Coccidiosis is a protozoal infection responsible for high morbidity and substantial economic loss to the poultry industry( Reference Adams, Vahl and Veldman 1 , Reference Williams 2 ). Pathogenesis of coccidiosis is attributed to damage of the mucosal lining, which results in growth depression and malabsorption of macro- and micronutrients( Reference Ruff and Wilkins 3 – Reference Bafundo, Baker and Fitzgerald 7 ). In broiler chickens, coccidial infection decreased levels of serum Fe, while increasing levels of Cu( Reference Southern and Baker 6 , Reference Bafundo, Baker and Fitzgerald 7 ). Plasma Zn was found decreased in birds inoculated with Eimeria acervulina or Eimeria tenella compared to their unchallenged cohorts( Reference Southern and Baker 8 – Reference Richards and Augustine 12 ). This decrease in plasma Zn corresponded with increased liver Zn content( Reference Bafundo, Baker and Fitzgerald 7 , Reference Richards and Augustine 12 ). Metallothionein (MT) is a cysteine-rich Zn binding protein, which is upregulated during the acute phase response through a mechanism associated with oxidative stress( Reference Andrews 13 – Reference Lahiri and Abraham 15 ). Infection with E. tenella increased Zn-bound MT content in the liver by 91 %( Reference Richards and Augustine 12 ). Observable increases in MT-bound Zn are not exclusive to coccidiosis. Immune stressors such as bacterial cell wall components and inflammatory cytokines increased liver Zn content( Reference Etzel, Swerdel and Swerdel 16 – Reference Klasing 18 ). While MT is the major eurokayotic storage protein for Zn, transport of Zn to cytosolic proteins is mitigated by two families of contra-directional Zn transporters.
Both Zn/Fe-regulated transporter (ZRT/IRT-like) proteins (Zip) and cation diffusion facilitator (CDF) Zn transporters (ZnT) are responsible for intracellular Zn trafficking( Reference Cousins, Liuzzi and Lichten 19 ). The evaluation of Zn transporters within the avian species is relatively new to the literature; however, Zn transporter function is highly conserved between species( Reference Cousins, Liuzzi and Lichten 19 , Reference Wang and Zhou 20 ). The current NCBI 2011 assembly for Gallus gallus has four complete coding sequences: ZnT5, ZnT7, Zip9 and Zip13. In the context of gastrointestinal function, ZIP4 and ZnT1 have been extensively studied in mammals( Reference Wang, Zhou and Kuo 21 – Reference McMahon and Cousins 23 ). However, ZnT5, ZnT7, ZIP9 and ZIP13 transporters are associated with the trans-Golgi network and are involved in major signalling pathways, for example phosphorylation of extracellular signal-regulated kinase, protein kinase B, and transforming growth factor-β( Reference Matsuura, Yamazaki and Yamaguchi-Iwai 24 – Reference Jeong, Walker and Wang 27 ). ZnT7 was found to be particularly critical for Zn uptake in the murine small intestine( Reference Huang, Yu and Kirschke 28 ). Therefore, it is likely that these transporters would be influenced by inflammation and Zn status in poultry. In confirmation of this observation, ZnT5 and 7 were found to be critical to the function of alkaline phosphatases (mucosal protective proteins)( Reference Suzuki, Ishihara and Migaki 29 , Reference Mizumori, Ham and Guth 30 ).
Influx of Zn into the cytosol is coordinated by ZIP proteins localised to the plasma membrane, vesicles, and/or the Golgi complex( Reference Cousins, Liuzzi and Lichten 19 , Reference Wang and Zhou 20 ). Efflux of Zn out of the cytosol and into vesicles and/or the Golgi complex is mediated by ZnT. Extracellular stimuli including but not limited to cytokines( Reference Liuzzi, Lichten and Rivera 31 ), glucose( Reference Aydemir, Chang and Guthrie 32 ) and estrogens( Reference Lopez and Kelleher 33 ) have been shown to alter Zn homeostasis via Zn transport proteins. Directionality of net Zn transport appears to be dependent on cell type: for example stimulated monocytes and granulocytes increased free intracellular Zn, activated dendritic cells decreased intracellular Zn, and lymphocytes appeared to have no significant change( Reference Haase, Ober-Blöbaum and Engelhardt 34 , Reference Kitamura, Morikawa and Kamon 35 ). The shift in intracellular Zn content was attributed to a shift in transporter expression( Reference Haase, Ober-Blöbaum and Engelhardt 34 , Reference Kitamura, Morikawa and Kamon 35 ). In dendritic cells, the control ratio of measured Zip:ZnT mRNA was 0·67, and when stimulated with lipopolysaccharide, this ratio decreased to 0·25( Reference Kitamura, Morikawa and Kamon 35 ). This shift led to an overall movement of Zn out of the cytosol, ultimately reducing free intracellular Zn( Reference Kitamura, Morikawa and Kamon 35 ).
