There is substantial epidemiological evidence (at least fifty studies(Reference Lee1)) to suggest that being physically active can reduce the risk of developing cancer, particularly of the breast and colon(Reference McTiernan, Ulrich and Slate2). Indeed, it has been estimated that high levels of physical activity may reduce the risk of colon cancer by up to 50 %(Reference Stein and Colditz3). However, physical activity may not help those with a family history of colonic adenomatous polyps(Reference Slattery, Levin and Ma4) or those who have had at least one polyp removed, as physical activity had no effect on polyp recurrence in the Polyp Prevention Trial(Reference Colbert, Lanza and Ballard-Barbash5). There may be sex differences in response to physical activity since there was an inverse association between physical activity and polyp recurrence in men only in the Calcium Polyp Prevention Study(Reference Wallace, Baron and Karagas6).
A limitation of such observational studies is that they demonstrate associations, but cannot prove causality, between physical activity and colon cancer, and there may be other aspects of lifestyle associated with physical activity (such as diet(7)) that are responsible for the protection against colon cancer. In addition, because physical activity is a complex and heterogeneous behaviour, it is difficult to characterise and quantify, and this imprecision in measurement of exposure exacerbates the difficulty in assessing observational data.
The amount or types of physical activity which may reduce risk of bowel cancer are poorly understood. Human epidemiological studies have used relatively crude instruments to quantify physical activity, including duration and intensity of leisure activity, occupational activity, activity in the past year and lifetime activity. Evidence of the benefit (or otherwise) of specific forms of exercise is obtained more readily from experiments in animal models (as reviewed by Basterfield et al. (Reference Basterfield, Reul and Mathers8)). Studies using carcinogen-treated rats have been unanimous in finding reduced colon cancer with both voluntary (wheel running) and forced (treadmill running) exercise(Reference Thorling, Jacobsen and Overvad9–Reference Reddy, Sugie and Lowenfels13). In contrast, the outcomes of studies of exercise in Min mice, which carry a truncating mutation in the Apc gene and develop multiple intestinal tumours spontaneously(Reference Moser, Pitot and Dove14), have been mixed with only a few studies showing protection against tumourigenesis in some circumstances (Reference Colbert, Davis and Essig15–Reference Baltgalvis, Berger and Pena20). The reasons for this heterogeneity in response are not known, but may be related to the use of different sexes, exercise protocols and/or diets. The type of physical activity (treadmill or wheel running), and how much, that protects against intestinal tumourigenesis in Min mice remains unclear, and the mechanisms responsible for protection remain to be discovered.
Poor sleep quality is associated with the metabolic syndrome(Reference Jennings, Muldoon and Hall21) and obesity(Reference Vorona, Winn and Babineau22), and obesity is known to be a risk factor for cancer(7). Interest in the association between sleep and cancer is developing(Reference Blask23), although results so far are conflicting(Reference Verkasalo, Lillberg and Stevens24–Reference Pinheiro, Schernhammer and Tworoger28). To date, the relationship between sleep and intestinal cancer risk has not been investigated using an animal model of tumourigenesis. The SCFA butyrate is another potentially protective factor which is under-investigated in exercise studies. SCFA are produced by the bacterial fermentation of carbohydrates in the large intestine, and butyrate is used as fuel by colonocytes(Reference Csordas and Hill29). The anti-inflammatory and anti-neoplastic properties of butyrate are well established(Reference Williams, Coxhead and Mathers30), but its potential role as a mediator of the effects of physical activity has not been investigated.
The primary purpose of the present study was to compare the effects of forced (treadmill running) and voluntary (wheel running) modes of exercise on tumourigenesis in Min mice. To maximise the potential for translation of the study outcomes to human health, the mice were fed a Western-style, high-fat diet. We hypothesised that mice on a wheel running exercise protocol would undertake a greater amount of physical activity than their treadmill-running counterparts, and so would have fewer, and smaller, tumours than either treadmill-running or unexercised control mice. Furthermore, we explored two novel protective mechanisms, namely increased butyrate proportion in the large bowel and, in a subset of mice, quantity of non-exercise physical activity and sleep.
