 , Reference Wirth, Burch and Shivappa 25 ), anthropometric measurements in Spain( Reference Ruiz-Canela, Zazpe and Shivappa 26 ), asthma in Australia( Reference Wood, Shivappa and Berthon 23 ), bone mineral density among postmenopausal women in Iran( Reference Shivappa, Hebert and Karamati 27 ), colorectal cancer in a case–control study in Spain( Reference Zamora-Ros, Shivappa and Steck 28 ) and in two cohort studies of women in the USA( Reference Shivappa, Prizment and Blair 29 , Reference Tabung, Steck and Ma 30 ) and pancreatic and prostate cancers in Italy( Reference Shivappa, Bosetti and Zucchetto 31 , Reference Shivappa, Bosetti and Zucchetto 32 ).
Our hypothesis is that a higher DII score (indicating a pro-inflammatory diet) may increase the risk for incident HCC. In the current study, we thus examined the association between DII and HCC using a multicentre, hospital-based case–control study conducted in Italy( Reference Franceschi, Montella and Polesel 33 ). This provided original information on a southern European population in which hepatitis B and C are common and dietary and lifestyle habits and awareness of diet-related health issues are different from those in North America and northern Europe.
Methods
Recruitment and questionnaire
A case–control study of HCC was conducted between January 1999 and July 2002 in the province of Pordenone in Northeast Italy and the city of Naples in South Italy( Reference Franceschi, Montella and Polesel 33 ). Cases were 258 patients under the age of 85 years with incident HCC, who had not yet received any cancer treatment at study entry. They were admitted to Centro di Riferimento Oncologico (CRO), National Cancer Institute, Aviano, to ‘Santa Maria degli Angeli’ General Hospital, Pordenone and to IRCCS ‘Pascale’ National Cancer Institute and four General Hospitals in Naples. Overall, twenty-nine cases did not provide a blood sample and forty-four did not provide data on dietary habits, thus leaving 185 eligible cases (median age 66, range 43–84 years) with available questionnaires and blood samples for the present analysis. Histologic or cytologic confirmation was available for 78·2 % of HCC cases, whereas for the remaining cases the diagnosis was based on ultrasound, tomography and elevated α-fetoprotein levels.
Controls were patients <85 years of age admitted for a wide spectrum of acute conditions to the same hospitals where HCC cases had been interviewed. Patients whose hospital admissions were due to diseases related to tobacco smoking or alcohol abuse were specifically excluded, as were those hospitalised for chronic diseases that might have led to substantial dietary modifications. However, comorbidity for such diseases was not an exclusion criterion. Blood samples were available for 431 of 462 controls; of these, 404 provided comprehensive questionnaire information on dietary habits and were included in the present analyses (median age 65, range 40–82 years). A total of 27 % was admitted for trauma, 24 % for non-traumatic orthopaedic diseases, 25 % for acute surgical conditions, 13 % for eye diseases and 11 % for other miscellaneous illnesses. Overall, 1 % of cases and controls contacted refused to participate. All study participants signed an informed consent form, according to the recommendations of the Ethical Committee of CRO, the National Cancer Institute at Aviano. Cases and controls in each study centre were interviewed in the hospital by uniformly trained personnel using a standardised, structured questionnaire designed to collect information on socio-demographic characteristics, lifestyle habits – such as tobacco smoking and alcohol drinking – and personal medical history, including history of cirrhosis and diabetes mellitus.
An interviewer-administered FFQ shown to have satisfactory reproducibility and validity( Reference Franceschi, Negri and Salvini 34 , Reference Decarli, Franceschi and Ferraroni 35 ) was used to assess participants’ habitual diet, including total energy. Average weekly frequency of consumption of sixty-three foods or food groups, as well as complex recipes, during the 2 years before cancer diagnosis or hospital admission (for controls) was assessed. To compute energy and nutrient intakes, an Italian food composition database, as well as information from additional sources, was used( Reference Gnagnarella, Parpinel and Salvini 36 ).
BMI was calculated as weight (kg) divided by height squared (m2) and was categorised into normal weight (BMI<25·0 kg/m2), overweight (25·0≤BMI<30·0 kg/m2) and obese (BMI≥30·0 kg/m2).
Dietary inflammatory index
FFQ-derived dietary data were used to calculate DII scores for each study participant. A complete description of the DII is available elsewhere( Reference Shivappa, Steck and Hurley 20 ). Briefly, on the basis of a search of the literature from 1950 to the end of 2010, we identified forty-five food parameters among foods, nutrients and other food components that were associated with six plasma inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP). We defined a specific DII score for each food parameter on the basis of the literature review and taking into account the quality and number of published papers (1943 articles were reviewed and scored).