These data show that cellular Zn homeostasis, controlled by ZIP and ZnT transporters as well as MT, is influenced by extracellular stimuli. These cellular changes can then lead to changes in tissue homeostasis( Reference Cousins 36 ). The afore-mentioned studies focus on changes in hepatic Zn during immune stimulus, and make a strong case for Zn sparing within the liver( Reference Kehl-Fie and Skaar 37 , Reference Corbin, Seeley and Raab 38 ). However, the liver is the downstream of the intestinal mucosa, which is coccidia's main effector tissue. Early studies were unable to determine any effect of coccidial infection on intestinal Zn content( Reference Bafundo, Baker and Fitzgerald 7 ). These studies were based on total Zn content within dry tissue, and were thus unable to differentiate between membrane- and/or protein-bound Zn and free intracellular Zn. The experiments presented herein were designed to test the hypothesis that Zn transport and immune function were altered with exposure to coccidial challenge and dietary Zn regimen. Flow cytometry was used to measure phagocytic capacity and intracellular Zn content in both jejunum and caecal tonsils. Additional measures of immune status included intracellular peroxidase and CD3+ in jejunum and caecal tonsils, respectively. In order to characterise Zn flux during coccidial exposure, transporter expression of ZnT5, ZnT7, ZIP9 and ZIP13 were also measured in both tissues.
Materials and methods
All procedures of animal care and use for this experiment were approved by the Purdue University Animal Care and Use Committee. Newly hatched, male Ross broiler chicks (708; Aviagen, Inc.) were used for the present study. All chicks were housed in electrically heated battery cages (model no. SB 4 T; Alternative Design Manufacturing) in an environmentally controlled room. Battery cage temperature was maintained at 37 ± 1°C for the first week, and decreased by 3°C each consecutive week until 24°C in the third week. Chicks were weighed and allocated to groups (six chicks/cage) in such a way that the initial weight of each group was the same. The chickens were provided ad libitum access to drinking water and feed.
Experimental design
Expt 1 and 2
In Expt 1, a 3 × 2 factorial was utilised with three dietary treatments and two vaccine exposures: (an unchallenged control or ten times the recommended dosage of Coccivac®-B (10CV); each treatment was replicated six times. In Expt 2, a 7 × 2 factorial was utilised with seven dietary treatments and two vaccine exposures (as in Expt 1); each treatment was replicated six times. Dietary regimens were designed to provide 90 mg of Zn/kg of diet from one of two Zn sources: zinc sulphate (ZnSO4) or a 1:1 blend of ZnSO4 and Availa®-Zn 100 (Zinpro Corporation). Availa®-Zn 100 (10 % Zn) is a proprietary metal amino acid complex. The Association of American Feed Control Officials describes the product as a complex of a soluble metal salt with an amino acid, where one Zn ion is bound within an amino acid complex. A basal maize–soyabean meal diet was formulated to provide Zn from feedstuffs alone and was, on average, 14 % below the National Research Council( 39 ) recommendations, which is 40 mg/kg for dietary Zn. Ca and non-phytate phosphorus were provided at 9·8 and 4·3 mg/kg, respectively. Zn provided from the maize–soyabean meal basal diet (30 mg/kg of dietary Zn), was taken into account when formulating Zn premixes, therefore reported inclusion levels are based on total dietary Zn. Expt 1 provided broilers with one of three dietary treatments: basal (30 mg/kg of dietary Zn provided by feedstuffs alone), 90ZnSO4 (90 mg/kg of total dietary Zn = 30 mg/kg Zn from basal+60 mg/kg supplemental Zn from ZnSO4), or 90Blend (90 mg/kg of total dietary Zn = 30 mg/kg Zn from basal+30 mg/kg supplemental Zn from ZnSO4+30 mg/kg supplemental Zn from Availa®-Zn 100). Expt 2 introduced additional supplemental Zn concentration inclusion levels by mixing portions of basal and 90 mg/kg of Zn to create intermediate concentrations of 45 and 70 mg/kg of total dietary Zn.
Coccivac®-B (a live oocyst vaccination containing strains of E. acervulina, Eimeria mivati, Eimeria maxima and E. tenella; Intervet, Inc.) administered 10CV was used as an immune stimulus. The vaccine was introduced to day-old Ross 708 broilers through oral gavage on day 1, 7, 14, and 22 (Expt 1). At 30 and 31 d of age (8 and 9 d post gavage) one bird per cage from six replicates of each treatment was CO2 asphyxiated, and proximal jejunal sections were removed for fluorophore conjugation and mRNA transcript analysis.
In a nearly identical experimental design, chicks in Expt 2 were orally gavaged at 1, 7, 11, and 17 d of age. At 26 and 27 d of age one bird per cage (day 9 and 10 post gavage) was CO2 asphyxiated, and caecal tonsils were removed for fluorophore conjugation and mRNA transcript analysis, as in Expt 1.
