Nutrition affects human growth and development from an early age. However, dietary habits of many children and adolescents are characterised by low consumption of fruit, vegetables, whole grains and fibre, and high intake of fast food, soft drinks and salty snacks( Reference Kafeshani, Sarrafzadegan and Nouri 1 , Reference Mesirow and Welsh 2 ). Over-consumption of energy-rich foods, along with low physical activity, can contribute to an increased risk of civilisational diseases, such as obesity( Reference Kar and Khandelwal 3 ). In recent years, researchers( Reference Kafeshani, Sarrafzadegan and Nouri 1 , Reference Pigeot, Barba and Chadjigeorgiou 4 ) have reported an alarming increase in the prevalence of childhood overweight and obesity. Globally, childhood overweightness and obesity affect many low- and middle-income countries, particularly in urban settings( 5 ). Ogden et al. ( Reference Ogden, Carroll and Kit 6 ) reported that, in the USA, approximately one-third of early adolescents (10–14 years) are overweight or obese. Overweightness and obesity also are a serious public health problem in Europe, where approximately 20 % of the early adolescents are overweight or obese( Reference Pigeot, Barba and Chadjigeorgiou 4 ). In addition, it is highly probable that overweight and obese children will stay obese during adulthood. It is also more likely that these individuals will develop non-communicable diseases, such as type 2 diabetes and cardiovascular diseases. In addition, it is well known that consumption of a high amount of fat, specifically fat characterised by high content of unhealthy fatty acids (SFA), causes deterioration of bone properties and consequently can cause osteoporosis, even at a young age( 5 ).
Because eating habits are difficult to change within a population, one of the ways to improve bone health may be probiotic supplementation. It is well known that the intestinal microbiota influences not only the health of the intestine but also affects the skin, lungs, arteries and bones( Reference Scholz-Ahrens, Ade and Marten 7 – Reference Weaver, Martin and Story 9 ). Inulin is one of the probiotics affecting the composition of gut microbiota and stimulates the growth of bifidobacteria and/or lactobacilli, which increase SCFA formation( Reference Pouyamanesh, Amoli and Yaghmaei 10 ). SCFA directly contribute to increased absorption of Ca, Mg and Fe in the intestines( Reference Yasuda, Roneker and Miller 11 ) and indirectly have an immunostimulating effect( Reference Watzl, Girrbach and Roller 12 ). Some studies suggest that feeding inulin to animals may affect bone quality( Reference Roberfroid, Cumps and Devogelaer 13 ) owing to increased mineral absorption. However, such conclusions were based on the concentration of minerals in the blood plasma and organs such as the liver, kidneys and heart( Reference Jolliff and Mahan 14 ), or were based on total mineral balance( Reference Demigné, Jacobs and Moundras 15 ). Thus, it is obvious that high consumption of unhealthy fat (rich in SFA) deteriorates bone quality, whereas inulin improves bones quality.
Most studies on the influence of direct effects of inulin on bone properties have been conducted on rodents fed a standard diet. In turn, the influence of high consumption of fat on bone quality usually has been compared with animals fed a standard diet. Recent studies have demonstrated that, for humans, pigs are a better model than rodents owing to several similarities in anatomy, physiology, metabolism and pathology( Reference Aigner, Renner and Kessler 16 – Reference Reinwald and Burr 18 ). Obviously, there are interspecies differences in the skeletal structure and load, but factors of research interest affect the properties of bones of both pigs and humans in a similar manner.
The aim of this study was to determine the effect of inulin on whole-body mineral content, bone density and femur properties in growing pigs fed a high-fat diet rich in SFA, having an unbalanced ratio of lysine:metabolisable energy (ME). It was hypothesised that inulin would reduce the negative effects of such a diet on bone health.
Methods
According to the 3R (Replacement, Reduction and Refinement) ethical principle, the design of the study and experimental techniques used through the analysis allowed to minimise the number of animals while maintaining high statistical precision. There is no way to completely replace live animals with another research model. Thus, pigs have been chosen as a model for humans because of several similarities in anatomy, physiology, metabolism and pathology.
Experimental procedures used throughout this study were performed in accordance with national/local ethical guidelines and approved by the III Local Ethics Committee on Animal Experimentation of the Warsaw University of Life Sciences – SGGW, Poland.