Experimental methods
Animals
All procedures complied with current UK government Home Office legislation, and were approved by the Newcastle University Ethics Committee. Female C57BL/6 mice (Harlan, Oxon, UK) were mated with male C57BL/6Min+/ − mice (Min mice) from the established Newcastle University colony, and offspring were genotyped at 4 weeks of age using DNA from tail snips according to established methods(Reference Su, Kinzler and Vogelstein31). For three separate studies, 155 Min mice (eighty-seven females and sixty-eight males) were available and randomised to one of three treatment groups at approximately 5 weeks of age: treadmill (TR) thirty-one females and twenty-nine males, wheel (WH) twenty-six females and seventeen males, or control (CON) thirty females and twenty-two males. TR mice were housed 1–6 in a cage, and were allowed to run on a treadmill (Exer-3/6 rodent treadmill, Columbus Instruments, OH, USA) for 5 d/week at 18–21 m/min for 30–60 min on a 5 % gradient for 10–12 weeks. The first 2 weeks were used to train the mice to run on the treadmill, and during this period, the speed and duration of running were increased gradually. WH mice were housed individually in a cage containing a running wheel to which they had free access. The running wheel had an internal diameter of 10 cm for the first 5 weeks and then changed to a 13 cm internal diameter wheel for the final 5 weeks. Numbers of wheel revolutions were recorded by a magnetically actuated counter. CON mice were housed 1–6 in a cage, and were removed to individual ‘control lanes’ (no imposed exercise), while the TR mice were on the treadmill. All mice were housed in the same room as the treadmill under a 12 h light–dark cycle, and were offered 6 g/d of a non-commercial ‘Western’-style(Reference Williamson, Kartheuser and Coaker32), high-fat diet containing (g/kg) casein high nitrogen 279·49, lard 250, maize flour 250, sucrose 148, mineral mix AIN-93-G-MX 35, gelatine 20, vitamin mix AIN-93-VX 10, l-cystine 3, choline bitartrate 2·5, Cr2O3 2 and t-butylhydroquinone 0·014. Due to its high fat content, this diet had a ‘paste-like’ consistency, which minimised scattering by the mice and facilitated the collection of uneaten food. Food refusals were weighed daily. Body weights were recorded at the beginning of each week.
Non-exercise physical activity and quantification of sleep
An IR-sensing device (‘Inframot’, TSE Systems, Bad Homburg, Germany) was used to quantify cage activity in a subsample of singly housed mice, seven TR (five males and two females) and five CON (three males and two females). Inframot detects movements of 5 ms length and greater, and stores the data accumulated in set time periods as ‘counts’. Each mouse was assessed for 23 h (i.e. when not on the treadmill/control lanes) to provide information on the total amount of activity and the activity during the light and dark phases. Activity was accumulated in 10 min bouts. Time spent sleeping was determined according to the strict criterion of zero movement counts recorded in each 10 min recording epoch(Reference Basterfield, Lumley and Mathers33).
Blood and tissue sampling
Mice were killed after 10–12 weeks of treatment. Anaesthesia was induced using 3 % isoflurane in oxygen followed by cardiac exsanguination and cervical dislocation. Blood was collected into EDTA for haematocrit measurement. The carcass was weighed, and a midline section was made. The intestinal organs and spleen were dissected out, weighed and examined for the presence of tumours by an observer blind to the treatment group. The small intestine was divided into equal-length proximal and distal portions and opened longitudinally. Size and site of all adenomas were recorded. The colon contents were mixed with de-proteinising solution (containing 100 g/350 ml H2O metaphosphoric acid and 50 mm 3-methyl valeric acid(Reference Mathers and Fotso Tagny34)) in preparation for SCFA determination by GC. Carcasses were freeze-dried, and body fat was measured gravimetrically after extraction by petroleum diethyl ether using the Soxhlet procedure.
Statistical methods
Data were tested for normality, and any data that were not normally distributed were transformed. Normally distributed and transformed data were investigated for effect of exercise using the general linear model of ANOVA, with sex included as a covariate. Comparisons between treatment groups (TR v. CON and WH v. CON) were investigated using Dunnett's test. As data from three replicate studies were combined in this analysis, ‘study’ was included in the analysis as a fixed factor. Normally distributed data are presented as mean values with their standard errors. The results for transformed data are summarised as geometric means and 95 % CI. The data for colon tumours and small tumours ( ≤ 1 mm diameter) were tested using the non-parametric Kruskal–Wallis test, and are presented as medians and ranges. The significance level was set at P ≤ 0·05.
