Skeletal muscle mass maintenance is regulated by the balance between muscle protein synthesis and breakdown rates. The stimulation of muscle protein synthesis by food intake (i.e. dietary protein ingestion and subsequent aminoacidemia) and physical activity (i.e. resistance type exercise) are key factors responsible for the maintenance of skeletal muscle mass. The amplitude and duration of the muscle protein synthetic response to protein ingestion is modulated by the amount(Reference Witard, Jackman and Breen1–Reference Pennings, Groen and de Lange3), source(Reference Pennings, Boirie and Senden4–Reference Wilkinson, Tarnopolsky and MacDonald7) and type of protein(Reference Pennings, Boirie and Senden4,Reference Burd, Yang and Moore8) that is consumed, as well as the matrix in which it is embedded(Reference Churchward-Venne, Snijders and Linkens9–Reference van Vliet, Shy and Abou Sawan11). In addition, the essential amino acid (EAA) composition of the protein that is consumed, and the leucine content in particular, plays a key role in the postprandial stimulation of muscle protein synthesis(Reference Volpi, Kobayashi and Sheffield-Moore12).
The consumption of plant-based protein sources and the use of plant-based protein isolates and concentrates in food formulations and products are increasing worldwide, which is mainly due to the increasing awareness regarding food sustainability and the lower production cost of plant-based proteins(Reference Willett, Rockström and Loken13). However, based on their digestibility and/or amino acid composition, plant-based protein sources are generally considered of a lesser quality when compared with animal-based proteins(Reference van Vliet, Burd and van Loon14–Reference Gorissen and Witard16). In accordance, the postprandial muscle protein synthetic response to the ingestion of plant-based proteins has been shown to be lower when compared with the ingestion of an isonitrogenous amount of animal-based protein(Reference Gorissen, Horstman and Franssen6,Reference Wilkinson, Tarnopolsky and MacDonald7,Reference Phillips17,Reference Yang, Churchward-Venne and Burd18) . The lesser anabolic properties of plant-based proteins have been attributed to the lower EAA content and the shortage of specific amino acids such as leucine, lysine and/or methionine(Reference van Vliet, Burd and van Loon14,Reference Gorissen, Crombag and Senden15,19,Reference Young and Pellett20) . Since all amino acids are required as precursors for muscle protein synthesis, the lack of one or more amino acids may compromise the postprandial muscle protein synthetic response. Though there are only a few studies that have assessed muscle protein synthesis rates following the ingestion of plant-based proteins; a lower muscle protein synthetic response to the ingestion of soy(Reference Wilkinson, Tarnopolsky and MacDonald7,Reference Phillips17,Reference Yang, Churchward-Venne and Burd18) and wheat(Reference Gorissen, Horstman and Franssen6) has been consistently reported when compared with the ingestion of animal-based protein sources such as milk or beef.
To compensate for the proposed lesser anabolic potential of plant-based proteins, more of the plant-based protein could be consumed to induce a similar postprandial increase in muscle protein synthesis rates when compared with a high-quality animal-based protein source(Reference Gorissen, Horstman and Franssen6). Although effective, increasing the dose of plant-based proteins to compensate for their lower anabolic properties may not always be practical or feasible. Other strategies to increase the anabolic potential of a plant-based protein source may be to fortify with specific amino acids or the use of specific blends of various plant-based proteins that have opposing differences in their specific shortages of one or more amino acids. Recent innovations in food processing and the selection of specific plant-based protein blends may optimise the quality of a plant-based protein meal and, as such, increase the postprandial muscle protein synthetic response(Reference Reidy, Walker and Dickinson21–Reference Reidy, Walker and Dickinson23). As a result, there is an extensive range of plant-based protein products (as alternatives to meat consumption) available on the market; their capacity to stimulate muscle protein synthesis, however, has not yet been assessed. We aimed to compare the muscle protein synthetic response following the ingestion of an ample amount of a plant-based, whole-food protein source (a meat alternative) with an equivalent amount of an animal-based protein source. We hypothesised that ingestion of a lysine-enriched, plant-based protein product can increase muscle protein synthesis rates in healthy individuals. Furthermore, we hypothesised that the postprandial muscle protein synthetic response following the ingestion of an ample amount of such a plant-based meat alternative would not differ from the ingestion of an isonitrogenous amount of chicken. To test our hypothesis, we assessed basal and postprandial muscle protein synthesis rates using contemporary stable isotope methodology following ingestion of 40 g of protein provided via a lysine-enriched, wheat and chickpea protein-based product or an isonitrogenous amount of chicken in twenty-four healthy, young men.
Methods
Participants
Twenty-four healthy, young, recreationally active men (aged 18–35 years, BMI 18–27·5 kg m−2) volunteered to participate in this parallel, double-blind, randomised controlled trial (recreationally active was defined as engaging in sports or structured exercise ≤ 3 d/week and not participating in any structured resistance type exercise programme). Participants’ characteristics are presented in Table 1. The flow chart of participant enrolment is shown in Supplemental Fig. 1. This study was registered at the Netherlands Trial Register (NTR6380) and was conducted between June 2017 and October 2017 at Maastricht University Medical Centre+, Maastricht, the Netherlands. All participants were informed on the purpose of the study, the experimental procedures and possible risks before providing informed written consent to participate. The procedures followed were in accordance with the ethical standards of the medical ethics committee of Maastricht University Medical Centre+ on human experimentation and in accordance with the Helsinki Declaration of 1975 as revised in October 2013. The study was independently monitored by the Clinical Trial Centre Maastricht.
Table 1. Subjects’ characteristics
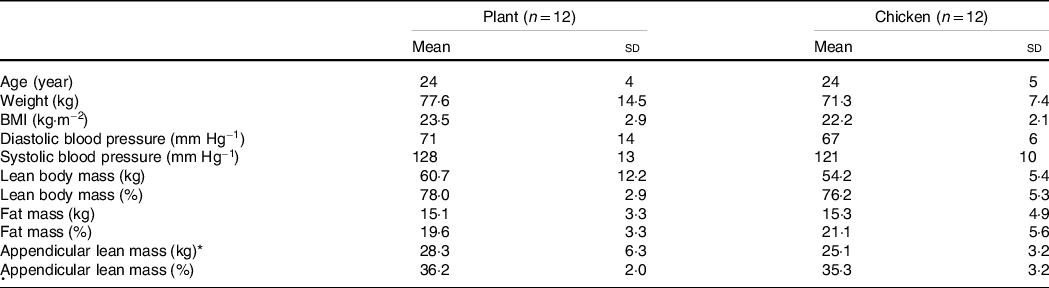
Values are mean and standard deviation.
* Appendicular lean mass was calculated by the sum of lean mass of both arms and legs. Data were analysed by unpaired Student’s t test, P < 0·05. No significant differences were observed between groups.
Pre-testing
Participants underwent an initial screening session to assess height, weight, blood pressure and body composition (by dual-energy X-ray absorptiometry; Hologic Inc., DXA; Discovery A, QDR series). Whole-body and appendicular (sum of lean mass of both arms and legs) lean mass and body fat was determined using the software package Apex (en-CORE 2005, version 4.0.2. Hologic) and reference values from the National Health and Nutrition Examination Survey (NHANES) population-based dataset(Reference Kelly, Wilson and Heymsfield24). Participants were deemed healthy based on their responses to a medical questionnaire and screening results. All participants were instructed to refrain from strenuous physical activity and alcohol consumption for 3 d before the experimental trial. On the evening before the experimental trial, all participants consumed a pre-packaged standardised meal (Maaltijdpannetje, Aviko) containing 55 % energy as carbohydrate, 30 % energy as fat and 15 % energy as protein before 20:00, after which they remained fasted.