Animals, diets and housing
This study was carried out at The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, on twenty-one cross-bred pigs (gilts) (male Duroc×female Polish Large White×Danish Landrace) growing from 50 to 120 d of age. At 50 d of age, animals were randomly allotted to three groups (n 7 per group): the control (C) group fed a standard diet (10 g of standardised ileal digestible lysine, 13·5 MJ ME per kg diet, and 0·72 of lysine:ME ratio) balanced according to nutrient requirements of swine( 19 ), two experimental (T and TI) groups fed a high-fat diet rich in SFA (141 g of diethyl ether extract, 7 g of standardised ileal digestible lysine, 15·4 MJ ME per kg diet, and 0·44 of lysine:ME ratio). Pigs fed diet TI received an extra supplement of inulin extracted from chicory root (100 % inulin content, 0 % sweetness level), with an average degree of polymerisation of at least 23 (Inulin Orafti®HPX; BENEO GmbH). A review of the literature indicated that supplementation of inulin in diets of pigs in previous studies ranged from 0·5 to 9 %( Reference Estrada, Drew and Van Kessel 20 , Reference Kjos, Øverland and Fauske 21 ). However, results from some studies( Reference Jolliff and Mahan 14 , Reference Kjos, Øverland and Fauske 21 ) indicated that the effect of inulin on the tested parameters depended on its supply. Generally, higher doses of inulin had a greater impact on tested parameters. On the basis of these findings, it was decided that pigs fed diet TI will receive an extra supply of inulin in an amount of 7 % of daily feed intake. This amount of inulin could be successfully used in food products for humans, among others as a component in baked goods( Reference Salinas, Hamet and Binaghi 22 , Reference Peressini and Sensidoni 23 ).
Mineral content in standard and high-fat diets was the same and balanced according to nutrient requirements of swine guidelines( 19 ). Amino acid content in the high-fat diet was not equalised to that in the standardised diet because the aim of the study was to compare the influence of an unbalanced ratio of lysine to ME and elevated consumption of SFA (similar to the Western-type diet in human nutrition) on bone health. Beef tallow, rich in SFA, was used at 13 % as fat supplement. Composition and nutritive value of diets are given in Table 1. The fatty acid content (%) of feeds is shown in Table 2 (data analysed). Pigs were fed semi ad libitum (95 % ad libitum intake) twice daily (08.00 and 14.00 hours). This facilitated full control of nutrient intake by pigs.
Table 1 Ingredients, chemical composition and nutritive value of diets

* Provided per kg diet: µg: vitamin A, 4500; vitamin D3, 50; vitamin E, 100; mg: Fe, 240; Zn, 150; Cu, 25; Mn, 20; I, 0·30; Se, 0·30; vitamin K3, 3·5; vitamin B1, 3·5; vitamin B2, 7·0; vitamin B6, 5·0; vitamin B12, 0·40; biotin, 0·20; folic acid, 3·0; nicotinic acid, 30; Ca -d pantothenate, 15; choline chloride, 400; g: Ca, 6·8 (premix A) and 6·6 (premix B); P, 2·9 (premix A) and 2·8 (premix B); essential amino acids: g: lysine, 4·51 (premix A) and 1·94 (premix B); methionine, 0·90 (premix A); threonine, 2·10 (premix A) and 0·25 (premix B).
Table 2 Fatty acid content of the diets (%)
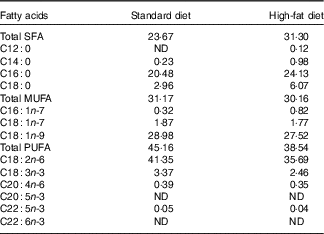
ND, value below 0·01 was classified as not determined.
Piglets were randomly selected from a population and kept individually on a concrete floor without straw in pens (3·3 m2) equipped with nipple drinkers. The environmental conditions in the piggery were as follows: air temperature of 18–20°C, humidity of 60–70 % and air flow of 0·2–0·4 m/s, which was regulated by a Fancom ventilation system (Fancom BV) and in accordance with the European Union law( 24 ). The pigs were maintained on a 12 h day–12 h night cycle, with lights on from 06.00 to 18.00 hours.