Results
Seven female mice and twelve male mice did not complete the initial 2-week training period on the treadmill satisfactorily, and were removed from the study, as per previous investigations(Reference Colbert, Mai and Perkins16, Reference Mehl, Davis and Clements17), leaving twenty-four female and seventeen male mice in the TR treatment group. A higher proportion of female (18/24) than of male (8/17) mice ran on every possible day (P = 0·048). There was no difference between the sexes in the median time spent on the treadmill in each session. As they completed the majority of the exercise protocol (twenty-six mice ran on all days, nine ran between 81 and 99 % of days, and six ran between 68 and 80 % of days), results from all these mice are included in this analysis (intention to treat analysis). During the 10-week study, female WH mice ran approximately twice as far (P = 0·002) in their wheels as did males, and the mean distances run were 276 (sem 26·0) v. 136 (sem 32·1) km, which equate to 3·97 and 1·92 km/d for female and male mice, respectively.
WH mice consumed significantly (P = 0·015) more food than CON mice (Table 1) whose intake was similar to that of TR mice. There was no difference between treatment groups for either carcass weight, weight gained over the study or body composition (Table 1). Weekly weight gain was similar for each treatment group, except at week 6 when CON mice gained 0·5 g more than both TR and WH mice (P = 0·017 for TR v. CON, P = 0·021 WH v. CON).
Table 1 Food intake, weight gain and percentage body fat of mice by exercise group
(Mean values with their standard errors and 95 % confidence intervals)

TR, treadmill running; WH, wheel running; CON, control (not exercised).
Total numbers of intestinal tumours were very similar for WH, CON and TR mice (Table 2), but TR mice had significantly (P = 0·042) fewer larger ( ≥ 2 mm) tumours than CON mice. TR mice showed a trend for fewer tumours in all sections of the intestine, but, with the exception of the colon (P = 0·049), the differences between TR and CON mice were NS (P>0·05) when analysed by anatomical site. For the WH mice, there was no correlation between distance run in wheels in 10 weeks and the total number of tumours (r − 0·096, P = 0·539), or the tumour burden (the sum of tumour diameters, r − 0·120, P = 0·444). Combined data showed a negative correlation between weight gained over the study and total tumour number (r − 0·372, P < 0·001).
Table 2 Tumour numbers and anatomical distribution of tumours in Min mice by exercise group
(Mean values and 95 % confidence intervals)

TR, treadmill running; WH, wheel running; CON, control (not exercised); SI, small intestine.
* Median and range.
† Sum of all tumour diameters.
The haematocrits of TR (29·6 (sem 1·32) %) and WH (28·4 (sem 1·49) %) mice were very similar, and both were significantly higher than that of CON (24·5 (sem 1·28) %) mice (P = 0·009 and 0·040, respectively). The spleens of the two exercising groups were significantly lighter (TR 197 mg, WH 247 mg) than those of the CON mice (334 mg; P = 0·007 and 0·055 for comparisons of TR and WH with CON, respectively). There was a strong inverse correlation (r − 0·580, P < 0·001) between spleen weight and haematocrit (Fig. 1), and spleen weight was positively correlated with tumour number (r 0·373, P = 0·002).
