With the increase in life expectancy in affluent societies, a growing part of the population in such societies will experience specific age-related disorders. Not all of these will be serious, but may nevertheless affect the quality of life. Common problems encountered in seniors are reduced bowel function and reduced immune function. Both may be associated with changes in the composition and activity of the intestinal microbiota(Reference Blaut, Marteau and Miller1, Reference He, Harmsen, Raangs and Welling2).
Furthermore, the common use of non-steroidal anti-inflammatory drugs (NSAID) among the elderly may affect their intestinal health. The most common adverse event caused by NSAID is damage to the mucosa in the gastrointestinal tract(Reference Bjarnason, Hayllar, MacPherson and Russell3, Reference Laine4). According to a recent study by Hartikainen et al. (Reference Hartikainen, Mantyselka, Louhivuori-Laako, Enlund and Sulkava5), 70 % of the over 75-year-olds in Kuopio, Eastern Finland (where the current study was performed) were taking at least one analgesic, of which NSAID were the most commonly used. A recent cross-sectional study indicated differences in microbial activity in elderly people, depending on the use of NSAID(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6).
The common opinion is that seniors have reduced levels of bifidobacteria(Reference Woodmansey7). Whether this really is true or whether this is related to differences in techniques used, culture or molecular-based, or is related to the country where the subjects live(Reference Mueller, Saunier and Hanisch8), remains to be determined. Bifidobacteria are in any case considered to be beneficial members of the intestinal microbiota and maintenance of sufficient high levels of bifidobacteria is considered important in this respect(Reference Crittenden, Salminen, von Wright and Ouwehand9).
Specific probiotics and prebiotics are able to increase the level of intestinal bifidobacteria(Reference Ouwehand, Nurminen, Mäkivuokko and Rautonen10, Reference Kruse, Kleessen and Blaut11), and improve bowel function(Reference Marteau, Cuillerier and Meance12) and immune function(Reference Gill, Rutherfurd and Cross13). The current study therefore aimed to investigate the influence of a combination of a probiotic and prebiotic on these parameters in healthy seniors. As probiotic, Lactobacillus acidophilus NCFM was chosen as it has been shown to improve intestinal microbiota metabolism(Reference Dunn, Simenhoff and Ahmed14) and immune function(Reference Foligné, Nutten and Grangette15). However, the strain has not been observed to increase faecal bifidobacteria levels(Reference Varcoe, Zook, Sui, Leighton, Busta and Brady16). As the prebiotic component, lactitol was chosen because it has been shown to be bifidogenic(Reference Ballongue, Schumann and Quignon17) and to increase bowel movements(Reference Delas, Gislon and Glikmanas18). Furthermore, since lactitol has been shown to be a good carbon source for lactobacilli in general and L. acidophilus in particular(Reference Kontula, Suihko, von Wright and Mattila-Sandholm19), and is utilised well by L. acidophilus NCFM (results not shown), it could provide a synbiotic combination.
Experimental methods
Study subjects
The main inclusion criteria of the study subjects were age over 65 years and regular use of NSAID (three or more times per week). Altogether fifty-one subjects were recruited from the city of Kuopio (Eastern Finland).
The subjects followed their habitual diet during the study. The diet in this part of Finland is typically rich in fibre (for males and females of that age group 25·7 and 21·0 g/d, respectively) originating mainly from rye bread, whole-grain porridge and berries. To reduce the variation of diets, the study subjects used the meal services offered by a local caterer (Kuopion Ateria). Subjects were asked about their pre-trial use of probiotic and prebiotic products and food products with high fibre content. The intake of fibre-rich food was not found to be different between the two groups. The main source of fibre was rye bread, providing on average 11·6 g fibre/d for subjects in the test group and 10·7 g fibre/d for subjects in the placebo group. The use of prebiotic- and probiotic-containing foods was not allowed during the study and was discontinued after the screening visit.