For each study participant, the dietary data were first linked to a global database that was developed on the basis of eleven data sets from around the world and thus provides a robust estimate of the means and standard deviations of these forty-five parameters( Reference Shivappa, Steck and Hurley 20 ). Each participant’s exposure relative to the ‘standard global mean’ was expressed as a Z score that was derived by subtracting the ‘standard global mean’ from the amount reported and then dividing this value by its sd. To minimise the effect of ‘right skewing’, this value was then converted to a centred percentile score. The participant’s DII score was computed by multiplying this value by the specific DII score for each food parameter and by summing together all forty-five values according to the following formula: DII=b1×n1+b2×n2+…+b45×n45, where b i refers to the literature-derived inflammatory effect score for each of the evaluated food parameter and n i refers to the food parameter-specific centred percentile, which were derived from the dietary data, per each i from 1 to 45. A higher DII score indicates a more pro-inflammatory diet. The DII computed on this study’s FFQ includes data on thirty-one of the forty-five food parameters comprising the DII, including carbohydrate, protein, fat, alcohol, fibre, cholesterol, SFA, MUFA, PUFA, n-3, n-6, niacin, thiamin, riboflavin, vitamin B6, Fe, Zn, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavanol, flavonol, flavonones, flavones, isoflavones, caffeine and tea. A flow chart of the DII methodology is shown in Fig. 1.

Fig. 1 Sequence of steps in creating the dietary inflammatory index (DII) in the Italian hepatocellular cancer case–control study. CRP, C-reactive protein.
The DII was analysed both as a continuous variable (i.e. for a one-unit increment in the DII corresponds to approximately 7 % of its global range) and by tertiles of exposure, determined on the basis of the entire study population. The DII also was examined across the strata of selected factors such as age, education, BMI and physical activity using the ANOVA test for continuous variables or the χ 2 test for categorical variables. OR and the corresponding 95 % CI were estimated using logistic regression models, adjusting only for age and then additionally for serum markers of hepatitis B and C infection, sex, study centre, education (<7, 7–11 and ≥12 years), BMI (<25·0, 25·0–29·9 and ≥30·0 kg/m2) and tobacco smoking (never smokers, ever smokers <15 cigarettes/d and ever smokers ≥15 cigarettes/d). Energy adjustment for DII was performed using the residual method( Reference Willett and Stampfer 37 ). Alcohol was not added as a covariate because alcohol is one of the components used for DII calculation. Linear tests for trend were performed using the median value within each tertile as an ordinal variable. The test for heterogeneity was carried out by including the interaction terms between DII and hepatitis status and sex in the model. Stratified analyses were carried out by hepatitis B and C infection status and sex. Statistical analyses were performed using SAS® 9.3 (SAS Institute Inc.).
Results
Controls were more often females and were younger compared with cases. Cases were more likely than controls to have a low level of education, to smoke cigarettes and to report heavy alcohol drinking( Reference Turati, Talamini and Pelucchi 38 ). The mean DII value among cases was 0·24 (sd 1·40), and among controls it was −0·11 (sd 1·37), indicating a slightly more pro-inflammatory diet for cases. Control characteristics across tertiles of DII are provided in Table 1. There were some differences in socio-demographic, anthropometric and lifestyle habits across DII tertiles. Compared with controls in tertile 1, participants in the highest tertile were younger and more likely to be former smokers, to have a higher BMI and to have educational attainment of <7 years.
Table 1 Participants’ characteristics across tertiles of dietary inflammatory index among 404 controls, Italy, 1999–2002 (Percentages; mean values and standard deviations)

CPD, cigarettes per d.
* ANOVA was used for continuous variables and χ 2 test was used for categorical variables.
† Hepatitis was defined as hepatitis B surface antigen and/or anti-hepatitis C virus positivity.
OR and 95 % CI of HCC according to tertiles of DII and continuous DII are shown in Table 2. When analyses were carried out using continuous DII, a significant positive association was observed with HCC risk (multivariable OR 1·30; 95 % CI 1·05, 1·60). When fit as tertiles, participants in tertile 3 were at higher risk for having HCC compared with participants in tertile 1 (ORtertile 3 v. 1 2·43; 95 % CI 1·27, 4·68; P trend=0·03).
Table 2 Risk for hepatocellular cancer and dietary inflammatory index (DII) both as continuous and expressed as tertiles, among 185 cases and 404 controls, Italy, 1999–2002 (Odds ratios and 95 % confidence intervals)
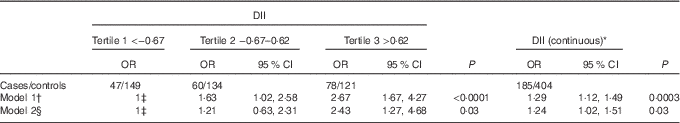
HBsAg, hepatitis B surface antigen; HVC, hepatitis virus C.