Dietary intervention
Participants were randomly assigned to consume 40 g of protein in the form of either 230 g of a baked lysine-enriched, plant-based meat substitute (Plant) or 174 g of baked chicken breast (Chicken). We selected an existing plant-based meat alternative (Tereos, France) typically available on the market. As plant-based meat substitutes usually provide more fat and/or carbohydrate relative to the amount of protein when compared with animal-based products, we compared products based on the same amount of protein provided. The lysine-enriched, plant-based protein product was composed of a blend of wheat and chickpea flour (60/40 ratio) and supplemented with 5 % free lysine/100 g (l-lysine monohydrochloride), up to about 200 % of the recommended levels of the FAO/WHO, in order to fortify the lysine content in the product that was naturally lacking in lysine and below the recommended intake levels according to the FAO standards. The product was produced by extrusion of the protein blend into small shredded, diced pieces at temperatures < 100°C followed by cooking at about 135°C. A staff member not involved in the study generated random assignment of the treatments and participant codes using a computerised list randomiser (www.random.org), and participants were sequentially allocated to a treatment according to the random assignment list that was stored in a closed cabinet. Meals were prepared by a staff member not involved in the study, served on an identical white plate and provided with the randomisation code, making them blinded to both participants and researchers. Both meals were presented in identical form and appearance (small, diced pieces) and baked for 9 min in 7 g of olive oil (15 % extra virgin olive oil) in a frying pan. No additional flavouring was added. Qualitative measurements on palatability were taken directly after consumption of the meals by providing participants with visual analogue scales and are presented in Online Supplemental Material. Macronutrient breakdown and amino acid composition are shown in Tables 2 and 3, and the plasma appearance of specific amino acids following ingestion of Plant and Chicken are provided in the Online Supplemental Material. The amino acid content of both interventions was determined as previously described(Reference Gorissen, Crombag and Senden15) and described in detail in Online Supplemental Material. In short, approximately 5 mg of freeze-dried Plant or Chicken was hydrolysed in 3 ml of 6 M HCl for 12 h at 110°C. After hydrolysis, HCl was evaporated under nitrogen stream, while heated to 120°C and the dried amino acids were reconstituted in 5 ml of 0·1 M HCl. Amino acids were measured by using ultra-performance liquid chromatography-MS (ACQUITY UPLC H-Class with QDa; Waters).
Table 2. Macronutrient composition of protein meals
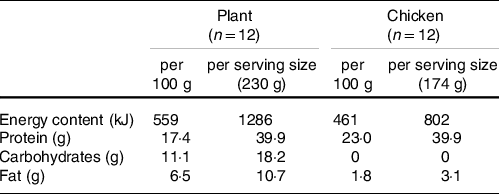
Table 3. Amino acid composition of raw product
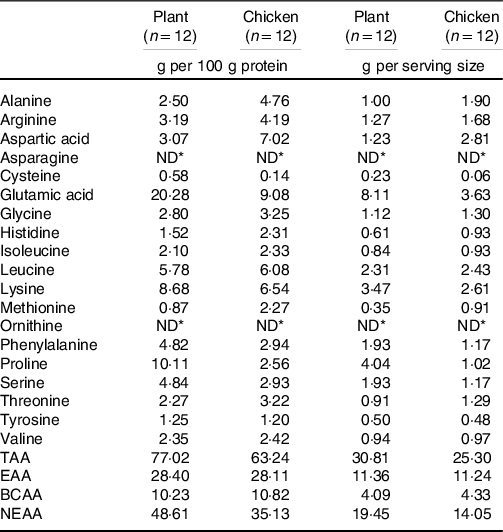
TAA, sum of total amino acids; EAA, essential amino acids; BCAA, branched-chain amino acids; NEAA, non-essential amino acids.
* Not detectable.
Experimental protocol
At 07:30, participants arrived at the laboratory after an overnight fast. A peripheral intravenous catheter was inserted into an antecubital vein for stable isotope amino acid infusion, and a second catheter was inserted into a dorsal hand vein on the contralateral arm for arterialised blood sampling (the hand was placed in a hot box (60°C) for 10 min before sample collection (24)). After taking a baseline blood sample (t = −180 min), the plasma phenylalanine pool was primed with a single dose of l-(ring-13C6)-phenylalanine (2·25 μmol kg−1), and subsequently, a continuous intravenous infusion of l-(ring-13C6)-phenylalanine (0·05 μmol kg−1 min−1) was initiated (t = −180 min) with use of a calibrated IVAC 598 pump. While resting in a supine position, blood samples were taken at t = −90, −60 and −30 min relative to meal ingestion. At t = 0 min, a blood sample and a muscle biopsy sample from the M. vastus lateralis of a randomly selected leg were collected to assess post-absorptive muscle protein synthesis. Subsequently, participants received a protein meal corresponding to their randomly assigned treatment (Plant (n =12) or Chicken (n = 12)). All subjects ingested a 150 ml water beverage (and were instructed to consume this consistently throughout their meal) with 3.85 % free, crystalline l-(ring-13C6)-phenylalanine to minimise dilution of the steady-state plasma l-(ring-13C6)-phenylalanine precursor pool implemented by the constant infusion. Arterialised blood samples were then collected at t = 15, 30, 60, 90, 120, 150, 180, 240 and 300 min. A second and third muscle biopsy sample were collected at t = 120 and t = 300 min to determine postprandial muscle protein synthesis rates from t = 0–120, 120–300 and 0–300 min. Blood samples were collected into EDTA-containing tubes and centrifuged at 1000 g for 15 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80°C. Muscle samples were collected with the use of a 5-mm Bergström needle custom-adapted for manual suction(Reference Bergstrom25). Samples were obtained from separate incisions from the middle region of the M. vastus lateralis, about 15 cm above the patella and about 3 cm below entry through the fascia, under 1 % xylocaine local anaesthesia with adrenaline (1:100·000). Muscle samples were freed from any visible non-muscle material, immediately frozen in liquid nitrogen and stored at −80°C until further processing.
Plasma and muscle analyses
Details of analysis related to the determination in plasma (glucose, insulin, and amino acid concentrations and plasma l-(ring-13C6)-phenylalanine enrichments) as well as muscle (mixed muscle protein-bound l-(ring-13C6)-phenylalanine enrichments and protein signalling) are presented in the Online Supplemental Material.
Calculations
The present study involved the infusion of l-(ring-13C6)-phenylalanine combined with muscle biopsy and arterialised venous blood sampling to determine the fractional synthesis rates (FSR) of mixed muscle proteins in the basal and postprandial state and were calculated by using the standard precursor-product equation:
where ΔEp is the increment in muscle protein-bound l-(ring-13C6)-phenylalanine enrichment after an incorporation period (in mole percent excess, MPE), E precursor is the weighted average plasma l-(ring-13C6)-phenylalanine enrichment during the tracer incorporation period (MPE) and t is the incorporation time (h). Weighted mean plasma enrichments were calculated by taking the measured enrichments between consecutive time points and correcting for the time between these sampling time points. For basal FSR, mixed plasma protein samples at t = –180 min and muscle biopsy samples at t = 0 min were used (single biopsy approach);(Reference Burd, Pennings and Groen26) for postprandial FSR, muscle biopsy samples at t = 0, 120 and 300 min were used.