Tissue processing for flow cytometry and transcript analysis
Expt 1
Six jejunal sections (3 cm on either side of the midpoint between the bile ducts and the Meckel's diverticulum) per treatment were collected from the broilers, and placed in ice-cold Tris(hydroxymethyl)-aminomethane-glycine buffer (TrisG; Thermo Scientific) before further processing. From each jejunal section, a 1 cm portion distal to the Meckel's diverticulum was placed into Trizol® (Life Technologies) for later transcript analysis. Jejunal sections were cut longitudinally and disrupted with a sterile cell sieve (CD-1™, 60 mesh screen 230 μm pore size; Sigma-Aldrich). Phosphate-free buffer, i.e. TrisG, was used to minimise Zn chelation during tissue processing( Reference Freitas, Porto and Lima 40 ). Cell homogenates were centrifuged at 800 g for 20 min, and the supernatant was decanted. The supernatant was centrifuged at 3560 g for 20 min; the pellet was retained and re-suspended in 2 ml of TrisG. The crude cell suspension was then incubated for 30 min at 37°C. Five hundred μl of each cell suspension was divided into four reagent tubes: an unlabelled tube (to determine background fluorescence) and three separate reagent tubes containing fluorescently conjugated cells. Fluorescent indicators included: 12·5 μl of FluoSpheres® (1·0 μm diameter; Invitrogen) for measurement of phagocytic activity( Reference Steinkamp, Wilson and Saunders 41 ); 100 μl of dihydrorhodamine-123 to report the presence of H2O2 and intracellular peroxidase( Reference Henderson and Chappell 42 ); 10 μl of Newport Green™ DCF diacetate (Invitrogen) for determination of intracellular Zn levels( Reference Sensi, Yin and Carriedo 43 ). A typical avian phagocyte ranges between 8 and 10 μm( Reference Aughey and Frye 44 ). The 1·0 μm diameter of carboxylate-modified microspheres was thought to be of adequate size to stimulate phagocytic response, yet small enough to allow for mechanical phagocytosis to occur( Reference Lee, Herant and Heinrich 45 ). All tubes underwent a final incubation for 30 min at 37°C. After incubation, cells were washed using a final 10 min 3000 g spin. The supernatant was discarded and the pellet was re-suspended in 1 ml TrisG. Cells were preserved with 500 μl of 2 % paraformaldehyde in Hanks' balanced salt solution (no Ca, no Mg; Life Technologies), and were stored at 3°C for next day analysis. Percent positive cells were calculated by bird, as the difference in fluorescence between cells only and fluorescently conjugated cells. Median fluorescent intensity (MFI) of fluorescently conjugated cells was also determined.
Expt 2
Tissue processing for Expt 2 was nearly identical to the protocol described in Expt 1; therefore, only the differences between the two experiments are described in this section. Caecal tonsils from six birds per treatment were collected. One caecal tonsil went into Trizol® for later transcript analysis. The remaining tonsils were processed for flow cytometry. Caecal tonsil cells are more lymphoid in nature than jejunal cells, and were therefore centrifuged at lower speeds: 500 g for 20 min. The supernatant was decanted and centrifuged at 1150 g for 20 min; the pellet was retained and re-suspended in 2 ml of TrisG. The crude cell suspension was then incubated for 30 min at 37°C. Five hundred μl of each cell suspension was divided into four reagent tubes: one unlabelled (to determine background fluorescence) and three separate reagent tubes (fluorescently conjugated cells). Fluorescent indicators included: 12·5 μl of FluoSpheres® (1·0 μm diameter; Invitrogen) for measurement of phagocytic activity( Reference Steinkamp, Wilson and Saunders 41 ); 5 μl of mouse anti-chicken CD3+–fluorescein isothiocyanate (FITC) conjugate to label the T cell receptor-associated CD3 complex (SouthernBiochem);10 μl of Newport Green™ DCF diacetate (Invitrogen) for determination of intracellular Zn levels( Reference Sensi, Yin and Carriedo 43 ). Caecal tonsil cells were preserved in 2 % paraformaldehyde and refrigerated (3°C) for next day analysis.
Flow cytometry parameters and data analysis
Expt 1 and 2 utilised a benchtop flow cytometer with 3-blue and 1-red lasers configured for excitation at 488 and 640 nm, respectively (C6 BD Accuri Cytometer, Inc.). The FL1 optical filter with emission detection of 522/30 nm was used for Newport Green™, CD3+–FITC, and dihydrorhodamine-123 analysis. The FL2 optical filter with emission at 585/40 nm was used to detect phagocytic microbeads. Data were collected on 20 000 cells per sample. Total cell population was examined on a scatter-height (FSC-H) v. side scatter-height (SSC-H) plot. Histogram overlays were generated using System II Software (Beckman Coulter Company). Histograms of optical filter by cell count were generated for cells-only and each measured fluorophore. For each bird, a fluorescently labelled cell population was overlaid against the unlabelled cells-only histogram. The shift in cell population between fluorescently conjugated cells and cells-only was calculated as percentage fluorescence difference. Fluorescence of cells-only and fluorophore-labelled cells were used to estimate MFI of FCS-generated histograms.