* One unit increase corresponding to approximately 12 % of its range in the current study.
† Estimated using age- and energy-adjusted logistic regression model. Energy intake (kcal), energy from alcohol excluded).
‡ Reference.
§ Additionally adjusted for age, sex, centre, BMI (<25·0, 25·0–29·9 and ≥30·0 kg/m2), smoking (non-smoker, current smoker, former smoker), education (<7, 7–11 and ≥12 years), hepatitis viruses (HBsAg+ and/or anti-HCV+ v. HBsAg– and anti-HCV–).
The interaction term for DII-by-sex was significant (P value=0·04) but not for hepatitis infection (P=0·09). Still, we carried out a stratified analyses. When stratified by sex, strong associations were observed only among males (ORtertile 3 v. 1 3·60; 95 % CI 1·65, 7·87; P trend=0·001). When stratified by hepatitis infection status, the DII was strongly associated with HCC among hepatitis B surface antigen (HBsAg)- and anti-hepatitis virus C2 (HCV2)-negative participants (i.e. the hepatitis-negative group), n 38 (ORtertile 3 v. 1 4·18; 95 % CI 1·53, 11·39; P trend=0·02). No statistically significant associations were observed among HBsAg+ and/or anti-HCV+ group, n 147 (Table 3).
Table 3 Risk for hepatocellular cancer (HCC) and dietary inflammatory index (DII) both as continuous and expressed as tertiles, in separate strata of hepatitis C and/or B virus infection and sex (185 cases and 404 controls), Italy, 1999–2002 (Odds ratios and 95 % confidence intervals)

HBsAg, hepatitis B surface antigen; HVC, hepatitis virus C.
* Reference.
† One unit increase corresponding to approximately 12 % of its range in the current study.
‡ Estimated using logistic regression model adjusting for energy intake (kcal, energy from alcohol excluded), sex, centre, BMI (<25·0, 25·0–29·9 and ≥30·0 kg/m2), smoking (non-smoker, current smoker, former smoker), education (<7, 7–11 and ≥12 years).
Discussion
In this case–control study, consuming a more pro-inflammatory diet – as reflected in higher DII scores – was associated with an increased risk for HCC. Previous results obtained in this case–control study showed deleterious effects of higher dietary glycaemic load (GL); the GL is a ranking of specific carbohydrate-rich foods based on the postprandial blood glucose response, which is expressed as a percentage of the glycaemic response to an equivalent amount of available carbohydrates from a reference food (e.g. white bread or glucose)( Reference Rossi, Lipworth and Dal Maso 39 ). From our prior research, we know that carbohydrate has a strong pro-inflammatory DII score( Reference Shivappa, Steck and Hurley 20 ). A diet rich in linoleic acid-containing foods (e.g. white meats and fish) and β-carotene was inversely related to HCC risk( Reference Polesel, Talamini and Montella 40 ). There is a favourable effect of coffee, although neither decaffeinated coffee nor tea( Reference Bravi, Bosetti and Tavani 19 , Reference Montella, Polesel and La Vecchia 41 ), on the risk for HCC. All of these nutrients and food items have anti-inflammatory values in the DII scoring( Reference Shivappa, Steck and Hurley 20 ).
Previous studies that have examined the effect of specific food items on HCC have reported an increased risk for high consumption of red meat, SFA( Reference Freedman, Cross and McGlynn 42 ) and excessive alcohol drinking( Reference Bosetti, Turati and La Vecchia 9 , Reference Kim, Ko and Han 43 , Reference Jee, Ohrr and Sull 44 ), whereas no association was observed with SFA in a European cohort( Reference Duarte-Salles, Fedirko and Stepien 45 ) and with red meat in this case–control study( Reference Talamini, Polesel and Montella 46 ). There is strong evidence showing coffee to be protective against HCC( 47 – Reference Bamia, Lagiou and Jenab 50 ). Inverse associations also have been observed for white meat and fish( Reference Freedman, Cross and McGlynn 42 , Reference Luo, Yang and Liu 51 ). A report from two cohort studies in China showed vitamin E to be protective against HCC( Reference Zhang, Shu and Li 18 ).
Two studies have been conducted to examine various other dietary patterns and indices in relation to HCC( Reference Li, Park and McGlynn 16 , Reference Zhang, Xiang and Li 17 ). In the National Institute of Health-American Association of Retired Professionals cohort, after adjustment for multiple confounders, significant inverse associations were observed between HCC incidence and the Healthy Eating Index-2005 and the alternate Mediterranean Diet Score( Reference Li, Park and McGlynn 16 ). In the Shanghai Women’s and Men’s Health Studies, fruit- and meat-based dietary patterns were not associated with liver cancer risk; however, a vegetable-based dietary pattern was associated with reduced liver cancer risk( Reference Zhang, Xiang and Li 17 ). In a large European cohort only vegetable consumption was associated with reduced risk, whereas no association was observed with fruit intake( Reference Bamia, Lagiou and Jenab 52 ).