Statistical analysis
All data are expressed as mean and standard deviation. Subjects’ characteristics and baseline data (including basal FSR and anabolic signalling) were analysed using unpaired, two-tailed Student’s t tests. The primary outcome of the study was mixed muscle FSR (change from the basal to postprandial period), secondary outcomes included plasma glucose, insulin, amino acid concentrations (changes over time, total AUC and time to peak) and anabolic signalling responses (changes over time). Two-factor repeated-measures ANOVA with time as within-subject factor and intervention as between-treatment factor was used to compare differences over time in plasma glucose, insulin, amino acid concentrations and enrichments, anabolic signalling, and FSR (basal to the 0–120 min and 120–300 min postprandial period, and basal to the cumulative 0–300 min postprandial period). In case of significant time × treatment interactions, separate analyses were performed to determine time effects for each treatment (one-factor repeated-measures ANOVA with Bonferroni post hoc tests to identify time differences) and between-treatment effects for each time point (unpaired, two-tailed Student’s t test). Peak values, time to peak and AUC were calculated for plasma time curves, and differences were determined using unpaired, two-tailed Student’s t tests. Based on previous studies(Reference Gorissen, Horstman and Franssen6), a sample size of twelve subjects per intervention including a 10 % dropout rate was calculated, using a unpaired, two-sided statistical test (P < 0·05, 95 % power, effect size 1·8), to detect differences in FSR between treatments. For all analyses, statistical significance was set at P < 0·05. All calculations were performed using SPSS (IBM Statistics, version 25.0, IBM Corp.).
Results
Plasma glucose and insulin
Plasma glucose and insulin concentrations are shown in Fig. 1. Following the ingestion of the 40-g protein meal, plasma glucose concentrations (Fig. 1(a)) increased to a greater extent in Plant when compared with Chicken (time x treatment P < 0·001). Plasma glucose concentrations reached peak values at 30 ± 0 min in Plant (6·1 ± 0·2 mmol l−1) and were higher when compared with peak values in Chicken (5·4 ± 0·4 mmol l−1 at 140 ± 59 min; P < 0·001). Following protein ingestion, plasma insulin concentrations (Fig. 1(b)) increased to a greater extent after Plant ingestion when compared with Chicken (time x treatment P < 0·001). Plasma insulin concentrations in Plant peaked at 38 ± 19 min, reaching concentrations of 205 ± 73 pmol l−1, and were higher when compared with Chicken, reaching peak values of 111 ± 39 pmol l−1 at 78 ± 37 min (P < 0·05). Plasma insulin responses (AUC) were higher at 0–2 h (P = 0·003) and 0–5 h (P = 0·031) in Plant when compared with Chicken.
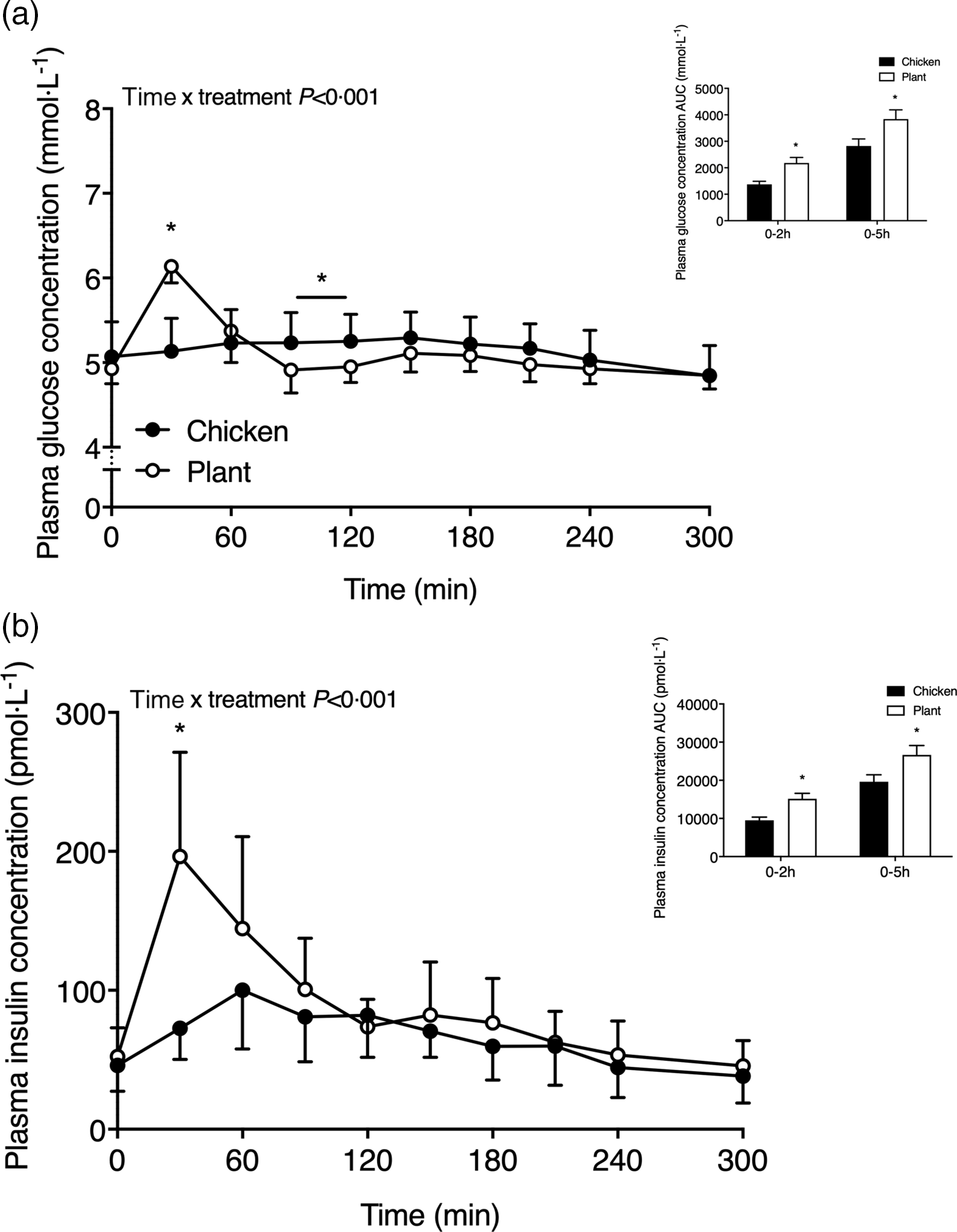
Fig. 1. Plasma glucose (a) and insulin (b) concentrations (mmol·l−1 and pmol·l−1, respectively) in twenty-four healthy, young men following the ingestion of 40 g of protein of either a lysine-enriched, wheat and chickpea protein product (Plant; n = 12) or chicken breast fillet (Chicken; n = 12). Values represent means and standard deviation. Insets represent AUC. Data were analysed by repeated-measures (time × treatment) ANOVA. Bonferroni post hoc test was used to locate differences over time. (a) Time × treatment interaction, P < 0·001. (b) Time × treatment interaction, P < 0·001. *A significant difference between treatments, P < 0·05.