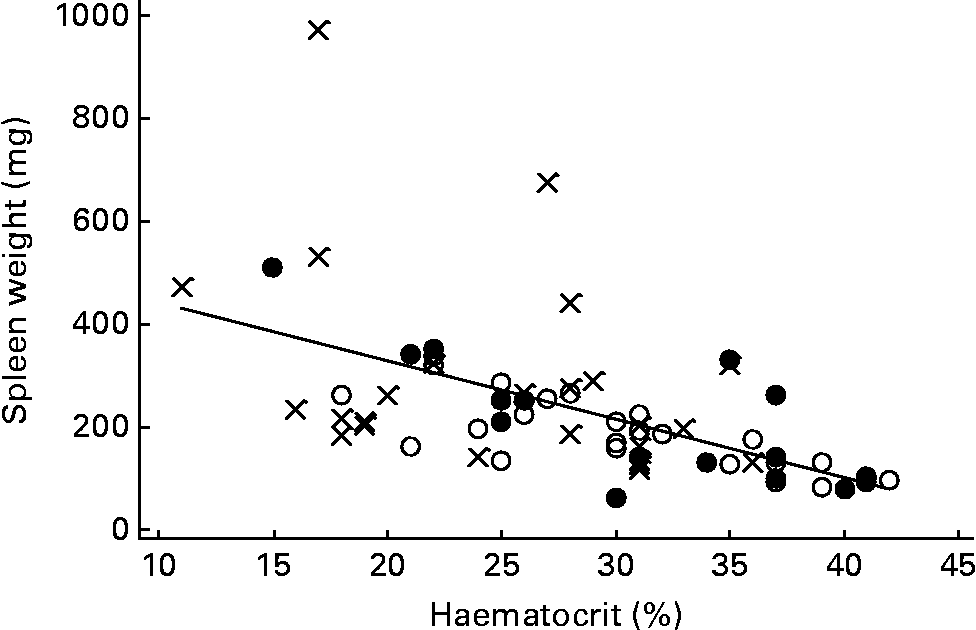
Fig. 1 Negative correlation (P < 0·001) between spleen weight and haematocrit in Min mice. Spleen weight = 554 (se 58) − 11 (se 2) × haematocrit. ●, Treadmill running (TR) mice; ○ wheel (WH) running mice, × , control (CON) or no exercise mice.
The type of exercise had a significant effect on the pattern of colonic SCFA (Table 3). For TR mice, the reduction in acetate molar proportion approached significance compared with the CON mice (P = 0·067), whereas butyrate molar proportion was increased by 50 % compared with the CON mice (P = 0·030). For WH mice, propionate was increased significantly (P = 0·019) compared with the CON mice. This observation of differential effects of exercise regimen on colonic SCFA pattern highlights an area of ignorance about the impact of physical activity on intestinal function. It may be relevant to note that treadmill running was restricted to the light phase of the day, whereas the majority of the wheel running occurred in the dark phase. Given the diurnal patterns of food consumption by mice that were fed ad libitum (most food is eaten in the dark phase of the day), there may be some interaction between timing of food ingestion, exercise and large bowel bacterial activity. When the treatment groups were combined, there was a negative correlation (r − 0·174, P = 0·061) between butyrate molar proportion and total tumour number.
Table 3 Effect of treadmill (TR) and wheel (WH) running on molar proportions (mmol/mol) of SCFA in the colon of Min mice compared with the control (CON) animals
(Mean values and 95 % confidence intervals)
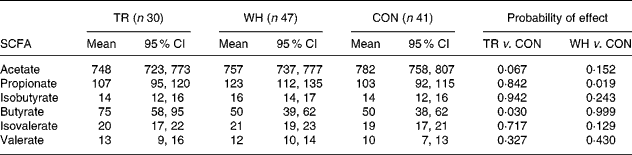
TR, treadmill running; WH, wheel running; CON, control (not exercised).
Non-exercise physical activity was measured in a subsample of twelve mice (seven TR and five CON), with a mean of five separate 23 h continuous recordings per mouse. With this subsample of mice, there was no significant (P>0·05) difference in tumour number or tumour burden between the groups, but the TR mice tended to have fewer tumours (12·1 (sem 4·3) for TR and 24·1 (sem 5·0) for CON, P = 0·104) in parallel with the findings of the main study. During the 23 h per day when they were in their home cage, there was a trend for TR mice to be more active than CON mice (1377 v. 757 × 103 counts, P = 0·084), with this difference in activity being confined to the dark period. There was no difference in total sleep time between the groups (P = 0·125), or in sleep during the dark (P = 0·111) or light (P = 0·793) phase. However, there were negative correlations between total activity and tumour burden (Pearson's r − 0·559, P = 0·059, Fig. 2(a)), total activity and tumour number (r − 0·558, P = 0·059, Fig. 2(b)), and dark activity and tumour burden (r − 0·559, P = 0·040). There were significant positive correlations between dark sleep and tumour burden (r 0·835, P = 0·001, Fig. 2(c)), total sleep time and tumour number (r 0·872, P < 0·001, Fig. 2(d)), and total sleep and tumour burden (r 0·841, P = 0·001). There was no correlation between activity and final body mass.

Fig. 2 Relationships between 23 h activity and (a) tumour burden (r − 0·559, P = 0·059) and (b) tumour number (r − 0·558, P = 0·059), and between (c) dark sleep (sleep during the dark period) and tumour burden (r 0·835, P = 0·001) and (d) total sleep and tumour number (r 0·872, P < 0·001) in a subsample of Min mice. Symbols refer to individual mice.