The exclusion criteria were critical illness, inflammatory bowel disease, coeliac disease and a major malignancy in the gastrointestinal tract. The use of antibiotics was not allowed for 1 month prior to and for the whole duration of the study. The inclusion and exclusion criteria were checked during the run-in period before randomisation. Background demographic information on the subjects from both groups is presented in Table 1.
Table 1 Subject characteristics

The study was approved by the Research Ethics Committee, Hospital District of Northern Savo (Finland) and written informed consent was obtained from the volunteers.
Study product
The study product (hereafter referred to as synbiotic) consisted of lactitol (Danisco Sweeteners, Redhill, UK) and Lactobacillus acidophilus NCFM (Danisco Cultures, Madison, WI, USA). Lactitol and L. acidophilus NCFM were milled to the same particle size and mixed to give a concentration of 2 × 109 colony-forming units (CFU)/g. The synbiotic was packed in sachets containing 5·0–5·5 g. The stability of the product was monitored for 3 months and no significant change in viable numbers was detected (results not shown). The placebo product consisted of 5 g sucrose milled to the same particle size and packed in identical sachets.
Study design
Study subjects were randomly assigned to either placebo or synbiotic group. Both study subjects and investigators were blinded to the nature of the product. The study was performed in a parallel manner, with a 2-week run-in period followed by a 2-week intervention period and finished with a 2-week wash-out period. Each 2-week periods ended with faecal sampling.
During the intervention period the study subjects were instructed to consume, on two separate occasions during the day, one sachet. The subjects were instructed to mix the test powders with, for example, yogurt or juice. Otherwise the subjects followed their habitual diet.
The subjects were carefully instructed to obtain the faecal samples according to the protocol. All faecal samples were stored in the subjects' home freezer, transferred to the laboratory within 12 h and stored at − 70°C until analysed.
During the study, subjects recorded in a study diary all changes in their medication, health status and bowel function as well as consumption of NSAID in case the consumption pattern was irregular. The use of test products was recorded daily and the records were checked by the study nurse at each visit. The average intake of test products was 1·99 (sd 0·05) sachets/d in the synbiotic group and 1·97 (sd 0·08) sachets/d in the placebo group.
Physico-chemical analyses
For the analysis of ammonia, 6 ml water were added to 1 g faeces. After thorough mixing, the sample was centrifuged at 5000 g for 5 min and 100 μl 1 m-NaOH was added to 1 ml of the supernatant. Ammonia was then measured potentiometrically using an NH3 selective electrode (Orion Research). Ammonium chloride was used as calibration standard.
For DM determination, approximately 1 g faecal sample was weighed, dried at 105°C for 16 h, cooled down in a desiccator to room temperature, reweighed and the DM content (%) was calculated.
Microbial activity was determined through measurement of SCFA and branched-chain fatty acids in faeces. Internal standard (1 ml 20 mm-pivalic acid) and 5 ml water were added to 1 g of the sample. After thorough mixing, the sample was centrifuged at 5000 g for 5 min. Following centrifugation, 250 μl saturated oxalic acid solution was added to 500 μl supernatant and the mixture was incubated at 4°C for 60 min and subsequently centrifuged at 16 000 g for 5 min. Supernatant (1 μl) was analysed by GC using a glass column packed with 80/120 Carbopack B-DA/4 % on Carbowax 20 M stationary phase (Supelco, Bellefonte, PA, USA) at 175°C and with helium as the carrier gas at a flow rate of 24 ml/min. The temperature of the injector and the flame ionisation detector were 200 and 250°C, respectively. The concentration of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, 2-methylbutyric acid and lactic acid was determined.
Biogenic amines in faeces were analysed as described previously by Saarinen(Reference Saarinen20).