Plasma amino acid concentrations
Plasma amino acid concentrations are shown in Figs. 2 and 3. Plasma leucine concentrations (Fig. 2(a)) increased following meal ingestion, but to a greater extent in Chicken when compared with Plant (time x treatment P < 0·001). Plasma leucine concentrations were higher in Chicken (peak values 290 ± 28 µmol l−1) when compared with Plant (peak values 200 ± 34 µmol l−1) from t = 60–240 min (P < 0·05). The AUC of plasma leucine concentrations was higher in Chicken in the 0–2 h and 0–5 h postprandial period when compared with Plant (both, P < 0·001). Plasma lysine concentrations (Fig. 2(b)) rapidly increased following Plant ingestion (time x treatment P < 0·001), reaching peak concentrations of 517 ± 77 µmol l−1, and were higher when compared with Chicken (peak values 324 ± 28 µmol l−1) throughout the postprandial period from t = 15–90 min (P < 0·001). Plasma methionine concentrations (Fig. 2(c)) increased to a greater extent following ingestion of Chicken when compared with Plant (time x treatment P < 0·001) and remained elevated in Chicken from 30–300 min following protein ingestion (P < 0·05).
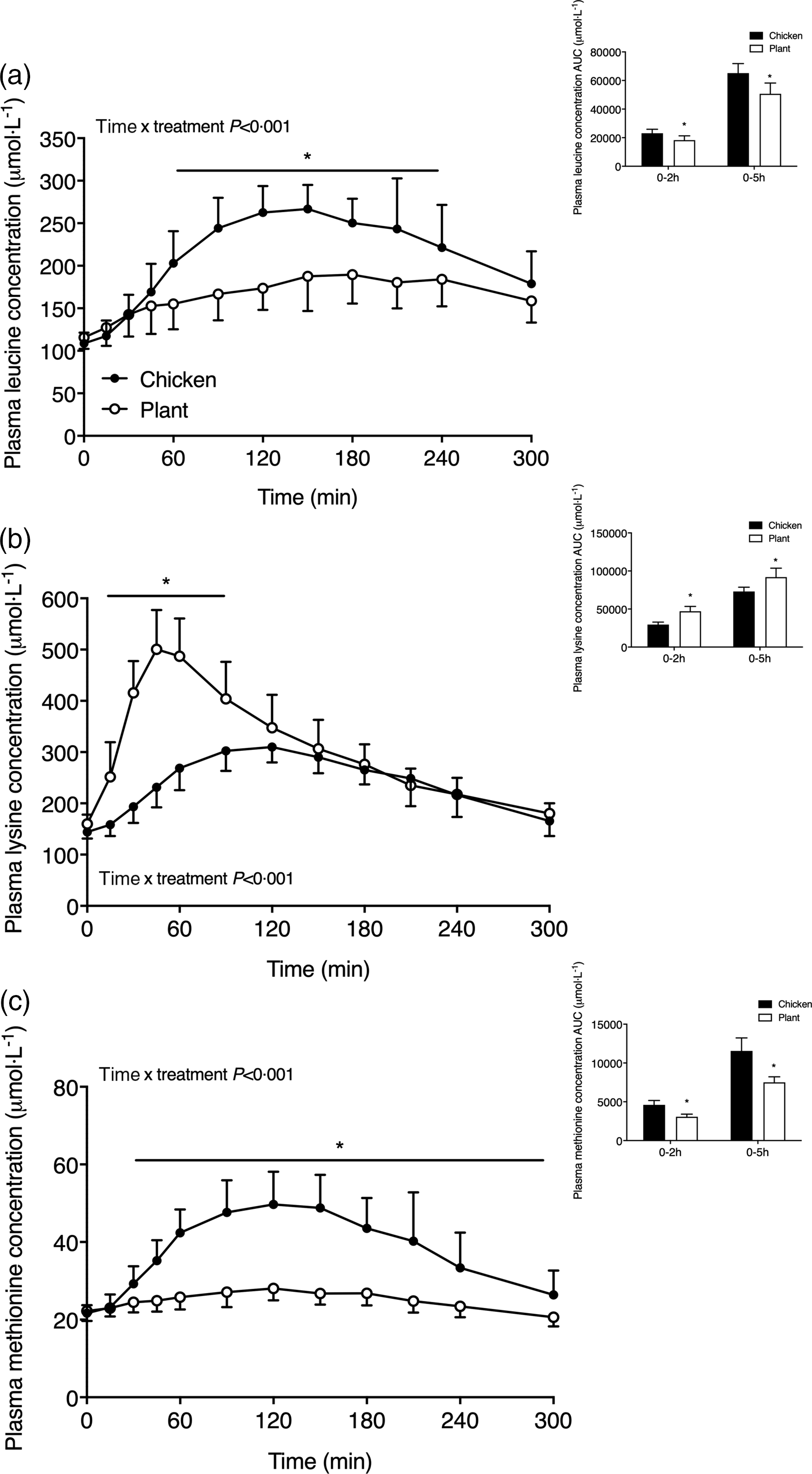
Fig. 2. Plasma leucine (a), lysine (b) and methionine (c) concentrations in twenty-four healthy, young men following the ingestion of 40 g of protein of either a lysine-enriched, wheat and chickpea protein product (Plant; n = 12) or chicken breast fillet (Chicken; n = 12). Values represent means and standard deviation. Insets represent AUC. Data were analysed by repeated-measures (time × treatment) ANOVA. Bonferroni post hoc test was used to locate differences over time. (a) Time × treatment interaction, P < 0·001. (b) Time × treatment interaction, P < 0·001. (c) Time × treatment interaction, P < 0·001. *A significant difference between treatments, P < 0·05.
