Oxidative stress refers to a state of imbalance between oxidative and antioxidant effects in the body(Reference Pisoschi and Pop1,Reference Kattoor, Pothineni and Palagiri2) , which involves an overproduction of reactive oxygen species or dysfunction of the antioxidant defence system, resulting in macromolecular damage and disruption of redox signalling and cellular control(Reference Sztretye, Dienes and Gönczi3), which in turn leads to inflammatory infiltration of neutrophils and production of a large number of oxidative intermediates(Reference Pisoschi and Pop1–Reference Saleem, Sabir and Niazi4). Oxidative stress plays an important role in many clinical conditions, and antioxidant therapy can have a positive effect on these diseases(Reference Liguori, Russo and Curcio5). Carotenoids are a class of natural pigments that are widely distributed in yellow, orange and red fruits and vegetables. α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene are all natural antioxidants(Reference Johra, Bepari and Bristy6), and they play an active antioxidant role in these diseases(Reference Johra, Bepari and Bristy6–Reference Du, Lv and Liu10). In addition, carotenoids are associated with the expression of superoxide dismutase, catalase and glutathione peroxidase and activate the body’s own antioxidant defence system by interacting with transcription factors(Reference Bohn11). Carotenoids also have powerful anti-cancer properties and have positive effects on the human body by participating in physiological activities such as intercellular signalling conduction, immune system regulation and inhibiting cell proliferation(Reference Yeum and Russell12,Reference Milani, Basirnejad and Shahbazi13) . Moreover, lungs have abundant blood flowing through and are constantly exposed to high levels of oxygen, and they are prone to inhaling harmful substances, which makes them highly susceptible to oxidative stress and developing lung diseases(Reference Rogers and Cismowski14,Reference Cannavò, Perrone and Viola15) . The AlphaTocopherol, Beta-Carotene Cancer Prevention (ATBC) and Beta-Carotene and Retinol Efficacy Trial (CARET) studies showed an increased risk of lung cancer rate when smokers or asbestos workers were supplemented with β-carotene(Reference Virtamo, Taylor and Kontto16). Oxidative stress induces airway hyperresponsiveness and neutrophilic inflammation, leading to cell death and chronic bronchial inflammation and emphysema(Reference Wiegman, Li and Ryffel17). It can also further cause cellular and molecular damage to DNA, proteins and lipids, making asthma worse(Reference Liu, Hua and Song18). Currently, a large number of studies have shown that carotenoids have positive effects on the human body(Reference Johra, Bepari and Bristy6,Reference Bohn11,Reference Thomson, Stendell-Hollis and Rock19–Reference Genç, Bardakci and Yücel21) , but there are some studies with inconsistent results(Reference Virtamo, Taylor and Kontto16,Reference Amengual, Lobo and Golczak22,Reference Borel, Grolier and Boirie23) . Despite the increasing interest in studying the role of serum carotenoids in respiratory health, population-based studies examining the relationship between serum carotenoid levels and respiratory mortality are limited(Reference Zhang, Li and Du24,Reference Min and Min25) . To further explore the connection, this study used data from the Third National Health and Nutrition Examination Survey (NHANES III), including data from over 18 000 participants, to investigate whether levels of serum α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene are associated with respiratory morbidity and mortality among American adults.
Method
Study population
The data used in this study came from the NHANES III, a total of 39 695 individuals aged 2 months and older. The study was conducted in two phases from 1988 to 1994. Phase I (1988–1991) and Phase II (1991–1994) were nationally representative samples, respectively. During the 6-year sample survey, 33 994 samplers were interviewed and 30 818 were inspected, with an overall inspection response rate of 78 %. We limited the study population to subjects ≥ 20 years of age at the time of the examination. Of the 18 805 subjects, 15 534 (82·61 %) had serum carotenoid levels available. Among them, we excluded 7650 patients who were missing data on smoking. The cohort analysis presented in this study was thus based on 7884 NHANES III participants.
Serum carotenoid measurements
Serum carotenoid levels are useful biomarkers of the total dietary intake of vegetables and fruits. Serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene are measured by using HPLC. The median inter-assay coefficients of variation were 9·4 % forα-carotene, 7·0 % for β-carotene, 8·7 % for β-cryptoxanthin, 11·0% for lutein/zeaxanthin and 7·7% for lycopene(Reference Gunter, Lewis and Koncikowski26).
Main outcomes
The main outcome variables were asthma, emphysema, chronic bronchitis and mortality of disease specific and all cause. The participants were considered to have asthma, emphysema and chronic bronchitis if they separately answered yes to the questions ‘Has a doctor or other health professional ever told you that you had asthma/emphysema/chronic bronchitis?’. These questions were included in the part of the medical conditions in the NHANES, and the participants’ conditions were based on the medical history record of their clinicians or medical departments.