To analyse d- and l-lactic acid, 1 g sample was weighed in a test tube and 6 ml water were added. The sample was shaken thoroughly and centrifuged at 10 000 g for 10 min. Supernatant (400 μl) was transferred into a 1·5 ml microfuge tube. To precipitate protein, 400 μl 0·4 m-HClO4 was added. The sample was kept on ice for 5 min and centrifuged (16 000 g for 5 min). To neutralise the sample, 600 μl supernatant was transferred into a new 1·5 ml microfuge tube and 70 μl 2 m-KOH was added. The sample was kept on ice for 5 min and centrifuged (16 000 g, 5 min). In the supernatant, d- and l-lactic acid were analysed enzymatically as instructed by the manufacturer (R-Biopharm E1112821).
Microbial analyses
The total bacteria counts in faecal samples were determined by flow cytometry (FACSCalibur; Becton Dickinson) as described previously(Reference Apajalahti, Kettunen and Kettunen21). In short, bacterial fractions were prepared by suspending faecal samples in sodium phosphate buffer (50 mm, pH 8), followed by centrifugation (30 000 g, 30 min); the pellet was washed three more times. The cell samples were diluted, fixed (37 % formaldehyde) and stained with a fluorescent nucleic acid binding dye SYTO 24 (Molecular Probes, Leiden, The Netherlands).
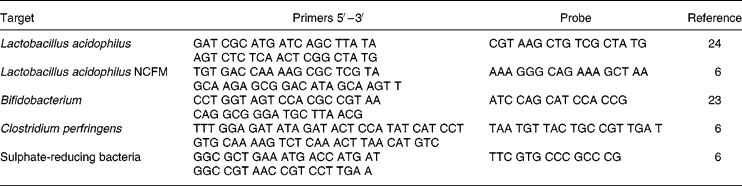
For quantification of Bifidobacterium, L. acidophilus, L. acidophilus NCFM, Clostridium perfringens and sulphate-reducing bacteria by real-time PCR (Table 2), DNA was extracted from bacteria isolated from faecal samples using the method described by Apajalahti et al. (Reference Apajalahti, Särkilahti, Mäki, Heikkinen, Nurminen and Holben22). In short, bacteria were subjected to five freeze–thaw cycles in 2-amino-2-hydroxymethyl-1,3-propanediol (10 mm) EDTA (1 mm) buffer (pH 8) and subsequently treated with lysozyme and proteinase K. The recovered bacterial DNA was used to enumerate total bifidobacteria with primers and probes designed from 16S rRNA gene as described by Mäkivuokko et al. (Reference Mäkivuokko, Nurmi, Nurminen, Stowell and Rautonen23). Primers and probe for the detection of sulphate-reducing bacteria were designed from the adenosine-5′-phosphosulphate reductase α gene of Desulfovibrio intestinalis using PrimerExpress software (Applied Biosystems, Foster City, CA, USA) as described by Tiihonen et al. (Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6), and primers and probe detecting C. perfringens were designed from phospholipase C gene as described by Tiihonen et al. (Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6). Total L. acidophilus was determined using primers described by Furet et al. (Reference Furet, Quénée and Taolliez24) with an in-house designed MGB-probe. L. acidophilus NCFM was quantified using primers and probes designed from the Clustered Regularly Interspaced Short Palindrome Repeats sequence of L. acidophilus NCFM(Reference Altermann, Russell and Azcarate-Peril25) using PrimerExpress software. For the PCR reactions 1 μg bacterial DNA was amplified with 300 nm primers for the analyses for C. perfringens and total bifidobacteria, and 900 nm primers for the analyses for D. intestinalis, L. acidophilus NCFM and total L. acidophilus (Medprobe, Normay, Oslo, Norway), and 200 nm probe (Medprobe or Applied Biosystems) in TaqMan Universal PCR Master Mix (Applied Biosystems). The assays were run on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) using the instrument's default settings. To prepare standard curves for absolute quantification, isolated chromosomal DNA either from D. intestinalis (DSM 11 275; DSMZ, Braunschweig, Germany), C. perfringens (ATCC 13 124; LGC Promochem AB, Borås, Sweden), Bifidobacterium adolescentis (DSM 20 083; DSMZ) or L. acidophilus NCFM (Danisco Cultures) were used. The mass for one bacterial chromosome was calculated by using the Avogadro constant and assuming the mean molecular weight of a base pair to be 650. Threshold cycle values from standard runs with bacterial chromosomal DNA were plotted against the number of bacteria corresponding to the mass of DNA added to the standard runs at 10-fold dilutions. The results are expressed as quantity of bacteria/g faeces.