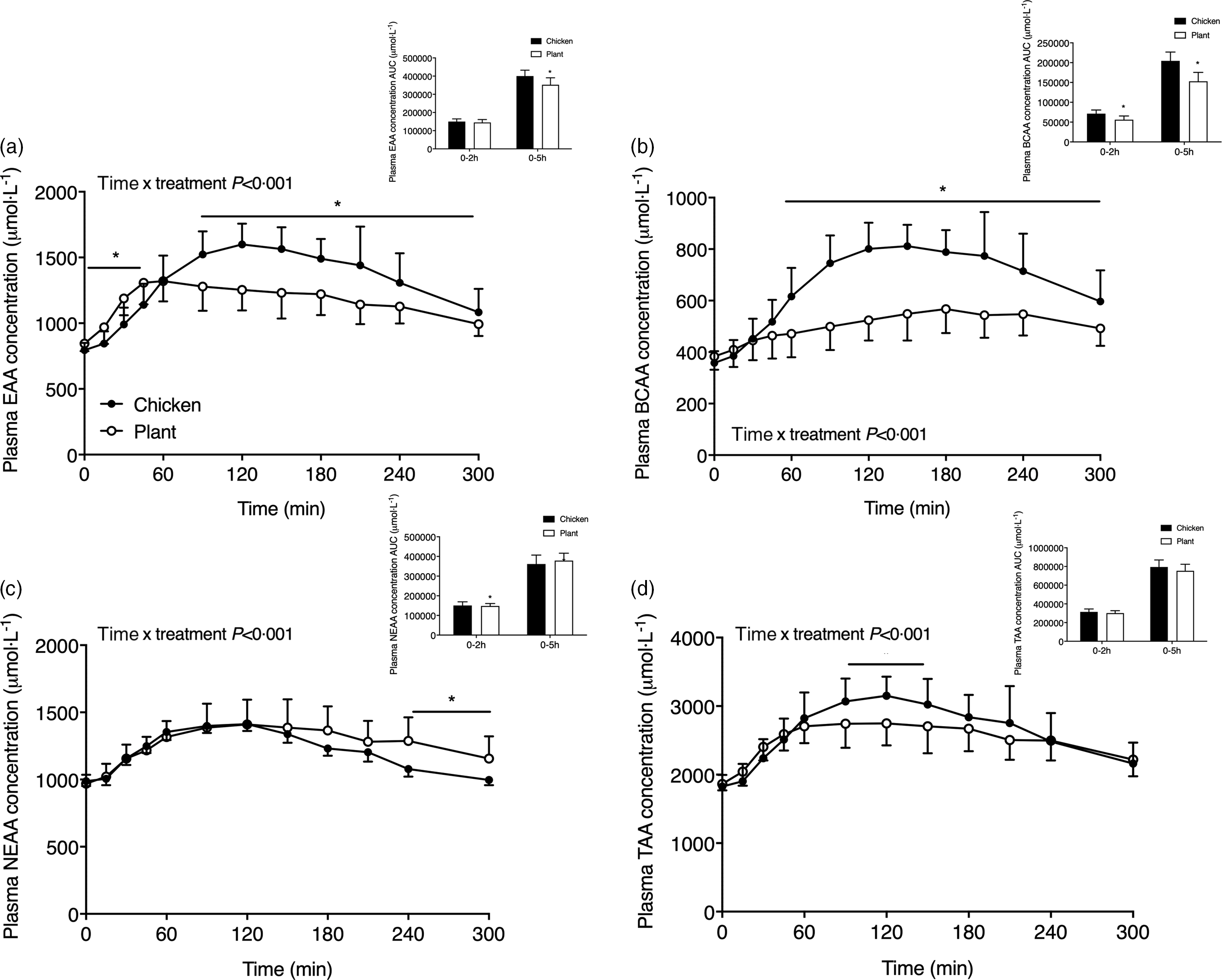
Fig. 3. Sum of plasma essential amino acids (a), branched-chain amino acids (b), non-essential amino acids (c) and the sum of all amino acids (d) in twenty-four healthy, young men following the ingestion of 40 g of protein of either a lysine-enriched, wheat and chickpea protein product (Plant; n = 12) or chicken breast fillet (Chicken; n = 12). Values represent means and standard deviation. Insets represent AUC. Data were analysed by repeated-measures (time × treatment) ANOVA. Bonferroni post hoc test was used to locate differences over time. (a) Time × treatment interaction, P < 0·001. (b) Time × treatment interaction, P < 0·001. (c) Time × treatment interaction, P < 0·001. (d) Time × treatment interaction, P < 0·001. *A significant difference between treatments, P < 0·05. EAA, essential amino acids; BCAA, branched-chain amino acids; NEAA, non-essential amino acids; TAA, sum of all amino acids.
The sum of specific subgroups of plasma amino acids is shown in Fig. 3. Plasma EAA (Fig. 3(a)) increased following protein ingestion in both interventions (time x treatment P < 0·001; time effect, both P < 0·001) and were higher following Plant when compared with Chicken from t = 0–45 min though conversely from t = 90–240 min (P < 0·05). The AUC of EAA did not differ during the 0–2 h postprandial period (P = 0·438) but was lower in Plant when compared with Chicken over the 5-h postprandial period (P < 0·01). Plasma branched-chain amino acids (Fig. 3(b)) were higher in Chicken when compared with Plant from 60 min after protein meal ingestion and throughout the remainder of the postprandial period time x treatment P < 0·001, post hoc all, P < 0·05). The AUC of the branched-chain amino acids was higher in Chicken when compared with Plant in both the 0–2 h and 0–5 h phase (P < 0·05). Plasma non-essential amino acids (Fig. 3(c)) differed between treatments (time x treatment P < 0·05) and were higher in Plant when compared with Chicken from t = 240–300 min following meal ingestion (all, P < 0·05). The sum of all amino acids Fig. 3(d)) differed between interventions (time x treatment P < 0·05) and were higher in Chicken when compared with Plant from t = 90–150 min following meal ingestion (all, P < 0·05). The AUC of non-essential amino acids and sum of all amino acids did not differ between interventions (both, P > 0·05). Individual plasma amino acid concentrations of alanine, arginine, asparagine, aspartic acid, β-alanine, cystine, glutamic acid, glycine, histidine, isoleucine, ornithine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine and valine are presented in Supplemental Figs. 2 and 3.
Muscle protein synthesis rates
Prior to ingestion of the meal, plasma l-(ring-13C6)-phenylalanine weighted mean enrichments averaged 6·6 ± 0·6 MPE in Chicken and 6·7 ± 0·4 MPE in Plant with no differences between interventions (P = 0·940; Supplemental Fig. 4). Plasma l-(ring-13C6)-phenylalanine enrichments increased directly following the ingestion of the protein meal (main time effect, P < 0·001) but returned rapidly to baseline steady-state levels. Postprandial plasma l-(ring-13C6)-phenylalanine weighted means averaged 6·5 ± 0·7 MPE in Chicken and 6·6 ± 0·4 in Plant, with no differences between interventions (time x treatment P = 0·323).
Mixed muscle protein synthesis rates are shown in Fig. 4. Basal muscle protein synthesis rates did not differ between interventions (Chicken: 0·031 ± 0·013 % h−1 and Plant: 0·031 ± 0·011 % h−1, P = 0·884). Muscle protein synthesis rates increased from the basal to the 0–5 h postprandial period (0·056 ± 0·015 % h−1 in Chicken and 0·046 ± 0·010 % h−1 in Plant; main time effect, P < 0·001; Fig. 4(a)) but did not differ between treatments (time x treatment P = 0·068, main treatment effect, P = 0·369). Similarly, postprandial FSR in the early, 0–2 h (0·057 ± 0·021 % h−1 in Chicken and 0·048 ± 0·016 % h−1 in Plant) and late, 2–5 h postprandial period (0·052 ± 0·023 % h−1 in Chicken and 0·044 ± 0·027 % h−1 in Plant) increased when compared with basal rates (main time effect, P < 0·001), with no differences between treatments (time x treatment P = 0·562, main treatment effect P = 0·261; Fig. 4(b)).