Gene expression analysis
Trizol® was used to extract total RNA from jejunal and/or caecal tonsil mucosa according to manufacturer's instructions. RNA samples were dissolved in nuclease-free H2O, and concentration was determined with a Nanodrop reader (Thermo Scientific). DNA was enzymatically degraded from RNA samples, using the TURBO DNA-free™ kit (Applied Biosystems). RNA samples underwent gel electrophoresis on 0·8 % agarose gel in 1 × Tris-acetate EDTA running buffer to check for integrity and genomic DNA contamination. Expression of ZnT genes was assessed through RT-PCR. Primers for G. gallus-specific ZnT5, ZnT7, ZIP9, and ZIP13 solute carriers were designed from provisional mRNA sequences from NCBI (http://www.ncbi.nlm.nih.gov). Each primer pair was designed to be at least 20 bp in length. The University of California, Santa Cruz (UCSC) database was used to blast primer pairs back to the chicken genome (http://genome.ucsc.edu). Primer pairs crossed an intron/exon boundary, and matched the target template sequence. Primer sequences, annealing temperatures and efficiencies are listed in Table 1. RNA samples were reverse transcribed using the MultiScribe™ reverse transcriptase kit (Applied Biosystems). PCR was performed using the Bio-Rad iCycler (BioRad). The PCR mix was composed of 0·5 μg of complementary DNA(cDNA), 0·075 nmol of each forward and reverse primer, and iQ SYBR green master mix (BioRad). Nuclease-free H2O was added for total reaction volumes of 25 μl. Reactions were initiated with a 5 min, 95°C incubation. Post incubation, reactions were cycled forty times using the following procedure: 10 s at 95°C, 20 s at primer-specific annealing temperature, 72°C. The Pfaffl method( Reference Pfaffl 46 ) was used for the relative quantification of real-time RT-PCR. The initial housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was found to be significantly affected by exposure to coccidial vaccine. Due to the inconsistency of housekeeping gene expression, the BestKeeper-Excel-based tool was used to evaluate potential genes and create an expression standard( Reference Pfaffl, Tichopad and Prgomet 47 ). Pair-wise correlation analysis of all pairs of candidate genes revealed that both hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA-binding protein (TBP) had the lowest between-sample variations, and high correlations with the BestKeeper index. The geometric mean of these two genes was used to normalise target gene expression.
Table 1 Primer pairs selected for zinc trafficking

ZnT5, Zn efflux transporter 5; ZnT7, Zn efflux transporter 7; Zip9, Zn influx transporter 9; Zip13, Zn influx transporter 13; HPRT1, hypoxanthine phosphoribosyltransferase 1; TBP, TATA-binding protein.
Statistical analyses
All data were analysed using the PROC MIXED procedures of SAS® (SAS Institute, 2012) as a completely randomised design. Cage was the experimental unit for all experiments discussed. For Expt 1, a 3 × 2 factorial was utilised, with three dietary regimens (maize–soyabean meal basal, 90 mg/kg dietary Zn from ZnSO4, or 90 mg/kg dietary Zn from a 1:1 blended source of Availa®-Zn and ZnSO4), and two vaccine exposures (an unchallenged control or a 10CV). Each treatment was replicated six times. For Expt 2, a 7 × 2 factorial was utilised with seven dietary regimens (maize–soyabean meal basal, 45, 70 and 90 mg/kg dietary Zn from ZnSO4, or Blend). Treatment comparisons were obtained through Tukey–Kramer means separation, and significance was established at P≤ 0·05.
Results
Mucosal response
Jejunum
Flow cytometry was used to characterise mucosal response to 10CV and dietary Zn source. There was no significant interaction between challenge and Zn treatment in any of the measured jejunal mucosal parameters. The data is expressed as both percentage of cells positive for fluorophore and the MFI of the fluorophore positive population. Unlabelled cells, from individual birds, were used to account for background fluorescence in each measurement. Exposure to 10CV decreased phagocytic capacity in jejunal cells by 2 %, with no change in MFI (Fig. 1(a) and (b)). In order to further characterise the mucosal response to 10CV, dihydrorhodamine-123 was included to measure peroxynitrite (a reactive oxygen intermediate) produced by phagocytic cells during oxidative burst. Repeated exposure to 10CV had no significant impact on percentage positive or MFI (Fig. 1(c) and (d)). Intracellular-free Zn was highly variable between birds, and therefore was not significantly different with 10CV (Fig. 1(e) and (f)). Dietary regimen did not impact any of the measured flow parameters for jejunal tissue.

Fig. 1 Jejunal mucosal response. The percentage of jejunal cells positive for the measured fluorophore (a, c and e) and median histogram fluorescent intensity of the cell population (b, d and f). Expt 1 labels include FluoSphere™ microbeads to measure percentage of cells positive (a) and median fluorescent intensity (MFI) (b) of phagocytic microbeads. Dihydrorhodamine-123 (DHR) was used to report the presence of H2O2 and intracellular peroxidase, expressed as both percent cells positive (c) and MFI (d). Newport Green™ indicator measured intracellular zinc content again expressed as both percent of cells positive for the Newport Green™ indicator (e) and the median shift in fluorescent intensity (f). Values represent mean response of six birds per zinc source for both control (Con) and 10CV (10 × dose of coccidial vaccine Coccivac®-B) birds. Significant (P≤ 0·05) main effect mean comparisons of zinc source, 10CV, and their interactions are indicated within each panel. Dietary regimens consisted of a basal maize–soyabean meal diet, or basal diet supplemented with either zinc from zinc sulphate (ZnSO4), or a blended source (1:1 blend of ZnSO4 and Availa®-Zn) to achieve 90 mg/kg of total dietary zinc. Birds were unchallenged (Con, □), or exposed to 10CV (![]() ) on days 1, 7, 14, and 22, and the final gavage occurred 10 d before tissue collection. Trt, treatment.
) on days 1, 7, 14, and 22, and the final gavage occurred 10 d before tissue collection. Trt, treatment.