Measurements and calculations
At 120 d of age, after 16 h of fasting, each pig was scanned using dual-energy X-ray absorptiometry (DXA) (Norland XR-800™ densitometer scanner with a ‘Whole-Body Scan’ type, Norland, A Cooper Surgical Company) to determine whole-body mineralisation – that is bone mineral content (BMC, g) and bone mineral density (BMD, g/cm2). Before scanning, animals were subjected to short-lasting sedation by injection using a mixture of ketamine hydrochloride (2 mg/kg body weight) and xylazine (0·2 mg/kg body weight)( Reference Skiba, Weremko and Sobol 25 ). Next, pigs were placed in a ventral position with all limbs extended. The DXA scans were obtained according to the standard procedures described by the manufacturer for scanning and analysis. A quality assurance test to verify the stability of the system calibration (control scans) was performed on a daily basis (as indicated by the manufacturers) to ensure precision. The daily calibration procedure was performed using QC Phantom and QA Calibration standard (Norland; A Cooper Surgical Company). The analysis was performed with the whole-body application. Two scans were performed on each pig.
After scanning, animals were slaughtered by exsanguination at the Institute’s experimental slaughter house. After slaughter, the femur was manually dissected from the right side of each carcass. After excision, the femur was cleaned of any remaining flesh, weighed and frozen (−20°C) for subsequent DXA scan (Norland XR-800TM densitometer scanner with a ‘Research Scan’ type, Norland). Specimens for scanning were thawed at room temperature (23°C) for 12 h before use. During scanning, the femur was positioned horizontally with the femoral head facing upwards and the condyles downwards and was scanned from the distal to the proximal end. All scans were performed in duplicate in order to avoid any rotation of the bone, because inconsistencies in their orientation adversely affect test precision. All scans were performed by the same operator. Femur BMC and BMD were recorded. After scanning, the three-point bending test was applied to determine the mechanical bone characteristics using a TA-HDi Texture Analyser (Godalming) with head speed of 1 kN, 10 mm/min. The relationship between loading force perpendicular to the longitudinal axis of the bone and the resulting displacement was determined graphically. The values of maximum strength (N) and maximum elastic strength (N) were determined. The distance between supports of the bone was set at 40 % of the femur length and the measuring head loaded bone samples at the midshaft with a constant speed of 50 mm/min. The geometrical properties of each femur were determined on the basis of the measurements of horizontal and vertical diameters (both external and internal) recorded after cutting the bone. The measurements were collected using an electronic ruler. The values of cross-sectional area (mm2), cortical wall thickness (mm) and cortical index (%) were determined in accordance with the following mathematical formulas:
 $$\eqalignno{ & {\rm Cross{\hbox-}section}\,{\rm area}\,\left( {{\rm mm}^{2} } \right)\,{\equals}\,\left[ {\pi \left( {HV{\minus}hv} \right)} \right]\,/\,4 \cr & {\rm Cortical}\,{\rm wall}\,{\rm thickness}\,\left( {{\rm mm}} \right)\,{\equals}\,\left[ {\left( {V{\plus}H} \right){\minus}\left( {v{\plus}h} \right)} \right]\,/\,4 \cr & {\rm Cortical}\,{\rm index}\,(\%)\,{\equals}\,\left\{ {\left[ {\left( {H{\minus}h\,/\,H} \right){\plus}\left( {V{\minus}v\,/\,V} \right)} \right]\,/\,{\rm 2}} \right\}100, $$
$$\eqalignno{ & {\rm Cross{\hbox-}section}\,{\rm area}\,\left( {{\rm mm}^{2} } \right)\,{\equals}\,\left[ {\pi \left( {HV{\minus}hv} \right)} \right]\,/\,4 \cr & {\rm Cortical}\,{\rm wall}\,{\rm thickness}\,\left( {{\rm mm}} \right)\,{\equals}\,\left[ {\left( {V{\plus}H} \right){\minus}\left( {v{\plus}h} \right)} \right]\,/\,4 \cr & {\rm Cortical}\,{\rm index}\,(\%)\,{\equals}\,\left\{ {\left[ {\left( {H{\minus}h\,/\,H} \right){\plus}\left( {V{\minus}v\,/\,V} \right)} \right]\,/\,{\rm 2}} \right\}100, $$
where V is the vertical external diameter (mm), H the horizontal external diameter (mm), v the vertical internal diameter (mm) and h the horizontal internal diameter (mm)( Reference Ferretti, Capozza and Mondelo 26 ).
Chemical analysis
DM, ash, crude protein, crude fibre, diethyl ether extract, simple sugar and starch contents in the diets were determined as detailed by Horwitz & Latimer( Reference Horwitz and Latimer 27 ) (procedures: 934.01, 942.05, 984.13, 978.10, 920.39, 974.06 and 920.40, respectively). Content of ME and standardised ileal digestible amino acids in the diets were calculated as detailed by the nutrient requirements of swine( 19 ).