Discussion
The present study adds to the body of work on the effects of exercise on tumourigenesis in Min mice by demonstrating a small reduction in larger tumours (P = 0·042) and in colon tumours (P = 0·049) following treadmill running. We report the novel observation of altered SCFA proportions in the colon, in particular, an increase in the anti-neoplastic butyrate, in response to treadmill running. Furthermore, we provide preliminary evidence that time spent sleeping is correlated strongly with both tumour number and tumour burden.
The aim of the present study was to help resolve controversies arising from the six previously published studies of the effect of exercise on tumourigenesis in Min mice, which have reported conflicting results (see Table 4 for summary). In contrast with the heterogeneity of outcomes from Min mouse studies, there were consistently protective effects of both voluntary and involuntary forms of exercise in previous experiments with carcinogen-treated rats(Reference Thorling, Jacobsen and Overvad9–Reference Reddy, Sugie and Lowenfels13). There is no readily apparent reason for the inconsistent effects of exercise in the Min mouse studies, but differences in experimental protocols between the studies, e.g. the ages at the start of the procedures, the length of the study and the number of tumours generated per mouse, may help explain the differing results (Table 4). In the rat studies where a protective effect of exercise was apparent, only males were used, whereas both male and female Min mice have been used in most studies. There may be interactions between sex and environmental factors on tumourigenesis in Min mice since McKay et al. (Reference McKay, Williams and Mathers35) observed more tumours in females than in males, and this difference and total tumour numbers were reduced by feeding a low-folate diet.
Table 4 Comparison of published studies of the effects of exercise on intestinal neoplasia in Min mice*

SI, small intestine.
* Adapted from Basterfield et al. (Reference Basterfield, Reul and Mathers8).
† Exact data not published.
The optimum mode of exercise (forced treadmill running or voluntary wheel running) to reduce tumourigenesis in Min mice has not been established yet. Although it seems counter-intuitive, treadmill running (which results in shorter running distances than wheel running) reduced tumour number in male Min mice, whereas wheel running had no effect in the study done by Mehl et al. (Reference Mehl, Davis and Clements17). This mode of exercise difference was confirmed in the present study where tumours were smaller and numbers were lower in all sections of the intestine of TR mice but not of WH mice (Table 2).
Increased adiposity is a risk factor for cancer(7), and energetic restriction studies (which reduce body mass and adiposity) decrease tumour risk in human subjects(Reference Spindler36) and Min mice(Reference Mai, Colbert and Berrigan37). Therefore, if exercise lowers body mass it may also lead to a reduction in tumour load. Four of five carcinogen-treated rat studies(Reference Thorling, Jacobsen and Overvad9–Reference Andrianopoulos, Nelson and Bombeck12) reported that exercise decreased body weight, and a similar effect was observed in two Min mice studies(Reference Colbert, Mai and Perkins16, Reference Colbert, Mai and Tooze18) (one of which found an inverse relationship between polyp number and percentage body fat(Reference Colbert, Mai and Tooze18)), but not in the other two studies, neither of which reports any associations between body weight and tumour multiplicity(Reference Colbert, Davis and Essig15, Reference Mehl, Davis and Clements17). Ju et al. (Reference Ju, Nolan and Cheh19) reported a reduction in body fat but not in body mass with wheel running exercise in female Min mice, and Baltgalvis et al. (Reference Baltgalvis, Berger and Pena20) did not observe a reduction in either body mass or fat pad mass with exercise. In the present study, we did not observe an effect of exercise on either final weight or body composition, in agreement with recent studies reporting no effect of wheel running on body mass or fat mass in female C57BL/6 mice(Reference Rogers, Berrigan and Zaharoff38). However, where adiposity is measured at the end of the studies of tumourigenesis in Min mice (i.e. all studies to date), outcomes should be interpreted with caution since cachexia accompanying intestinal tumourigenesis may have profound effects on food intake and body composition. Colbert et al. (Reference Colbert, Mai and Tooze18) observed that male wheel-running mice had higher levels of body fat, and suggested that this may indicate that WH mice were healthier than the CON mice which showed signs of cachexia (loss of muscle and fat tissue)(Reference Colbert, Mai and Tooze18, Reference Mehl, Davis and Berger39). A recent study(Reference Baltgalvis, Berger and Pena40) has suggested that the severest cachectic symptoms are observed in Min mice with the highest levels of circulating IL-6 and greatest tumour multiplicity. Although we did not find a significant correlation between tumour number and body fat (r − 0·214, P = 0·098), an observation also noted by Ju et al. (Reference Ju, Nolan and Cheh19) and Baltgalvis et al. (Reference Baltgalvis, Berger and Pena20), there was a significant negative correlation between total tumour number and weight gained over the study (P < 0·001).