Caecal tonsils
In contrast to jejunal cells, a significant interaction occurred between 10CV and Zn treatment in caecal tonsil cells (P< 0·0001), with no significant change in MFI (Fig. 2(a) and (b)). While the main effect mean of Zn treatment was not significant (P= 0·1), 10CV had a significant impact on phagocytic capacity (P= 0·001). This interaction indicates that the phagocytic capacity of caecal cells significantly increased with the 10CV, with the magnitude of increase being more pronounced with higher levels of Zn (70 and 90 mg/kg, regardless of source). The possibility of a shift in cell population was investigated with a fluorophore, designed to conjugate to the T-cell specific (CD3+) receptors. There was no significant effect of 10CV on percentage of cells positive for CD3+ conjugation; however, the MFI of CD3+ was reduced by 94 % (P= 0·001) with 10CV (Fig. 2(c) and (d)). The percentage of caecal cells positive for Newport Green™ decreased by an average of 27 % with 10CV (P< 0·0001). This reduction in percentage positive was coupled with an 86 % decrease (P< 0·0001) in MFI (Fig. 2(e) and (f)). As with jejunal cells, Zn treatment had no effect on the measured flow parameters.

Fig. 2 Caecal tonsil mucosal response. The percentage of caecal tonsil cells positive for the measured fluorophore (a, c and e) and median histogram fluorescent intensity of the cell population (b, d and f). Expt 2 labels include FluoSphere™ microbeads to measure percentage of cells positive (a) and median fluorescent intensity (MFI) (b) of phagocytic microbeads. Mouse-anti-chicken CD3+–fluorescein isothiocyanate (FITC) conjugate, used to label the CD3+T cell receptor complex, is expressed as both percent cells positive (c) and MFI (d). Newport Green™ indicator measured intracellular zinc content again expressed as both percent of cells positive for the Newport Green™ indicator (e) and the median shift in fluorescent intensity (f). Values represent mean response of six birds per zinc regimen for both control (Con) and 10CV (10 × dose of coccidial vaccine Coccivac®-B) birds. Significant (P≤ 0·05) main effect mean comparisons of zinc regimen, 10CV, and their interactions are indicated within each panel. Dietary regimens consisted of a basal maize–soyabean meal diet, or basal diet supplemented with either zinc from zinc sulphate (ZnSO4), or a blended source (1:1 blend of ZnSO4 and Availa®-Zn) to achieve 45, 70, or 90 mg/kg of total dietary zinc. Birds were unchallenged (Con, □), or exposed to 10CV (![]() ) on days 1, 7, 11, and 17, with the final gavage occurring 10 d before tissue collection. Trt, treatment.
) on days 1, 7, 11, and 17, with the final gavage occurring 10 d before tissue collection. Trt, treatment.
Zinc trafficking
Jejunum
A significant interaction between dietary regimen and 10CV occurred in Zip13 expression (Fig. 3). Jejunal tissue from birds consuming 90ZnSO4 had a 4-fold increase in Zip13 expression with exposure to coccivac. Zip9 expression was not altered by 10CV. Compared to control tissues, 10CV reduced ZnT7 expression (P= 0·02) by 50 %. The ratio of Zip:ZnT mRNA was increased by 75 % with 10CV (Fig. 5). With the exception of Zip13, dietary Zn treatment did not impact transporter expression.

Fig. 3 Jejunal zinc transporter expression. (a) ZnT5, (b) ZnT7, (c) ZIP9 and (d) ZIP13. Zinc transporter expressions in jejunal mucosal (Expt 1) from 30 and 31 d-old broilers consuming different dietary zinc sources and exposed to 10 × dose of coccidial vaccine Coccivac®-B (10CV). Values represent mean response of six birds per zinc source for both control (Con) and 10CV birds. Significant (P≤ 0·05) main effect mean comparisons of zinc source, 10CV, and their interactions are indicated within each panel. Dietary regimens consisted of a basal maize–soyabean meal diet, or basal diet supplemented with either zinc from zinc sulphate (ZnSO4), or a blended source (1:1 blend of ZnSO4 and Availa®-Zn) to achieve 90 mg/kg of total dietary zinc. Birds were unchallenged (Con, □), or exposed to 10CV (![]() ) on days 1, 7, 14, and 22, and the final gavage occurred 10 d before tissue collection. Figure depicts the expression of the target genes against the geometric mean of two housekeeper genes hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA-binding protein (TBP) as selected by the BestKeeper index(
Reference Pfaffl, Tichopad and Prgomet
47
). Trt, treatment.
) on days 1, 7, 14, and 22, and the final gavage occurred 10 d before tissue collection. Figure depicts the expression of the target genes against the geometric mean of two housekeeper genes hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA-binding protein (TBP) as selected by the BestKeeper index(
Reference Pfaffl, Tichopad and Prgomet
47
). Trt, treatment.
Caecal tonsil
A significant interaction between dietary regimen and vaccine exposure occurred in Zip13 expression (Fig. 4). In contrast to jejunal tissue where 90ZnSO4 maximised Zip13 expression, birds consuming the 90Blend treatment had a 27-fold increase in Zip13 caecal tonsil expression. Zip9 expression within caecal tonsil cells was increased 2-fold with 10CV. Caecal ZnT expression was not significantly impacted by Zn treatment or 10CV (Fig. 4). The ratio of Zip:ZnT mRNA was increased 16-fold with 10CV (Fig. 5). As in the jejunum, Zip13 expression was the only measured transporter impacted by dietary Zn source.