Table 2 Primer and probe sequences as used in the current study
Immunological analyses
Changes in the immunological status of the intestine were monitored by measuring the concentrations of IgA, TNF-α, calprotectin and PGE2 from the soluble fraction of faeces. For IgA, TNF-α and PGE2 measurements, the frozen samples were thawed and extracted with bovine serum albumin as described previously and stored at − 20°C before analysis(Reference Peuranen, Tiihonen, Apajalahti, Kettunen, Saarinen and Rautonen26). Concentrations of IgA, TNF-α and PGE2 were then determined by ELISA according to the manufacturer's instructions (E80-102, Bethyl Laboratories Inc., Montgomery, TX, USA; R&D Systems Inc., Minneapolis, MN, USA; and Cayman Chemical Company, MI, USA, respectively). The concentrations of calprotectin were determined by ELISA following the manufacturer's instructions (Calpro AS, Oslo, Norway). The results were expressed as μg/g fresh weight (IgA and calprotectin) or pg/g fresh weight (TNF-α and PGE2).
Statistical analysis
To determine differences between the groups and over time, data were analysed with repeated measures ANOVA with a linear mixed-effects model, having effects for time, group, and their joint effect. Individual baseline differences were taken into account by including the first time-point as a covariate into the model. Log-transformed data were used when residual plots did not indicate constant variance. L. acidophilus NCFM data could not be analysed as described above and were analysed using baseline corrected data with between-group t tests.
Correlations between L. acidophilus NCFM and other parameters were calculated using the Pearson test for both log-transformed and original data. Residuals of the Pearson test were plotted and checked for homogeneity. Data not appearing homogeneous even after log transformation were analysed with Spearman rank correlation.
To test the dependency of the side-effects to the grouping of the individuals into synbiotic or placebo group, Fisher's exact test was used.
P values smaller than 0·05 were considered significant. All statistical analyses were performed with statistical language R, version 2.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Forty-seven subjects out of the fifty-one recruited completed the study. Two subjects were prescribed antibiotics before the last sampling and were excluded for that reason. Furthermore one subject, although completing the study protocol otherwise, was excluded from the final tables due to lack of NSAID use during one of the study periods.
At run-in, the groups had a mean defecation frequency of 1·2 (sd 0·9) and 1·3 (sd 0·6) per d for placebo and synbiotic, respectively. At the end of intervention, the mean defecation frequency the placebo group was 1·0 (sd 0·6) per d while it remained 1·3 (sd 0·5) per d for the synbiotic group. After correction for the baseline difference, the difference at the end of the intervention was statistically significant (P = 0·009).
The results of the intestinal microbiota analyses are shown in Table 3. Differences between the groups and over time were calculated using repeated measures ANOVA with linear mixed-effects model. After correction for baseline differences at the end of the run-in period, there was a difference over time for the total microbial numbers (P = 0·00 465). The numbers were reduced in both groups towards the wash-out period. Bifidobacteria were higher in the synbiotic group after intervention (7·8 × 109 CFU/g) as compared to the placebo group (3·8 × 109 CFU/g) (P = 0·0102 after baseline correction). Bifidobacterium levels declined for both groups after wash-out to 1·8 × 109 and 1·7 × 109 CFU/g for the placebo and synbiotic group, respectively (P < 0·00 001 after baseline correction). The level of sulphate reducers changed significantly over time between the intervention and wash-out period (P = 0·0058 after baseline correction); for both groups the mean levels increased (Table 3). The differences for L. acidophilus NCFM could not be modelled with repeated measures ANOVA with linear mixed-effects differences and were therefore calculated from the log-transformed data using a baseline-corrected pair-wise t test. Mean counts of L. acidophilus NCFM are 1·5 × 107 and 4·8 × 106 CFU/g for the placebo and synbiotic group, respectively. However, after log transformation and baseline correction, faecal L. acidophilus NCFM counts at the end of the intervention period were 5·63 and 3·67, for the synbiotic and placebo group, respectively (P = 0·000015). No other significant differences or changes in the measured microbiota components were observed.