Inter-study differences in tumour outcome with different modes of exercise may be explained by differential effects on the energy balance of the experimental mice. Most previous studies of effects of exercise in Min mice have used low-fat diets, but tumour number can be increased by feeding a higher fat diet(Reference Wasan, Novelli and Bee41). Our mice were given a high-fat diet that reflected human Western diets, as high-fat diets are associated with increased risk of colon cancer in human subjects(42). WH mice, but not TR mice, ate significantly more than CON mice, presumably to compensate for their extra energy expenditure. This greater energy intake by the WH mice may explain the lack of difference in body weights compared with CON mice. The provision of energy-dense food ad libitum in the present study may have enabled our exercising mice to match their greater energy outputs with higher energy intakes, so preventing any change in energy balance, and this may explain the more modest effects of exercise on tumourigenesis seen in the present study. In contrast, some previous studies have ‘pair-matched’ food intake of exercising mice with that of non-exercising controls to induce negative energy balance(Reference Colbert, Mai and Perkins16, Reference Colbert, Mai and Tooze18). Mehl et al. (Reference Mehl, Davis and Clements17) who fed their mice ad libitum also found reduced tumour numbers with only treadmill running, and reported increased food intake by WH-running mice. Baltgalvis et al. (Reference Baltgalvis, Berger and Pena20) showed that male TR mice on a low-fat diet had fewer tumours than controls, an effect that was negated by feeding a high-fat diet(Reference Baltgalvis, Berger and Pena20). Thus, changes in energy balance may be more important than energy intake per se in determining effects on intestinal tumourigenesis, and this area deserves further exploration.
Insulin-like growth factor 1 (IGF-1)(Reference Colbert, Mai and Perkins16, Reference Colbert, Mai and Tooze18), IL-6(Reference Mehl, Davis and Clements17), corticosterone(Reference Colbert, Mai and Perkins16, Reference Colbert, Mai and Tooze18), inflammation(Reference Mehl, Davis and Clements17, Reference Baltgalvis, Berger and Pena20) and leptin(Reference Colbert, Mai and Perkins16, Reference Baltgalvis, Berger and Pena20) have been investigated as possible mechanisms or mediators for the protective effect of exercise, but with no clear results. In the present study, we investigated the possibility that exercise alters the exposure of intestinal epithelial cells (the cells from which tumours develop) to pro- or anti-neoplastic substrates. We focused on butyrate which is one of the most potent anti-neoplastic components of the intestinal milieu and which suppresses tumour cell proliferation, increases apoptosis, induces differentiation and enhances immunosurveillance(Reference Williams, Coxhead and Mathers30). Although several factors in food are known to alter SCFA concentrations(Reference Mathers and Fotso Tagny34, Reference Goodlad and Mathers43) and changes in the microbiota in the large intestine in response to diet are associated with reduced tumour number in Min mice(Reference Mai, Colbert and Perkins44), the present study is the first to report effects of exercise on colonic SCFA in Min mice. We observed that the colon contents of TR mice contained a significantly (P = 0·030) greater proportion of butyrate than those of CON mice, and this greater exposure to butyrate may help to explain the reduced colonic tumour number and smaller tumours in TR mice. There was a negative correlation (r − 0·174, P = 0·061) between butyrate molar proportion and total tumour number in the present study. The mechanism through which exercise alters intestinal production of butyrate (and other SCFA) is not known, but evidence from rats indicates that changes in the caecal microbiota may be critical in mediating increases in caecal butyrate(Reference Matsumoto, Inoue and Tsukahara45). There are several potential means by which exercise could affect intestinal microbiota, including changes in intestinal transit time(Reference Mathers and Dawson46), intestinal immune system or endogenous substances such as mucin(Reference Matsumoto, Inoue and Tsukahara45). In the present study, we found no effect of exercise on caecal transit time (data not shown). However, these observations suggest that future intervention studies should also investigate butyrate production.