Fig. 4 Caecal tonsil zinc transporter expression. (a) ZnT5, (b) ZnT7, (c) ZIP9 and (d) ZIP13. Zinc transporter expressions in caecal tonsil mucosa (Expt 2) from 26 and 27 d-old broilers consuming different dietary zinc sources and exposed to 10 × dose of coccidial vaccine Coccivac®-B (10CV). Values represent mean response of six birds per zinc source for both control (Con) and 10CV birds. Significant (P≤ 0·05) main effect mean comparisons of zinc source, 10CV, and their interactions are indicated within each panel. Dietary regimens consisted of a basal maize–soyabean meal diet, or basal diet supplemented with either zinc from zinc sulphate (ZnSO4), or a blended source (1:1 blend of ZnSO4 and Availa®-Zn) to achieve 45, 70, or 90 mg/kg of total dietary zinc. Birds were unchallenged (Con, □), or exposed to 10CV (![]() ) on days 1, 7, 11, and 17, with the final gavage occurring 10 d before tissue collection. Figure depicts the expression of the target genes against the geometric mean of two housekeeper genes hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA-binding protein (TBP) as selected by the BestKeeper index(
Reference Pfaffl, Tichopad and Prgomet
47
). Trt, treatment.
) on days 1, 7, 11, and 17, with the final gavage occurring 10 d before tissue collection. Figure depicts the expression of the target genes against the geometric mean of two housekeeper genes hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA-binding protein (TBP) as selected by the BestKeeper index(
Reference Pfaffl, Tichopad and Prgomet
47
). Trt, treatment.
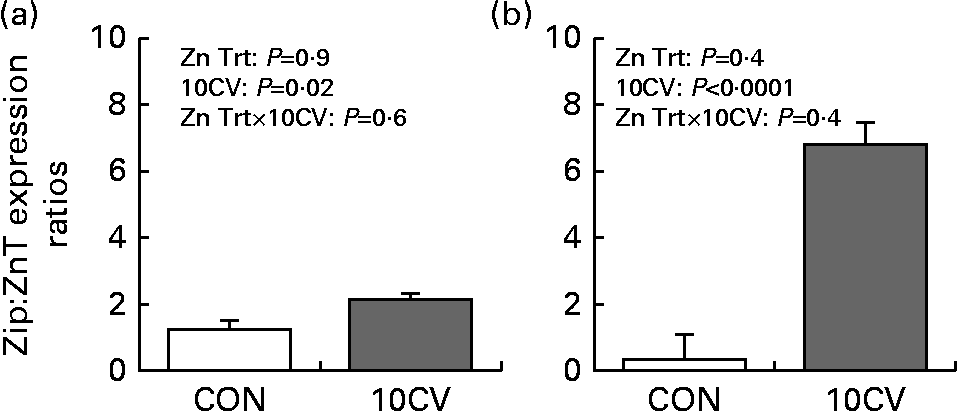
Fig. 5 Ratio of zinc influx transporter (Zip):zinc efflux transporter (ZnT) expression. Zip:ZnT expression in jejunal (a) and caecal (b) cells. Mean values with their standard errors represent ratios which were calculated from expression data, by cage as: (Zip9+Zip13)/(ZnT5+ZnT7). The main effects of dietary treatment (Expt 1 (a) basal maize–soyabean meal diet, or basal diet supplemented with either Zn from zinc sulphate (ZnSO4), or a blended source, 1:1 blend of ZnSO4 and Availa®-Zn, to achieve 90 mg/kg of total dietary Zn. Expt 2 (b) was nearly identical with the exception of two additional supplemental levels at 45 and 70 mg/kg dietary Zn), and vaccine exposure (unchallenged control (CON), or 10CV (10 × dose of coccidial vaccine Coccivac®-B)) were analysed. Vaccine exposure (10CV) had a significant effect on Zip:ZnT expression ratio and P values are indicated within each panel. Trt, treatment.