Lipids from the diets were saponified with potassium hydroxide according to the method described by Folch et al. ( Reference Folch, Lees and Sloane Stanley 28 ). Next, methyl esters were prepared by esterification with thionyl chloride (4 % in methanol) and extracted with n-heptane. Fatty acid methyl esters were analysed using a GC-2010AF Shimadzu Gas Chromatograph (Shimadzu Europa GmbH) equipped with a flame ionisation detector. The derivatives were separated on a capillary column (BPX70, 60-m length, 0·25-mm internal diameter and 0·25-μm film thickness; Phenomenex). The operating conditions were as follows: carrier gas, helium; split ratio, 1:100; injector and detector temperature, 260°C; the initial column temperature of 110°C was held for 5 min, then increased to 200°C at a rate of 3·5°C/min, and was held for 2·5 min, then increased to 205°C at a rate of 0·3°C/min, then increased to 215°C at a rate of 1·5°C/min and was held for 3 min. Individual fatty acid peaks were identified in comparison with the commercial standard, Supelco 37 Component FAME Mix (SUPELCO).
Statistical analysis
Statistical analyses were performed using Statgraphics Centurion (version 16.1.18, 2011) software (StatPoint Technologies Inc.). With an α level of 0·05, power established at 80 % and an effect size of 0·90, the required total sample size was 24 (i.e. n 8/group). The hypothesised effect size of 0·90 was calculated from the descriptive statistics of a previous study( Reference Grela, Pietrzak and Sobolewska 29 ). Post hoc calculations using the hypothesised effect size and the total sample size of twenty-one (i.e. n 7/group) indicated that the actual power achieved in this study was 79·7 %. Data are presented as means and standard deviations. The effect of diets on performance, whole-body mineralisation and femur properties of pigs was analysed using a one-way ANOVA. When the F ratio was significant, Tukey’s HSD post hoc analysis was performed. Statistical significance was set at P<0·05. A borderline significant trend was set at P<0·09.
Results
Final body weight did not differ among groups C and T; however, body weights of pigs in groups C and T were lower than pigs in group TI (P=0·0211) (Table 3). Daily feed intake ranged from 1·58 kg for pigs in group T to 1·69 kg for pigs in group C (P=0·0128). Pigs in groups T and TI had similar, but higher, daily ME intake from the feed than pigs in group C (P=0·0037). In addition, pigs in group TI received daily approximately 116 g of extra inulin supply, which is 0·72 MJ/d. Pigs in groups T and TI had similar, but lower, total feed intake (P=0·0115) and total lysine intake (P=0·0000) than pigs in group C. Total energy intake from the feed did not differ among groups. Average daily gain was higher (P=0·0184) for pigs in group TI than those in groups C and T; however, the feed conversion ratio ranged from 1·88 kg of feed/kg gain for pigs in group T to 2·03 kg of feed/kg gain for pigs in group C (P=0·0434). Whole-body BMC (P=0·0054) and BMD (P=0·0322) were higher for pigs in groups TI and C compared with pigs in group T (Table 4).
Table 3 Performance of pigs during experimental period (Mean values and standard deviations)
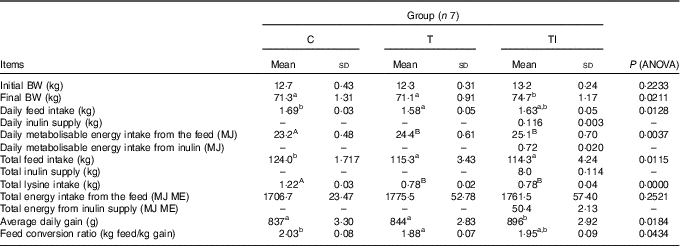
C, pigs fed a standard diet; T, pigs fed a high-fat diet rich in SFA; TI, pigs fed a high-fat diet rich in SFA and supplemented with inulin; BW, body weight; ME, metabolisable energy.
A,B Mean values within a row with unlike superscript letters were significantly different (P<0·01). a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Table 4 Whole-body mineralisation performed at the end of the study (at 120 d of age) (Mean values and standard deviations)

C, pigs fed a standard diet; T, pigs fed a high-fat diet rich in SFA; TI, pigs fed a high-fat diet rich in SFA and supplemented with inulin; BMC, bone mineral content; BMD, bone mineral density.