One of the possible mechanisms for the observed positive association between the DII and HCC is through the indirect effect of pro-inflammatory diet due to enhanced production of the tumour-promoting cytokines IL-6 and TNF, which cause hepatic inflammation and activation of the oncogenic transcription factor, signal transducer and activator of transcription 3 (STAT3)( Reference Park, Lee and Yu 53 ). Consumption of a diet rich in SFA increases pro-inflammatory responses through the process of peroxidation of lipids in cells, which then exacerbates liver injury leading to HCC( Reference Hill-Baskin, Markiewski and Buchner 54 ). Our finding that the positive association with the DII was restricted to the HBsAg- and anti-HCV2-negative group may indicate either that these viral infections operate through mechanisms independent of inflammation or that other factors related to these infections overwhelm inflammatory effects. Consequently, it may be that the effect of DII is more apparent in the absence of such infections. In the face of intense virus-induced inflammation, pro-inflammatory diet might have little impact. As this is the first study showing this result, the association has to be explored further in other studies.
Because of the relative rarity of the disease, the study of HCC in high-income countries has traditionally only been feasible using case–control designs. However, pooling of results across cohort studies make it feasible to explore HCC in combined data of several cohort studies too( Reference Bamia, Lagiou and Jenab 50 , Reference Bamia, Lagiou and Jenab 52 ). In this case–control study, both cases and controls came from comparable catchment areas and were interviewed by uniformly trained interviewers in their respective hospital settings. To limit possible sources of bias, we included in the control group patients admitted for a wide spectrum of acute, non-neoplastic conditions, unrelated to the major risk factors for HCC. The practically complete participation rate and the comparable catchment areas of cases and controls contributed to reduce any potential selection bias. Cases may recall history of other diseases more frequently than did controls. However, the hospital setting should have improved the comparability of information, as cases and controls are interviewed under similar conditions. Participants were unaware of any particular study-related hypothesis in relation to diet and HCC, thereby reducing potential selection and information bias( Reference D’Avanzo, La Vecchia and Katsouyanni 55 ). The FFQ was satisfactorily reliable( Reference Franceschi, Negri and Salvini 34 ) and validated( Reference Decarli, Franceschi and Ferraroni 35 ).
Other limitations are the non-availability of the remaining thirteen food parameters for the DII calculation. DII calculated from these thirty-one food parameters has not been validated with inflammatory markers. However, in previous validation studies, the DII has been calculated from food parameters ranging from 17 to 44 and produced reasonably uniform results with minimal reduction in predictive ability( Reference Shivappa, Steck and Hurley 21 , Reference Shivappa, Hebert and Rietzschel 22 ). Moreover, there could be a possible overestimation due to the inclusion of food items in the DII calculation that are also a source of nutrients. It also should be noted, however, that each of these food items has an inflammatory effect score, which is derived from an extensive review of the literature looking at the association between these foods and inflammation. Another limitation could be the absence of evidence of DII being associated with inflammatory markers in this study, although the case–control nature of the study is not amenable to adding such measures during the aetiologically relevant period in any event. Our results were adjusted for the majority of known risk factors for HCC, including education, tobacco smoking, BMI and energy intake.
In conclusion, Italian men and women who consumed a more pro-inflammatory diet high in components of dietary GL and low in PUFA, vitamin A and β-carotene were at increased risk for HCC compared with those who consumed a more anti-inflammatory diet. The results suggest that encouraging intake of more anti-inflammatory dietary factors – such as coffee, white meat in place of red meat and plant-based foods rich in vitamin E and phytochemicals – and reducing intake of pro-inflammatory factors – such as red meat rich in SFA – may be a strategy for reducing risk for HCC.
Acknowledgements
This study was supported by the Italian Foundation for Research on Cancer (FIRC) by the Italian Ministry of Health, General Directorate of European and International Relations and by the National Cancer Institute grant number R01 CA39742. N. S. and J. R. H. were supported by grant number R44DK103377 from the US National Institute of Diabetes and Digestive and Kidney Diseases. None of the funding organisations had any role in the design, analysis or writing of this article.
A. Z., M. M., C. L. V., S. F. and D. S. designed and conducted the case–control study; N. S. conducted the analyses and wrote the first draft of the manuscript; M. R., J. R. H., C. L. V., J. P., A. C., M. M., S. F., and A. Z. provided suggestions and revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr N. S. is an employee of CHI.