Discussion
Mucosal response of jejunal and caecal tonsil tissues
It is known that repeated exposure to oocysts results in increased cellular infiltration, and faster resolution of infection( Reference Rose, Hesketh and Ogilvie 48 , Reference Laurent, Mancassola and Lacroix 49 ). In the present study, repeated coccivac exposure within the jejunum decreased the phagocytic population. Respiratory burst, a process through which bactericidal superoxide and peroxides are produced, is the primary mechanism through which phagocytes induce lysis( Reference Morel, Doussiere and Vignais 50 ). In Expt 1, there was no effect of Zn source or challenge on dihydrorhodamine-123 signal (Fig. 1(c) and (d)). In poultry, secondary coccidial infections are characterised by high concentrations of CD8+ T cells within intraepithelial cell infiltrates( Reference Jeurissen, Janse and Vermeulen 51 – Reference Lillehoj, Kim and Keeler 54 ). During the course of infection, T cells produce interferon-γ, a cytokine which recruits leukocytes and enhances the lysosomal activity of macrophages. Laurent et al. ( Reference Laurent, Mancassola and Lacroix 49 ) found that transcript levels of interferon-γ peaked 7 d post infection and returned to baseline levels 13 d post-infection. In the present study, tissues were repeatedly exposed to coccivac and collected on day 8 and 9 post gavage. Therefore, in Expt 1, the innate macrophage response may have given way to an adaptive cellular immune response by the time of collection. Jejunal intracellular Zn, measured by Newport Green™, was widely variable between birds, and therefore the drop in intracellular Zn with 10CV was not significant. However, other studies have reported decreases in intracellular Zn with infection( Reference Ranaldi, Caprini and Sambuy 55 , Reference Thambiayya, Wasserloos and Huang 56 ); it is said that the drop in intracellular Zn acts as a protective mechanism through the induction of apoptosis. Thambiayya et al. ( Reference Thambiayya, Wasserloos and Huang 56 ) found that Zn binding can inhibit caspase 3, a pro-apoptotic protein. Limiting labile Zn within the cell may release inhibition of caspase 3 and promote apoptosis during oxidative stress.
Caecal tonsil cells (Expt 2) expressed a significant interaction between Zn treatment and 10CV. Exposure to coccidia (10CV) increased phagocytic capacity; however, the magnitude of increase from unchallenged and challenged groups was more pronounced with 70 and 90 mg/kg of supplemental Zn. Dubben et al. ( Reference Dubben, Hönscheid and Winkler 57 ) found that chelating Zn out of solution was found to enhance monocyte (modelled using the HL-60 cell line) differentiation and phagocytic potential. Therefore, if Zn has a negative impact on monocyte differentiation, results of Expt 2 may reflect lower steady state monocyte activity within caecal tonsils. This steady state population of monocytes did not negatively impact the phagocytic capabilities of 10CV. Furthermore, caecal tonsils are known to contain a high concentration of macrophages; macrophage inflammatory protein (a macrophage recruiting chemokine) was upregulated 80-fold in E. tenella-infected caecal cells( Reference Laurent, Mancassola and Lacroix 49 ). Laurent et al. ( Reference Laurent, Mancassola and Lacroix 49 ) found that the upregulation of inflammatory cytokine expression was similar between E. tenella-infected caecal tonsils and E. maxima-infected jejunum; however, the caecal response was more pronounced. Several studies have noted that poultry have an increased cellular immune response (systemically and within the small intestine and caecal tonsils) to coccidial infection( Reference Rose, Hesketh and Ogilvie 48 , Reference Laurent, Mancassola and Lacroix 49 , Reference Jeurissen, Janse and Vermeulen 51 – Reference Lillehoj, Min and Dalloul 53 ). Given the importance of the cellular immune response during coccidial infection( Reference Jeurissen, Janse and Vermeulen 51 – Reference Shanmugasundaram, Sifri and Selvaraj 60 ), we chose to focus on CD3+ expression in caecal tonsils. Though we found no change in the population of cells positive for CD3+, there was a significant reduction in the MFI. The CD3+ marker is a critical inducer of the signalling cascade necessary to activate T cells. In human T cell lines, the CD3+ marker becomes internalised and eventually degraded, resulting in a loss of CD3+ signal( Reference Valitutti, Müller and Salio 61 , Reference Sullivan and Coscoy 62 ). This mechanism is similar in chickens, as the T cell receptor/CD3 complex is known to contain an internalisation motif( Reference Göbel and Dangy 63 ). Furthermore, Göbel & Dangy( Reference Göbel and Dangy 63 ) determined that CD3 was downregulated in αβ T cells during stimulation. Therefore, while the results of the present study reflect no change in total T cell population, they do suggest an increase in activated T cells within 10CV caecal tonsils. Caecal tonsils are the largest lymphoid organ in the chicken, and they can function in a manner similar to that of mammalian Peyer's patches( Reference Lillehoj and Trout 64 ). Histologically, caecal tonsils contain germinal centres and IgA-positive B cells, which survey the intestinal immune environment, and aid in the development of intestinal immunity( Reference Liu, Cui and Peng 65 ). The jejunum also contains a large amount of lymphocytes within the epithelium and lamina propria( Reference Lillehoj and Trout 64 ). However, between the two organs, caecal tonsils contain twice the amount of lymphocytes as the jejunum does( Reference Uddin, Khan and Islam 66 ). Therefore, the difference in phagocytosis between jejunum and caecal tonsils may lie in the variability of cell type within those organs. The amount of free Zn (Newport Green™) within caecal tonsils was significantly decreased with 10CV. The decrease of free Zn within caecal tonsil cells is presumably a protective mechanism, promoting apoptosis and limiting tissue damage.