A,B Mean values within a row with unlike superscript letters were significantly different (P<0·01). a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Mass and length of femurs did not differ between treatments (Table 5). Pigs of groups TI and C had similar, but higher, femur BMC compared with group T (P=0·0009). Femur BMD was highest for pigs in group C, followed by pigs in group TI, and lowest for pigs in group T (P=0·0001). Femurs of pigs in groups TI and C had similar, but higher, maximum strength compared with group T (P=0·0082), whereas maximum elastic strength was highest for pigs in group C, followed by pigs in group TI, and lowest in group T (P=0·0001). Femur cross-sectional area tended to be lower for pigs in group TI than for pigs in group C (P=0·0753). Femurs of pigs in groups TI and T had similar, but lower, cortical wall thickness than femurs of pigs in group C (P=0·0100). However, the cortical index ranged from 42·3 % for pigs in group T to 45·8 % for pigs in group C (P=0·0366).
Table 5 Femur morphometric, geometric, densitometric and mechanical properties at the end of the study (at 120 d of age) (Mean values and standard deviations)
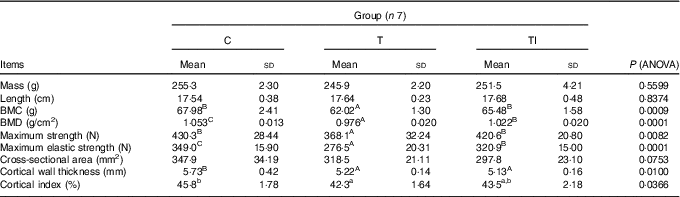
C, pigs fed a standard diet; T, pigs fed a high-fat diet rich in SFA; TI, pigs fed a high-fat diet rich in SFA and supplemented with inulin; BMC, bone mineral content; BMD, bone mineral density.
A,B,C Mean values within a row with unlike superscript letters were significantly different (P<0·01). a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Discussion
Composition of pig diets
Studies carried out on animals( Reference Bielohuby, Matsuura and Herbach 30 – Reference Yanagihara, Shimano and Tida 33 ) and humans( Reference Griel, Kris-Etherton and Hilpert 34 ) indicated that the amount and source of dietary fat may affect bone metabolism by changing Ca absorption, osteoblast formation, lipid oxidation and synthesis of pro- and anti-inflammatory eicosanoids. However, the aim of this study was to compare the influence of an unbalanced ratio of lysine:ME and elevated consumption of SFA (similar to the Western-type diet in human nutrition) on bone properties.
Performance of pigs
The results of the present study show that pigs fed a high-fat diet supplemented with inulin grew faster than those fed either a high-fat diet that was not supplemented with inulin or a standard diet. However, the feed conversion ratio differed only between pigs fed a standard diet and a high-fat diet not supplemented with inulin. Some authors( Reference Estrada, Drew and Van Kessel 20 , Reference Grela, Pietrzak and Sobolewska 29 ) previously found that feed supplementation with inulin increased mass gain in pigs. However, these authors used only a standard diet supplemented with inulin and suggested that this gain resulted from a favourable impact of inulin on the population of beneficial micro-organisms in the alimentary tract, which in turn protect the host against development of pathogenic strains. Consequently, this protection leads to higher growth rates and better feed utilisation( Reference Pouyamanesh, Amoli and Yaghmaei 10 , Reference Estrada, Drew and Van Kessel 20 ). On the other hand, studies carried out on rodents by Duan et al. ( Reference Duan, An and Li 35 ), Kumar et al. ( Reference Kumar, Ward and Brown 36 ) and Pouyamanesh et al. ( Reference Pouyamanesh, Amoli and Yaghmaei 10 ) showed that inulin supplementation to a high-fat diet resulted in weight gain attenuation in rodents compared with rodents fed a high-fat diet that was not supplemented with inulin. Discrepancies between our results and results of previous studies may be owing to differences in the kind and amount of fat supplementation, as well as the level of inulin addition. Other reasons for differences between the results of our study and previous studies could be because of differences in tolerance of species for high amounts of dietary fat. Comparing data in the literature concerning the proportion of energy from fat to total energy in the diet, it seems that rodents are able to tolerate feed with a higher ratio of fat (even 60 %) compared with pigs (approximately 28 % in the present study).