Table 3 Microbiota measurements (microbes/g wet weight faeces)*
(Mean values and standard deviations)

* For details of subjects and procedures, see Table 1 and Experimental methods.
† P value (sed of log-transformed results).
‡ As the data were corrected for baseline differences, differences between time-points concern intervention and wash-out only.
After baseline correction, no differences in the levels of faecal SCFA and branched-chain fatty acids could be observed between the two study groups or between intervention and wash-out period (Table 4).
Table 4 Faecal SCFA and branched-chain fatty acids (μmol/g) and d/l-lactic acids (nmol/g)*
(Mean values and standard deviations)
* For details of subjects and procedures, see Table 1 and Experimental methods.
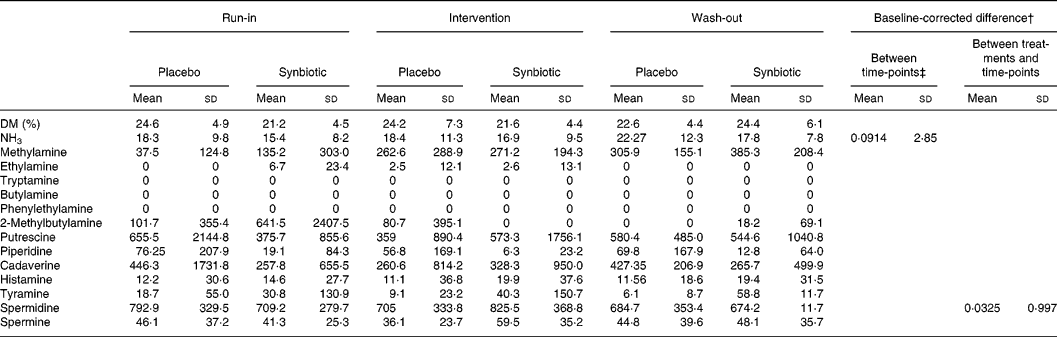
After baseline corrections, there was a change in spermidine levels (P = 0·0325), appearing higher in the synbiotic group. In addition, there was a trend for change in faecal NH3 level (P = 0·0914). This may relate to the increase in levels in both groups. No other significant differences in levels of DM, ammonia and biogenic amines could be observed between the groups or over time, after correction for baseline difference (Table 5).
Table 5 Faecal ammonia (μmol/g) and biogenic amines (nmol/g)*
(Mean values and standard deviations)
* For details of subjects and procedures, see Table 1 and Experimental methods.
† P value (sed).
‡ As the data were corrected for baseline differences, differences between time-points concern intervention and wash-out only.
Faecal concentrations of IgA were found to change significantly over time after correction for baseline differences (P = 0·0241). However, there was no difference in IgA concentrations between both groups. In contrast, the concentrations of PGE2 were affected by the synbiotic treatment. Although they tended to be already higher in the synbiotic group during run-in, they were significantly higher even after correcting for this baseline difference (P = 0·0281). There was also a trend (P = 0·0821) for differences in calprotectin concentrations between the placebo and synbiotic group after correction for baseline differences. No other significant differences in the measured faecal immune markers could be observed between the groups or over time, after correction for baseline difference (Table 6).
Table 6 Faecal immune markers*
(Mean values and standard deviations)

* For details of subjects and procedures, see Table 1 and Experimental methods.