The mortality of all-cause and respiratory diseases was determined by the National Death Index, which is widely used to identify deaths. Causes of death were determined by using the International Classification of Diseases Tenth Revision (ICD-10) codes. Deaths that occurred before 1999 were originally coded by using a previous version of the classification code (i.e. ICD-9) and now use the ICD-10 code recorded by the National Center for Health Statistics. Chronic lower respiratory disease mortality was defined as death from asthma (J45–J46), emphysema (J43), bronchitis (chronic or other; J40–J42) or other chronic lower respiratory disease (J44 and J47).
Covariates
The participants were interviewed in NHANES III to obtain information on age (years), sex (men or women), race (White, Black, Hispanic or other), annual household income and education (less than high school, high school graduate, or college or more). Drinking condition was assessed by using the question, ‘Had you had drinks for at least 12 times in the last 12 months?’. The response was yes or no. The exercise was assessed by using the question, ‘Have you participated in exercise in the past month?’ The response was yes or no. Smoking-related variable (Do you smoke cigarettes now?). The response was yes, no or blank but applicable. The BMI was calculated by dividing the weight in kilograms by the height in metres squared. The BMI was categorised into three groups (< 18·5, 18·5–24·9 and ≤ 25·1 kg/m2). Energetic intake (kcal/d) was measured by using a single 24-h diet recall. Marriage (married – spouse in the household, married – spouse not in the household, living as married in the household, widowed in the household, divorced in the household, separated in the household, never married, blank but applicable, Don’t know). Diabetic condition, ‘Ever been told you have sugar/diabetes?’. High blood pressure condition, ‘Doctor ever told you had hypertension/HBP?’. Asthma condition, ‘Doctor ever told you had asthma?’. Emphysema condition, ‘Doctor ever told you had emphysema?’. Chronic bronchitis condition, ‘Doctor ever told you had chronic bronchitis?’.
Statistical analysis
The baseline characteristics in terms of demographics, lifestyle and biochemical indices were presented as mean (standard deviation) and numbers (percentage). The serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene were categorised into tertiles. General linear models and a χ 2 test were used to compare the differences for baseline characteristics by tertiles of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene, respectively. Logistic regression models were performed to evaluate the OR and 95 % CI for the association of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene with the prevalence of asthma, emphysema and chronic bronchitis. Cox proportional hazards models were performed to evaluate the association of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene with mortality of all-cause and chronic lower respiratory diseases. In these Cox models, follow-up began at time 0 at study entry and continued until the event of interest (death from chronic lower respiratory disease) or censored event (death from other causes or death on 31 December 2015), the event that occurred first prevailed. Due to the NHANES data being cross-sectional, covariates were measured when participants were first enrolled and therefore did not change in these models. Restricted cubic spline (RCS) was used to visualise the dose–response association of the significant association found in the logistic regression or Cox proportional hazards models by setting 4 knots at the 5th, 25th, 75th and 95th percentiles.
We also controlled a series of confounders, including age, sex, race, marriage, education level, regular exercise, drinking, smoking, BMI, annual family income, energetic intake, vitamin E intake, vitamin C intake, fruit intake, vegetable intake, diabetes, hypertension, asthma, emphysema and chronic bronchitis in all models. All statistical analyses were conducted by R 4.2.2, and P-values < 0·05 were considered statistically significant.
Sensitive analysis
Three sensitivity analyses were performed. The first analysis excluded the participants whose follow-up duration was less than 2 years, to examine whether the severe illness would influence the results. The second analysis excluded the participants who had the extreme values in α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene to examine the stability of the results. The third analysis evaluated whether age (age > 45 years old), BMI ( < 25 and ≥ 25 kg/m2) and smoking status had a modification effect on these relationships.
Results
Baseline characteristics
Table 1 shows the differences in the baseline characteristics in terms of demographic, anthropometric, lifestyle and biochemical indicators across tertiles of baseline α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene. Participants with higher α-carotene were more likely to be non-Hispanic white with higher levels of education, income and fruit intake, and a lower percentage of smoking rate, drinking rate and low levels of BMI (all the P < 0·05). Meanwhile, participants with higher β-carotene were older and more likely to be non-Hispanic white and at higher levels of education and income, as well as a higher prevalence of hypertension with a lower percentage of smoking rate, drinking rate and low levels of BMI (all the P < 0·05). Reversely, participants with higher β-cryptoxanthin were younger and more likely to be men with high levels of BMI and fruit intake, and low levels of education and income as well as a lower prevalence of chronic bronchitis (all the P < 0·05). Meanwhile, participants with higher lutein/zeaxanthin were men and high levels of BMI, smoking rate and drinking rate as well as a higher prevalence of hypertension with a lower percentage of smoking rate and drinking rate (all the P < 0·05). Similarly, participants with higher lycopene were younger and more likely to be men with higher levels of BMI, income, smoking rate and drinking rate, and a lower prevalence of hypertension, fruit intake, vegetable intake and emphysema (all the P < 0·05). Table 2 shows the concentration range of carotenoids in serum.