Whole-body mineralisation
On performing DXA analysis, we were able to determine the BMC and BMD of each pig and relate it to the dietary treatment that was of interest in our study. Recent studies have demonstrated that, for humans, pigs are a better model than rodents owing to several similarities in anatomy, physiology, metabolism and pathology( Reference Aigner, Renner and Kessler 16 ). Moreover, pigs have similar lamellar bone structure and similar rates of bone regeneration, cortical bone mineralisation and bone remodelling rates as humans( Reference Pearce, Richards and Milz 17 , Reference Reinwald and Burr 18 ). Obviously, there are interspecies differences in the skeletal structure and load, but factors of research interest affect the properties of bones of both pigs and humans in a similar manner. We found that inulin supplementation to a high-fat diet increased the whole-body BMC and BMD compared with animals fed a high-fat diet that was not supplemented with inulin. However, whole-body mineralisation did not differ among pigs fed a standard diet and a high-fat diet supplemented with inulin. Few data have been published on the influence of inulin (and other oligosaccharides) supplementation on the whole-body bone parameters measured by DXA. Studies dealing with this issue have been carried out mainly on rodents( Reference Weaver, Martin and Story 9 , Reference Roberfroid, Cumps and Devogelaer 13 , Reference Devareddy, Khalil and Korlagunta 37 , Reference Jamieson, Ryz and Taylor 38 ); however, a major part of these studies only examined the use of a standard diet. Some authors found that the addition of inulin improved whole-body bone parameters( Reference Roberfroid, Cumps and Devogelaer 13 ). A similar finding was reported by Devareddy et al. ( Reference Devareddy, Khalil and Korlagunta 37 ) using a standard diet supplemented with fructo-oligosaccharides. Furthermore, inulin-type fructan supplementation increased whole-body BMC and BMD compared with a placebo group during a long-term study on human adolescents( Reference Abrams, Griffin and Hawthorne 39 ). In contrast, some authors( Reference Weaver, Martin and Story 9 , Reference Jamieson, Ryz and Taylor 38 ) did not find any effect of inulin supplementation to a standard diet on the whole-body bone parameters of rats.
Numerous studies performed using animal and human models showed that beneficial effects of fructose-based oligosaccharide supplementation (among other types of inulin) resulted from stimulation of mineral absorption, mainly Ca, but also Mg, Zn and Fe( Reference Abrams, Hawthorne and Aliu 40 , Reference Wang, Dellatore and Douard 41 ). Furthermore, by increasing the absorption of minerals, BMC and BMD improved. Simultaneously, peak bone mass also increased, which reduces the risk of developing osteopaenia and osteoporosis( Reference Roberfroid, Cumps and Devogelaer 13 , Reference Kruger, Brown and Collett 42 ). Mechanisms of this response could be associated with lower luminal pH, resulting from SCFA produced by Bifidobacterium after prebiotic fermentation( Reference Scholz-Ahrens, Ade and Marten 7 ). Reduced pH also changed speciation and solubility of minerals and increased their bioavailability( Reference Kaur and Gupta 43 ). Few minerals are essential co-factors for enzymes involved in the synthesis of collagen and other bone matrix constituents that are required to build up the organic bone matrix, which is the precondition for mineral accretion (e.g. Mg, Zn, B and Mn)( Reference Scholz-Ahrens, Ade and Marten 7 ).
On the basis of the literature data, several hypotheses have been suggested to explain how inulin enhances mineral absorption, mainly Ca as the main bone component. First, increased Ca solubility in the colon resulted from pH reduction as a consequence of inulin fermentation( Reference Scholz-Ahrens, Ade and Marten 7 , Reference Kaur and Gupta 43 ). Second, osmotic effects increase fluid transfer in the colonic lumen and, as a consequence, increase the permeability between intracellular enterocyte junctions that facilitates diffusion( Reference Roberfroid and Delzenne 44 ). Third, Ca/H exchange in the distal part of the colon is activated by absorption of short-chain carboxylic acids( Reference Yasuda, Roneker and Miller 11 , Reference Scharrer and Lutz 45 ).
Our results showed that feeding pigs a high-fat diet rich in SFA and not supplemented with inulin resulted in strong deterioration of whole-body bone parameters compared with a standard diet. Fehrendt et al. ( Reference Fehrendt, Linn and Hartmann 32 ) found similar results in mice.