† P value (sed).
‡ As the data were corrected for baseline differences, differences between time-points concern intervention and wash-out only.
Because L. acidophilus NCFM was fed to the volunteers in the synbiotic group, its faecal level was correlated to the other parameters measured at the end of the intervention period. Positive correlations were observed between log-transformed data of L. acidophilus NCFM and histamine (Spearman rank correlation; R 0·419, P = 0·037), and between log-transformed data of L. acidophilus NCFM and log-transformed data of spermine (Pearson correlation; R 0·41, P = 0·047). During run-in and wash-out, log-transformed data of L. acidophilus NCFM correlated positively with log-transformed data of L. acidophilus (Pearson correlation; R 0·73, P < 0·0001).
No significant differences in side-effects, temporary constipation, temporarily increased flatulence, temporary loose stools, combination of diarrhoea, constipation and loose stools, were observed between the two groups during the intervention period.
Discussion
Elderly subjects have specific ailments. The expected increase in the ageing population in affluent societies may give an increasing burden to health care services. Although many of these cases do not require medication, they may affect their quality of life. The present study investigated the effect of a probiotic and prebiotic combination (synbiotic) of L. acidophilus NCFM and lactitol on parameters of intestinal and immune health, to parameters commonly degenerated at old age. Faecal Bifidobacterium levels, microbial metabolites such as organic acids, branched chain fatty acids and biogenic amines were determined as measures for intestinal microbiota function and bowel function was recorded. Further more, faecal immune markers were determined.
It cannot be avoided that there may be differences at run-in between the study groups for the parameters under investigation. Therefore, a correction of baseline values was applied and all statistical analyses are corrected. Some of the observed changes, from run-in to intervention, may be caused by changes in the environment or indeed be a placebo effect. These are also corrected for by the used analysis model.
One of the main intervention parameters assessed was bowel function. Stool frequency was indeed found to be slightly higher in the synbiotic group as compared to the placebo group. This is in agreement with the observation that lactitol improves bowel function(Reference Delas, Gislon and Glikmanas18). The difference between the groups was modest; this is most likely due to the fact that the recruited subjects had a normal bowel function, with a mean defecation frequency of slightly more than one per day, which was similar to that observed for other NSAID users(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6). This most likely relates to the relatively high fibre intake in this population. In this respect, it is also important to note that no side-effects were experienced, although this has been reported for lactitol(Reference Morgan, Alonso and Stanger27). While not specifically investigated, it was interesting that a number of subjects from the synbiotic group enquired whether and where the product could be purchased as it made them ‘feel good’.
Synbiotic intervention increased the level of L. acidophilus NCFM numbers, indicating compliance by the volunteers. Curiously, the levels of observed L. acidophilus NCFM were on some occasions higher than observed total L. acidophilus amount. As a possible explanation it can be speculated either that the primers used for detecting total L. acidophilus are not broad enough and/or that primers designed for detecting L. acidophilus NCFM also amplify selected strains other than L. acidophilus NCFM. The levels of L. acidophilus NCFM strongly correlated with total L. acidophilus, indicating that indeed the same organisms were detected. Synbiotic consumption also increased the faecal level of total bifidobacteria, also this is in agreement with earlier observations for lactitol(Reference Ballongue, Schumann and Quignon17). Bifidobacterium levels were relatively high as compared to some earlier reports(Reference Hopkins and Macfarlane28). This may relate to differences in DNA extraction procedures or indicate differences in microbiota composition in different countries, as has been observed in particular for bifidobacteria(Reference Mueller, Saunier and Hanisch8). The proportion of bifidobacteria from the total microbiota (0·7–2·8 %) was, however, similar to what has been reported earlier, 1·1–3·5 %(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6, Reference Mueller, Saunier and Hanisch8). Although increases in faecal levels of the consumed probiotic (L. acidophilus NCFM) were observed, the results from the present study do, however, not allow us to conclude whether there was an interaction between both components of the fed synbiotic. Levels of sulphate reducers increased from intervention to wash-out. Since this occurred for both groups, it is not likely to be an effect of the intervention.