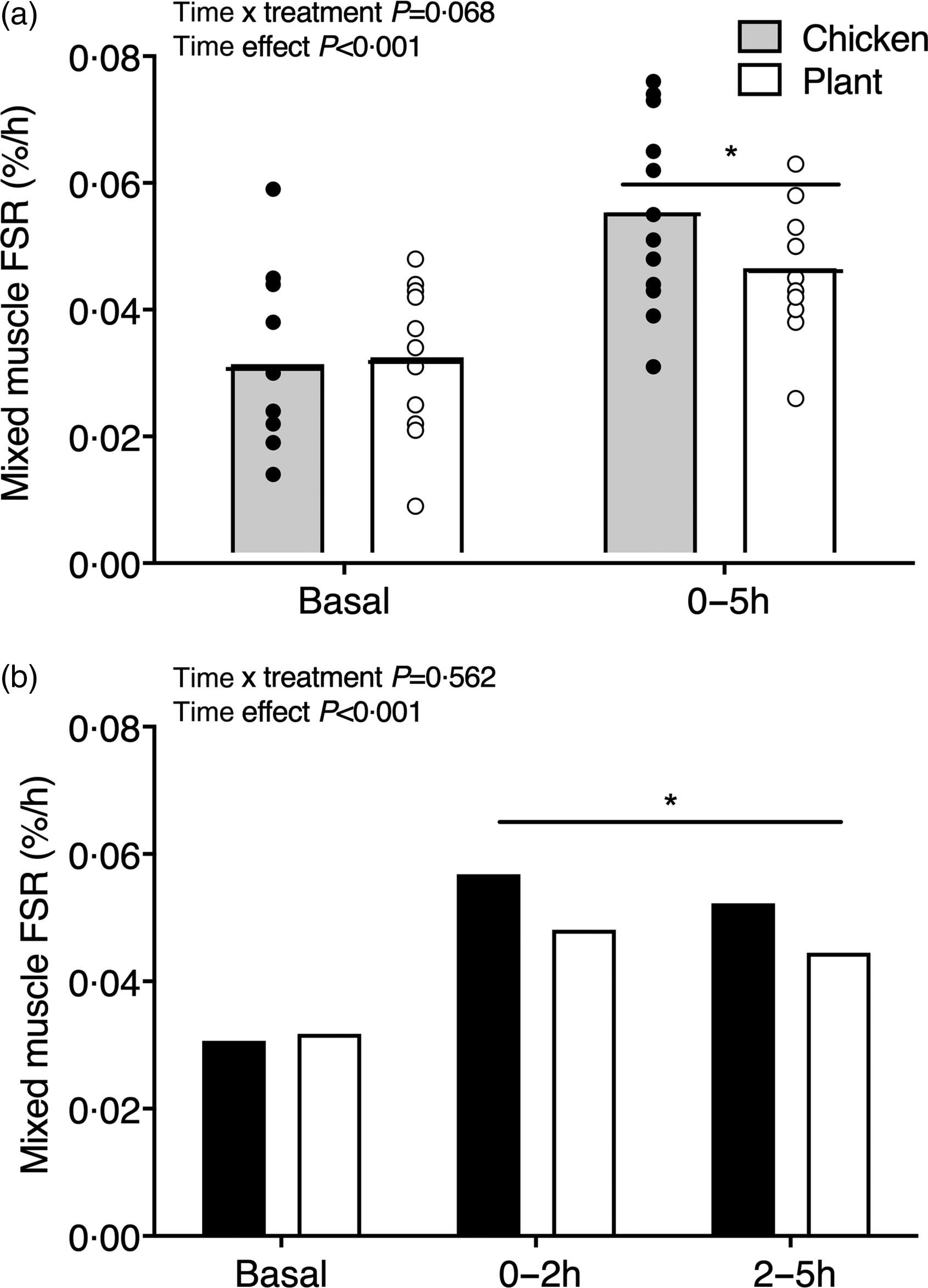
Fig. 4. Mixed muscle protein fractional synthesis rates (%·h−1) during the basal and 0–5 h postprandial period (a) and the early (0–2 h) and late (2–5 h) postprandial period (b), using intravenous L-(ring-13C6)-phenylalanine infusions in twenty-four healthy, young men following the ingestion of 40 g of protein of either a lysine-enriched, wheat and chickpea protein product (Plant; n = 12) or chicken breast fillet (Chicken; n = 12). Bars are means and dots represent individual values. Data were analysed with unpaired Student’s t test (between treatments) and repeated-measures (time × treatment) ANOVA. (a) Time × treatment interaction P = 0·068; main time effect, P < 0·001, main treatment effect, P = 0·369. (b) Basal between treatments, P = 0·884; time × treatment interaction, P = 0·562, main time effect, P = 0·006, main treatment effect, P = 0·261. FSR, fractional synthesis rates.
Muscle protein signalling
Key anabolic muscle signalling proteins are shown in Fig. 5. No differences over time or between groups were observed in phosphorylation status of mammalian target of rapamycin (mTORSer2448), p70 ribosomal protein S6 kinase (p70S6kThr389), ribosomal protein S6 (rS6Ser235/236) and eukaryotic initiation factor 4E binding protein-1 (4E-BP1Thr37/46) at 2 and 5 h after protein ingestion (P > 0·05).
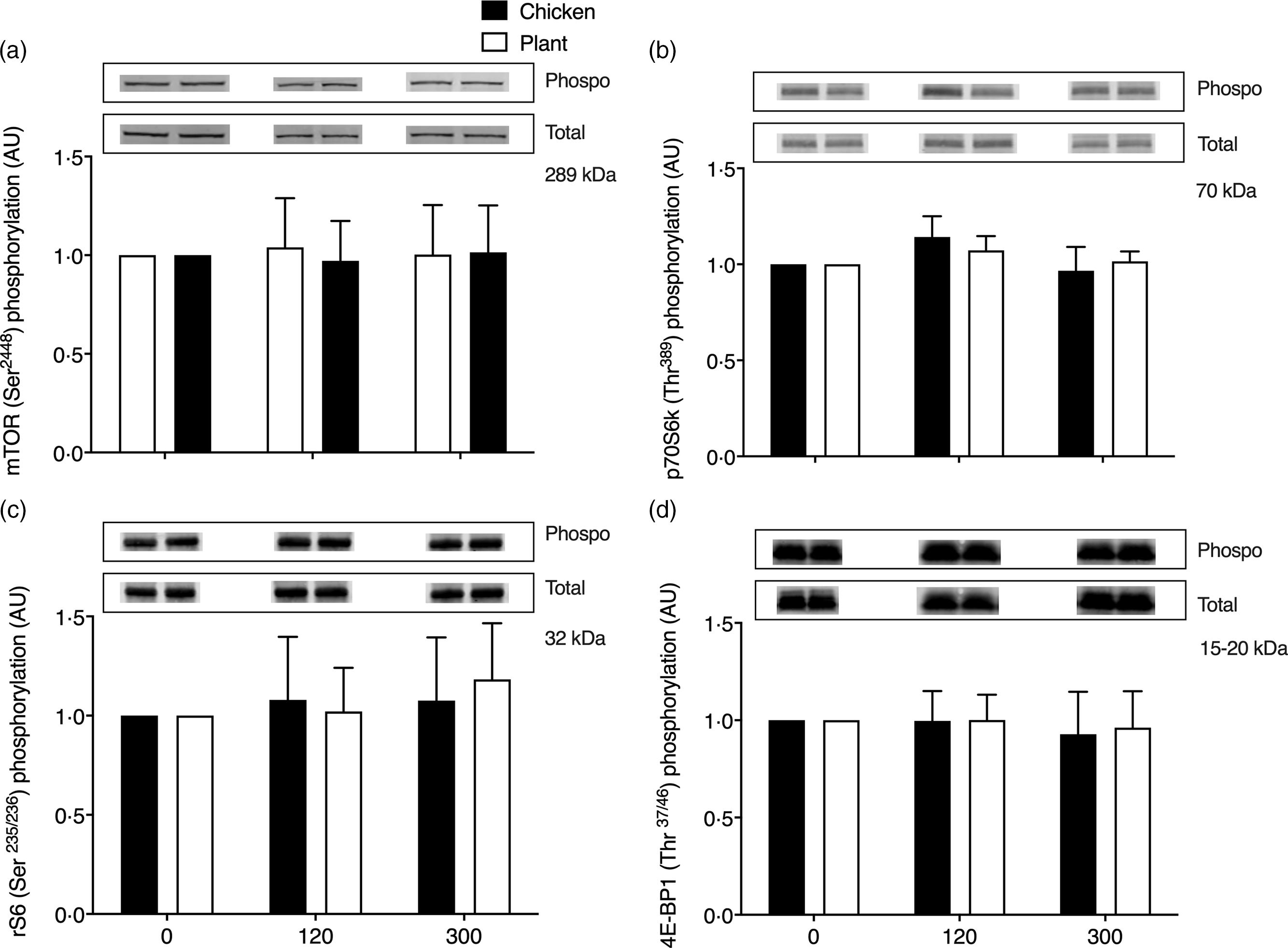
Fig. 5. Muscle protein expression (ratio between phosphorylated/total protein content) of mammalian target of rapamycin (mTORSer2448; (a)), phosphorylation of p70 ribosomal protein S6 kinase (p70S6kThr389; (b)), ribosomal protein S6 (rS6Ser235/236; (c)) and eukaryotic initiation factor 4E binding protein-1 (4E-BP1Thr37/46; (d)) in twenty-four healthy, young men in the post-absorptive state (0) and 120 and 300 min following the ingestion of 40 g of protein of either a lysine-enriched, wheat and chickpea protein product (Plant; n = 12) or chicken breast fillet (Chicken; n = 12) with representative blots for phosphorylated and total protein expression of each protein. Values represent means and standard deviation. Data were analysed with unpaired Student’s t test (between treatments) and repeated-measures (time × treatment) ANOVA. No significant main effects were detected. AU, arbitrary units.