Data in the literature show that fatty acid composition of the fat source in the diet also had a significant influence on bone health. In a study by Wang et al. ( Reference Wang, Dellatore and Douard 41 ) on mice, a high-fat diet rich in SFA resulted in lower values of bone mineralisation compared with mice fed a standard diet. These authors did not find such negative effects when a high-fat diet was rich in MUFA. Other authors also found that SFA may have effects that could impair bone health( Reference Oh, Sul and Kim 46 , Reference Estadella, da Penha Oller do Nascimento and Oyama 47 ), whereas MUFA and PUFA, in particular n-3 PUFA, have a beneficial effect on bone health( Reference Griel, Kris-Etherton and Hilpert 34 ).
Properties of femur
Densitometry measurements of whole-body bone parameters cannot be compared with data on selected bones because they represent an average value that can mask differences between rapid- and slow-growing bones( Reference Roberfroid, Cumps and Devogelaer 13 ). Therefore, despite whole-body mineralisation, femur properties also were determined.
A review of the available literature did not provide information on the effects of inulin supplementation to a high-fat diet on the bone health of growing pigs. The present study showed that neither a high-fat diet nor inulin supplementation affected mass and length of femur bones. Inulin supplementation to a high-fat diet, however, improved femur parameters, resulting in values similar to those obtained in pigs fed a standard diet. However, cortical wall thickness and cross-sectional area were similar to those of pigs fed a high-fat diet that was not supplemented with inulin. The results of this study were consistent with those of Fehrendt et al. ( Reference Fehrendt, Linn and Hartmann 32 ), who showed negative impacts of a high-fat diet on bone microstructure in mice, including a missarrangement of cell–cell and cell–matrix contacts, osteoblast and trabecular structure, and collagen-1 and osteoid synthesis. Bielohuby et al. ( Reference Bielohuby, Matsuura and Herbach 30 ) and Patsch et al. ( Reference Patsch, Kiefer and Varga 48 ) also showed an adverse effect of high-fat diet on BMD, bone microarchitecture (i.e. trabecular number and connectivity density) and bone strength (i.e. maximum fracture load, bone stiffness and energy to failure) compared with a standard diet. Bielohuby et al. ( Reference Bielohuby, Matsuura and Herbach 30 ) suggested that lower BMD and mechanical strength of femurs of rats fed a high-fat diet resulted from lower serum concentration of markers of bone formation and, simultaneously, unchanged serum concentration of markers of bone resorption. Moreover, lower rates of mesenchymal cells differentiating into osteoblasts might be explained by reductions of approximately 70–80 % in the expression of transcription factors influencing osteoblastogenesis in bone marrow. Furthermore, in studies carried out on mice, some authors found that a high-fat diet reduced femoral trabecular bone mass owing to an increased trabecular separation and a reduced trabecular number and connectivity density( Reference Cao, Sun and Gao 31 ). However, a considerable amount of dietary fat was lard, which is characterised by high content of SFA( 19 ). It seems that the amount of fat consumed, as well as its fatty acid profile, could play an important role in organismal response. Results of the present study clearly showed that pigs fed a high-fat diet (rich in SFA) had worse parameters of femurs compared with pigs fed a standard diet. Wang et al. ( Reference Wang, Dellatore and Douard 41 ) also found that rats fed a high-fat diet rich in SFA had lower values of femur volumetric BMD than those fed a control or a high-fat diet rich in MUFA. The negative effect of SFA on bone metabolism can be achieved by several mechanisms. It has been suggested that SFA may interfere with osteoblastogenesis and bone formation( Reference Parhami, Tintut and Beamer 49 ). Other authors showed that high content of SFA could increase bone resorption by elevating the expression of inflammatory cytokines and the receptor activator of NF-κβ ligand( Reference Oh, Sul and Kim 46 ) or that SFA can participate in osteoclastogenesis resulting in decreased osteoclast apoptosis( Reference Zhong, Xiu and Wei 50 ).
In conclusion, inulin supplementation to a high-fat diet rich in SFA reduced the negative effect of such a diet on whole-body mineralisation, as well as on femur mineral status and strength.
Acknowledgements
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
M. S., S. R. and G. S. conceived and designed the study, interpreted the data and drafted the manuscript. M. S. conducted the research and acquired the data and performed the statistical analysis.
None of the authors has any conflicts of interest to declare.
The amount of inulin used in animal studies could reasonably be expected to be achieved in the human population, for example, in food products, among others as a component in baked goods or dairy products.