As far as the metabolic activity of the intestinal microbiota is concerned, only minor changes in the levels of SCFA and branched-chain fatty acids were observed and these were not different between both treatment groups (Table 4), although the levels were similar as reported earlier in an NSAID-user population(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6). This is in contrast to earlier reported increases in faecal acetic and lactic acid after lactitol supplementation(Reference Ballongue, Schumann and Quignon17, Reference Hotten, Marotta and Naito29). A possible explanation for this is that in the current study substantially lower levels of lactitol were consumed than in earlier studies (2 × 5 v. 2 × 10 and 3 × 20 g, respectively). Of the studied biogenic amines, spermidine levels appeared to be increased at the end of intervention in the synbiotic group. This may therefore be associated with the synbiotic treatment. Furthermore, faecal L. acidophilus NCFM levels correlated positively with spermine. Increase in polyamines such as spermine and spermidine have also been observed to correlate positively with the consumption of Bifidobacterium lactis LMK 512 and has been suggested to be associated with reduced inflammation(Reference Matsumoto and Benno30), mutagenicity(Reference Matsumoto and Benno31) and improved epithelial cell growth(Reference Osborne and Seidel32).
Although immune senescence has been described in the elderly, the changes in the intestinal immune functions in relation to the intestinal environment have not been studied extensively previously(Reference Guigoz, Dore and Schiffrin33). Faecal concentrations of IgA and TNF-α have been reported not to alter in the elderly population(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6, Reference Arranz, O'Mahony, Barton and Ferguson34). Also the calprotectin concentrations appeared normal when compared with those reported in healthy adults(Reference Maiden, Thjodleifsson, Theodors, Gonzalez and Bjarnason35). Instead PGE2 levels appeared lower in the elderly than in young adults(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6). In addition to immune modulation, PGE2 has a central role in the normal physiological gastrointestinal functions including cytoprotection, e.g. against NSAID-induced injury, and motility(Reference Dey, Lejeune and Chadee36). Reduction in PGE2 concentrations in the elderly may thus indicate lowered motility as well as more vulnerable intestinal mucosa. This is well in line with the reduced motor function in the elderly(Reference Madsen and Graff37) as well as more frequent chronic conditions. It therefore appears to indicate a beneficial change that the consumption of the synbiotic was able to induce – a modest increase in the faecal PGE2 levels, although the concentrations did not quite reach the levels found in young adults during the 2-week intervention period(Reference Tiihonen, Tynkkynen, Ouwehand, Ahlroos and Rautonen6).
In conclusion, the consumption of lactitol in combination with L. acidophilus NCFM twice daily was associated with modest improvement in stool frequency without any side-effects. Furthermore, it increased faecal numbers of L. acidophilus NCFM, bifidobacteria and faecal concentrations of spermidine and PGE2 were increased. The present results indicate improved microbiota composition and mucosal functions.
Acknowledgements
Jaana Oksanen, Kirsi Stenström, Brita Mäki and Jaana Larsson-Leskelä are acknowledged for skilful technical assistance. Dr Janne Nikkilä is acknowledged for statistical analyses and Dr Essi Sarkkinen, MSc Henna Karvonen and MD Sakari Nieminen for organising the study. Financial support for the study was received from the National Technology Agency of Finland (TEKES). The authors are employees of Danisco. Danisco has partially funded the study and is marketing the components tested: Lactobacillus acidophilus NCFM and lactitol. A. C. O., K. T. and N. R. designed the study, interpreted the results and wrote the manuscript. M. S. performed and oversaw the physico-chemical analyses and wrote the corresponding section of the manuscript. H. P. performed and oversaw the microbial analyses and wrote the corresponding sections of the manuscript.