Discussion
In the present study, we compared the muscle protein synthetic response following the ingestion of 40 g of protein in the form of a lysine-enriched, wheat and chickpea protein-based product with the ingestion of an isonitrogenous amount of chicken in healthy, young men. The ingestion of an ample amount of both the plant-based protein product and an isonitrogenous amount of chicken strongly increased postprandial muscle protein synthesis rates when compared with post-absorptive muscle protein synthesis rates, with no differences observed between the protein sources.
The interest in plant-based products as alternative protein sources is increasing worldwide due to their proposed contribution to better health and greater sustainability(Reference Willett, Rockström and Loken13). However, it is generally reported that plant-based proteins have lesser anabolic properties when compared with animal-based proteins. This has been attributed to the lower EAA contents (leucine in particular) and deficiencies in specific amino acids (lysine and methionine) in various plant-based proteins. The combination of different plant-based protein sources and the fortification with deficient free amino acids have been suggested as effective strategies to increase the anabolic properties of plant-based protein sources. Such plant-based protein food products, aiming to replace meat or poultry, are becoming increasingly popular. However, their capacity to stimulate postprandial muscle protein synthesis has never been investigated. Therefore, in the present study, we assessed postprandial muscle protein synthesis rates following ingestion of an ample, 40 g amount of protein, provided in the form of 230 g of baked meat substitute (composed of a lysine-enriched blend of wheat and chickpea protein) or 174 g of baked chicken breast fillet. The products were matched for the amount of protein ingested and, as such, differed in carbohydrate, fat and total energy content (Plant: 559 kJ and Chicken: 461 kJ per serving). To our knowledge, this is the first study to compare postprandial protein handling following ingestion of a whole-food plant-based protein product with an equivalent amount of protein derived from animal-based origin.
Following protein ingestion, greater increases in plasma glucose and insulin concentrations were observed following consumption of the plant-based product when compared with an isonitrogenous amount of chicken (Fig. 1). Plasma EAA and branched-chain amino acid concentrations increased to a greater extent following ingestion of the 40 g of protein as chicken (Fig. 3), with higher postprandial plasma leucine and methionine concentrations when compared with the ingestion of the plant-based protein source (Fig. 2). To compensate for any potential limiting effect of the low lysine and EAA content of wheat and chickpea protein(Reference Gorissen, Crombag and Senden15), the plant-based protein product was fortified with free lysine at about 200 % of the recommended levels of the FAO/WHO ((18); Table 3) and as such suitable for consumers as a meat substitute. As a result, postprandial plasma lysine concentrations further increased following the ingestion of Plant when compared with Chicken. No differences between interventions were observed in postprandial non-essential amino acid concentrations or the sum of all amino acids when assessed over the entire 5-h postprandial period (Fig. 3). As such, despite that the products were protein-matched and had similar EAA contents (about 28 g/100 g, Table 2), the higher energy content and the greater amount of fat and carbohydrate of the plant-based protein product likely attenuated protein digestion and amino acid absorption contributing to the attenuated postprandial rise in plasma amino acid availability when compared with the ingestion of the isonitrogenous amount of baked chicken(Reference Burd, Gorissen and Van Vliet10,Reference Gorissen, Burd and Hamer27–Reference Hamer, Wall and Kiskini30) .
The few studies that assessed the muscle protein synthetic response following plant-based protein ingestion have shown lower postprandial muscle protein synthesis rates when compared with animal-based proteins(Reference Gorissen, Horstman and Franssen6,Reference Wilkinson, Tarnopolsky and MacDonald7,Reference Phillips17,Reference Yang, Churchward-Venne and Burd18) . The lesser anabolic properties of plant-based protein isolates (such as wheat) compared with high-quality animal-based protein sources (such as milk) may be compensated for by ingesting more protein(Reference Gorissen, Horstman and Franssen6). However, simply increasing the protein dose may not always be a feasible and practical strategy to increase the anabolic properties of a plant-based protein meal, since this would further increase both the volume and the energy content of a meal. In the present study, ingestion of 40 g of protein in the form of the plant-based protein product increased muscle protein synthesis rates by about 68 % when compared with post-absorptive muscle protein synthesis rates (Fig. 4(a); P < 0·001). Clearly, a measurable increase in muscle protein synthesis rates can be observed following the consumption of an ample amount of a plant protein-based meat substitute. The selected plant-based meat alternative was produced using a blend of wheat and chickpea protein isolates and further fortified with lysine to achieve levels recommended by the FAO/WHO(Reference Yang, Churchward-Venne and Burd18); Table 3). Whether the lysine fortification was required to support the postprandial increase in muscle protein synthesis cannot be derived from this study design. More work will be needed to assess whether plant-derived protein blends with or without (free) amino acid fortification are required to allow significant increases in muscle protein synthesis rates following ingestion of more moderate amounts and different compositions of plant-based protein products as well as more complete, mixed meals.