Zinc trafficking within jejunum and caecal tonsils
ZnT measured in Expt 1 and 2 are localised to the endoplasmic reticulum/Golgi complex but expressed different directionality of transport( Reference Cousins, Liuzzi and Lichten 19 , Reference Wang and Zhou 20 ). In mammals, ZnT5 and ZnT7 are specifically thought to transport Zn from the cytosol to the Golgi, while ZIP9 and ZIP13 are responsible for vesicular Zn influx into the cytoplasm( Reference Matsuura, Yamazaki and Yamaguchi-Iwai 24 – Reference Jeong, Walker and Wang 27 ). In Expt 1, Zip13 mRNA was significantly upregulated in the 90ZnSO4 treatment in the jejunum during challenge, but not by the 90Blend treatment. This finding suggests differences in availability between Zn sources( Reference Star, van der Klis and Rapp 67 ). ZnT7 expression in Expt 1 jejunum, was significantly decreased with 10CV. This is in contrast to liver expression where ZnT5 and ZnT7 have been shown to increase with inflammation induced by lipopolysaccharide( Reference Aydemir, Chang and Guthrie 32 ). Upregulation of Zip transporters coupled with decreased ZnT expression suggests that trafficking from cytosolic compartments to the Golgi was decreased with exposure to 10CV. However, these responses are tissue-specific, and the intestine is known to have a unique ZnT response during inflammation. Guthrie et al. ( Reference Guthrie, Aydemir and Troche 68 ) noted degradation of ZIP14 with lipopolysaccharide (as opposed to the upregulation noted in liver); it was hypothesised that highly challenging immunoenvironment was responsible for the differential responses between the jejunum and liver. In keeping with the general downregulation of ZnT during coccidial challenge, a recent report noted a significant decrease in the expression of ZnT1 with Eimeria infection( Reference Su, Miska and Fetterer 69 ). A decrease in ZnT1 along with the finding of the present study of a decrease in jejunal ZnT7 would suggest that cells do limit Zn efflux into intracellular compartments( Reference Su, Miska and Fetterer 69 ). This conclusion is strengthened by the observed trend of decreased free intracellular Zn in the present study (as measured by Newport Green™). This indicates that within the jejunum, movement of Zn into the cytoplasm was upregulated during repeated exposure to coccidial vaccine (10CV).
Caecal tonsils followed a similar pattern. In comparison to the jejunum, 10CV appeared to have little impact on measured ZnT mRNA expression in caecal tonsils. Thus, very little has been reported on ZnT expression within caecal tonsils. Though the expression of measured ZnT was not downregulated with 10CV, the expression of both Zip9 and Zip13 were significantly upregulated. In contrast to jejunal cells, the greatest expression of Zip13 was observed in 90Blend treatment. To our knowledge this is the first report that has linked the expression of ZnT to a dietary Zn source. Star et al. ( Reference Star, van der Klis and Rapp 67 ) found that the bioavailability of Availa®-Zn, as measured through broiler chick Zn tibia content, was higher than that of ZnSO4. Castillo et al. ( Reference Castillo, Martin-Orue and Taylor-Pickard 70 ) reported the use of an organic Zn source tended to reduce enterobacteria levels in weanling pig jejunums. It is therefore possible that the observed changes in Zip13 expression in Expt 1 and 2 were due to altered bioavailability and/or altered microbial load. Overall, our data shows that the ratio of Zip:ZnT expression was significantly increased due to 10CV (Fig. 5). This suggests movement of Zn from intracellular compartments to the cytoplasm. As Newport Green™ only measures free, not bound, Zn, this increase in Zip:ZnT ratio suggests that Zn may be incorporated into cytosolic proteins (e.g. MT or other metal-regulatory protein). Our hypothesis is that cells upregulate ZIP transporters in an effort to promote cell/tissue protective processes during coccivac exposure, i.e. apoptosis. However, this does not exclude the possibility that cells are simply Zn starved, and upregulate ZIP transporters in an effort to compensate. A model outlining the findings of the present study and potential hypotheses is included in Fig. 6. In conclusion, repeated exposure to coccidial challenge decreased free intracellular Zn, and concurrently increased the ratio of measured ZIP:ZnT transporters. This response appears to be a compensatory effect for reductions in intracellular-free Zn.

Fig. 6 Model of intracellular zinc trafficking during repeated exposure to coccidial vaccine. Amended mechanism for zinc transporter expression. Coccidiosis had a larger impact on transporter expression than dietary zinc regimen. Zinc influx transporter (Zip):zinc efflux transporter (ZnT) ratio increased with challenge in jejunal cells and caecal tonsils. However, flow cytometry reported a decrease in intracellular-free zinc with challenge. The upregulation of Zip transporters coupled with lower intracellular-free zinc suggests that the cell (1) may have shuttled zinc into cytosolic proteins during coccidial challenge, or (2) upregulated Zip transporters in an effort to replenish lost cytosolic zinc.
Acknowledgements
The authors would like to thank Zhengyu Jiang and Liting Xu for their assistance with tissue processing and sample preparation during the flow cytometry experiments. We also thank Kolapo Ajuwon for the generous use of his lab facilities and expertise with PCR analysis. The authors thank Zinpro Corporation (Eden Prarie, Minnesota, USA) for partial funding for the present research.
Partial funding for the present research reported herein was provided by the Zinpro Corporation, Eden Prairie, MN, USA as an unconditional research gift. Zinpro Corporation had no role in the design, analysis or writing of this article. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. United States Department of Agriculture is an equal opportunity employer.
The authors' contributions are as follows: C. T., S. D. E., and T. J. A. were responsible for the design of the research; C. T. carried out the research and prepared the manuscript; C. T., S. D. E., and T. J. A. reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
The authors have no conflicts of interest to declare.