There is increasing interest in the role of sleep in modulating cancer risk with evidence that increased sleep may reduce the risk of both breast(Reference Verkasalo, Lillberg and Stevens24, Reference Wu, Wang and Koh25, Reference Kakizaki, Kuriyama and Sone27) and prostate cancers(Reference Kakizaki, Inoue and Kuriyama26). In contrast, other studies have reported no convincing evidence for a relationship between sleep duration and breast cancer risk(Reference Pinheiro, Schernhammer and Tworoger28) or a modest increased risk with greater sleep duration(Reference McElroy, Newcomb and Titus-Ernstoff47). Relationships between sleep and bowel cancer risk are not known. We observed strong positive correlations between sleep duration and tumour multiplicity (P < 0·001) and sleep during the dark phase and tumour burden (P = 0·001; Fig. 2). It has been hypothesised that there is mutual reinforcement between circadian rhythms of melatonin production, the sleep/wake cycle and immune function(Reference Blask23) and evidence that biological clock proteins may interact with the cell cycle/DNA damage response pathway to alter cancer risk. Others(Reference Jenkins, Omori and Guan48) have observed an increase in sleep with increased body weight in mice fed a high-fat diet, and the complex interrelationships between physical activity, sleep, energy balance and cancer risk are a potentially important new research focus. The availability of robust techniques for quantifying sleep duration in animal models such as Min mice provides an exciting opportunity to investigate the putative relationship between sleep patterns and development of cancer.
Stress is another potentially important mediator of the effects of exercise regimens on intestinal tumourigenesis. TR mice were potentially exposed to extra stress during the current regimen, as the treadmill protocol occurred during the light phase of the day (when mice are often asleep), whereas most WH activity took place during the dark phase. Our investigation of non-exercise physical activity, however, suggests that TR mice did not behave differently from CON mice after they were returned to their home cages, as the amount of activity and sleep during the light period (when the intervention occurred) was not different between the two groups. This suggests that treadmill running is no more stressful than moving the CON mice to the individual control lanes, but the effect of exercise in the light phase v. dark phase of the day on intestinal tumourigenesis remains to be investigated. Our observation that greater non-exercise physical activity was associated with reduced tumourigenesis is difficult to explain in light of the lack of effect of wheel running, and warrants further investigation.
Chronic inflammation increases the risk of colorectal cancer(Reference Coussens and Werb49) possibly through oxidative damage from reactive oxygen and nitrogen species generated by neutrophils and macrophages in the inflamed tissue(Reference Itzkowitz and Yio50). An enlarged spleen is symptomatic of chronic inflammation, as demonstrated by the development of colitis in IL-2-deficient mice(Reference Garrelds, Van Meeteren and Meijssen51). Mehl et al. (Reference Mehl, Davis and Clements17) observed lower spleen size with exercise in male, but not in female, Min mice, and the authors suggested that reduced systemic inflammation in male mice may have been partially responsible for the reduction in tumour number. Our pooled data showing a positive correlation between spleen weight and tumour number support this hypothesis. In female C57BL/6 mice, Rogers et al. (Reference Rogers, Berrigan and Zaharoff38) observed that wheel running reduced spleen weight and increased mucosal cytokine production and T-cell proliferation in Peyer's patch cells.
In summary, treadmill` running, but not wheel running, reduced slightly the number of larger ( ≥ 2 mm) intestinal tumours, and tended to reduce tumour multiplicity in the colon (P = 0·049) of Min mice. We have observed for the first time that treadmill exercise increases colonic butyrate in Min mice, and that there was an inverse correlation between butyrate and tumour multiplicity. Finally, we present novel observations of relationships between sleep duration and both tumour multiplicity and tumour burden. These findings offer novel avenues for exploration of mechanisms through which exercise (and other lifestyle interventions) may prevent intestinal tumourigenesis.
Acknowledgements
The authors thank Adéle Kitching, Fiona Maclachlan and Emilie Guillaume for technical assistance. The present project was funded by the World Cancer Research Fund, project number 2001/38. L. B. and J. C. M. were involved in the study design, analysis and paper writing, and L. B. was involved in data collection. There are no conflicts of interest to declare.