To allow a comparison of the postprandial muscle protein synthetic response to the ingestion of a plant-based meat substitute with a high-quality animal-based protein source, we included a control trial in which we provided young individuals with an isonitrogenous amount of chicken. Despite the greater postprandial rise in plasma EAA, and leucine in particular, following the ingestion of chicken, we observed no significant differences in postprandial muscle protein synthesis rates assessed over the 0-5-h between the interventions (Fig. 4(a); time x treatment P = 0·068, main treatment effect P = 0·369). Though we did observe a trend of a greater overall response in muscle protein synthesis rates in the Chicken treatment, this trend was no longer present when we assessed the postprandial muscle protein synthetic response in the early (0–2 h) and late (2–5 h) postprandial phase (Fig. 4(b); time x treatment P = 0·237 and 0·394, respectively). In line, we did not detect any substantial differences in myocellular anabolic signalling following ingestion of the plant-based protein product and chicken (P > 0·05, Fig. 5). No detectable rise in phosphorylation status of mTOR, p70S6k, rS6 and 4E-BP1 was observed 2 and 5 h after protein ingestion in either treatment. These findings may seem inconsistent to the observed substantial postprandial rise in muscle protein synthesis rates. However, it should be noted that signalling responses merely provide snapshot measurements in time and do not necessarily serve as a proxy for the rise in muscle protein synthesis rates. It is likely that transient differences in anabolic signalling occurred prior to the biopsy collection at 2 h following protein ingestion. Nevertheless, these data clearly show that the ingestion of an ample amount of plant-based meat substitute has the capacity to stimulate muscle protein synthesis to an extent similar to the ingestion of an equivalent amount of animal-based protein source. It is evident that more work will be required to define the factors, such as the amount of protein consumed, that may contribute to the presence or absence of differences in the postprandial muscle protein synthetic response to the ingestion of plant v. animal-based protein foods. Relevant factors will likely include the dose(Reference Witard, Jackman and Breen1,Reference Pennings, Groen and de Lange3,Reference Gorissen, Horstman and Franssen6,Reference Yang, Breen and Burd31) , protein source(Reference Moore, Robinson and Fry2,Reference Tang, Moore and Kujbida5–Reference Wilkinson, Tarnopolsky and MacDonald7,Reference Yang, Churchward-Venne and Burd18) , matrix of the food(Reference Churchward-Venne, Snijders and Linkens9–Reference van Vliet, Shy and Abou Sawan11), food processing(Reference Barbe, Ménard and Le Gouar32,Reference Nyakayiru, van Lieshout and Trommelen33) and preparation of the foods(Reference Pennings, Groen and van Dijk34–Reference Remond, Machebeuf and Yven36), as well as the population consuming these products(Reference Wall, Gorissen and Pennings37).
There is a growing popularity and accessibility of plant-based protein sources, and the consumption of plant-based proteins has increased with campaigns, such as ‘Meatless Mondays’ and ‘flexitarianism’ that are advocating a more plant-based diet(Reference Rose, Heller and Roberto38–Reference Lemken, Spiller and Schulze-Ehlers40). As a response, the industry has been investing in the development and production of a growing range of plant-based protein food products(Reference Willett, Rockström and Loken13,Reference Curtain and Grafenauer41) . Previous studies that assessed muscle protein synthesis rates following the ingestion of plant-based protein sources have generally been limited to the ingestion of protein isolates derived from soy(Reference Tang, Moore and Kujbida5,Reference Wilkinson, Tarnopolsky and MacDonald7,Reference Phillips17,Reference Yang, Churchward-Venne and Burd18) , wheat(Reference Gorissen, Horstman and Franssen6), or blends of casein, whey and soy(Reference Reidy, Walker and Dickinson21–Reference Reidy, Walker and Dickinson23) in the form of a liquid protein drink. Moreover, commercially available plant-based protein products are naturally higher in carbohydrate and fat (and consequently energy content) when compared with animal-based protein sources and, therefore, might be less effective in their capacity to stimulate muscle protein synthesis. To date, studies assessing postprandial muscle protein synthesis rates following protein-containing whole-food products or meals are lacking. We(Reference Gorissen, Remond and van Loon42,Reference Trommelen, Betz and van Loon43) and others(Reference Vliet, Beals and Martinez44,Reference Gwin, Church and Wolfe45) have defined several dietary factors that can modulate protein digestion and amino acid absorption and the subsequent muscle protein synthetic response to protein ingestion. Such factors include the amount and type of protein, macro- and micronutrient composition of the meal, food density and meal composition, food texture, food matrix, food processing, food preparation and temperature (i.e. heating or cooling), and mastication. While most of these modifications seem to affect protein digestion and amino acid absorption kinetics(Reference Burd, Gorissen and Van Vliet10,Reference Kim, Shin and Schutzler29,Reference Pennings, Groen and van Dijk34) , their impact on postprandial muscle protein synthesis rates remains to be resolved(Reference Burd, Gorissen and Van Vliet10,Reference van Vliet, Shy and Abou Sawan11,Reference Kim, Shin and Schutzler29,Reference Pennings, Groen and van Dijk34) . We matched the meals for protein content and provided an ample amount that would be typically ingested during dinner. Here, we show that when an isonitrogenous amount of plant-based protein is consumed and the deficit of one specific amino acid is replaced, a plant-based meat replacement can be as effective as an animal-based protein source to stimulate muscle protein synthesis in healthy, young adults. Though the long-term effect of plant-based protein consumption on protein metabolism needs to be further explored; plant-based meat substitutes may be applied in a regular diet without compromising the capacity to support muscle mass maintenance in young individuals. Whether the fortification of plant-based meat substitutes with other specific amino acids is required to induce a proper anabolic response and how different plant-based protein blends can be combined to improve the amino acid profile and maximise the anabolic properties of plant-based meat replacers remains questions to be addressed in further studies.
In conclusion, the ingestion of an ample amount of lysine-enriched, plant-based protein product increases muscle protein synthesis rates in healthy, young men. The muscle protein synthetic response to the ingestion of an ample amount of protein (i.e. 40 g) of such a lysine-enriched, plant-derived protein blend does not differ from the ingestion of an isonitrogenous amount of chicken. Plant-based protein products sold as meat replacers may be as effective as animal-based protein sources to stimulate postprandial muscle protein synthesis rates in healthy, young individuals.
Acknowledgements
We greatly appreciate the assistance of Ino van der Heijden, Linda Mans, Michelle E.G. Weijzen, Lisanne H.P. Houben and Joey S.J. Smeets in the execution of the experiments. Technical expertise from Hasibe Aydeniz, Stefan H.M. Gorissen, Joy P.B. Goessens, Annemarie P. Gijssen, Henk Schierbeek and Wendy E. Sluijsmans during the sample analyses was greatly appreciated.
Financial support and food items for this research were obtained from Tereos (Marckolsheim, France). The project is organised by and executed under the auspices of TiFN (Wageningen, the Netherlands), a public–private partnership on precompetitive research in food and nutrition. The researchers are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The industrial partners within TiFN have contributed to the project through regular discussion and were involved in the study design. The funders had no role in data collection and analysis, decision to publish or preparation of the manuscript.
I. W. K. K., C. L. B., L. C. P. G. M. dG., L. V. and L. J. C. vL. formulated the research question and designed the study. I. W. K. K., P. J. M. P. and T. S. conducted the experimental trials. J. M. X. vK. and A. H. Z. performed the blood and muscle analyses. I. W. K. K. performed the (statistical) data analysis and data interpretation and wrote the manuscript together with L. V. and L. J. Cv. L. I. W. K. K. and L. J. Cv. L. had primary responsibility for final content. All authors read and approved the final content of the manuscript.
C. L. B. is employee of Tereos. L. J. Cv. L. has received research grants, consulting fees, speaking honoraria or a combination of these, from Friesland Campina, Tereos and Nutricia Research. I. W. K. K. and L. V. have received speaking honoraria from Nutricia Research. None of the other authors have disclosed any conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521004906