Table 1. Characteristics of study participants according to tertiles of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene
(Numbers and percentages; mean values and standard deviations)

Continuous variables are presented as mean values and standard deviations. Categorical variables are presented as numbers and percentages.
Table 2. Concentrations of carotenoids in the serum of the study participants (n 7884)
(Mean values and standard deviations)

Continuous variables are presented as mean values and standard deviations.
Association between serum total carotenoids and respiratory morbidity, all-cause and disease-specific mortalities
Table 3 shows that emphysema morbidity was significantly associated with serum total carotenoid levels (OR = 0·61, 95 % CI 0·41, 89). The hazard ratio (HR) and 95 % CI for the association of tertiles of serum α-carotene with mortality of all cause and respiratory disease are presented in Table 4. Compared with the lowest tertile of serumα-carotene, the participants in the highest tertile had lower mortality of all-cause (HR = 0·62, 95 % CI 0·42, 0·90) and respiratory diseases (HR = 0·74, 95 % CI 0·67, 0·81) in Table 4. Due to the inverse association of tertiles of total carotenoids with the prevalence of all-cause and respiratory diseases, the RCS was used to flexibly model for visualising the above association, which is presented in Fig. 1. Meanwhile, sensitivity analyses were performed, and none of them affected the above results.
Table 3. OR and 95 % CI for the association of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene with the prevalence of asthma, emphysema and chronic bronchitis
(Odds ratios and 95 % confidence intervals)

Data are odds ratios and 95 % confidence intervals.
Model 1 was adjusted for age, sex, race, marriage and education.
Model 2 was model 1 with additional adjustments for sport, drink, smoke, BMI, income, energy, vitamin E, vitamin C, fruit intake and vegetable intake.
Model 3 was model 2 with additional adjustments for diabetes, hypertension, asthma, emphysema and chronic bronchitis.
Table 4. HR and 95 % CI for the association of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene with all-cause and disease-specific mortalities
(Hazard ratios and 95 % confidence intervals)

Data are hazard ratios and 95 % confidence intervals.
Model 1 was adjusted for age, sex, race, marriage and education.
Model 2 was model 1 with additional adjustments for sport, drink, smoke, BMI, income, energy, vitamin E, vitamin C, fruit intake and vegetable intake.
Model 3 was model 2 with additional adjustments for diabetes, hypertension, asthma, emphysema and chronic bronchitis.
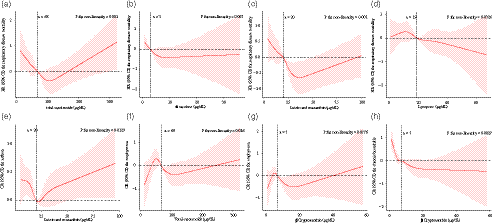
Fig. 1. RCS analysis between serum carotenoids levels and respiratory disease morbidity and mortality. (a) RCS analysis of the relationship between total carotenoids and respiratory disease mortality. (b) RCS analysis of the relationship between α-carotene and respiratory disease mortality. (c) RCS analysis of the relationship between lutein and zeaxanthin and respiratory disease mortality. (d) RCS analysis of the relationship between lycopene and respiratory disease mortality. (e) RCS analysis of the relationship between lutein and zeaxanthin and the prevalence of asthma. (f) RCS analysis of the relationship between total carotenoids and the prevalence of emphysema. (g) RCS analysis of the relationship between β-cryptoxanthin and the prevalence of emphysema. (h) RCS analysis of the relationship between β-cryptoxanthin and the prevalence of chronic bronchitis. RCS, restricted cubic spline.
Association between serum α-carotene and respiratory morbidity, all-cause and disease-specific mortalities
Respiratory morbidity was not associated with serumα-carotene levels (Table 3). Compared with the lowest tertile of serumα-carotene, the participants in the highest tertile had lower mortality of all-cause (HR = 0·76, 95 % CI 0·69, 0·84) and respiratory diseases (HR = 0·54, 95 % CI 0·36, 0·82) in Table 4. RCS curve results are shown in Fig. 1. Similarly, sensitivity analyses were performed, and none of them affected the above results.
Association between serum β-carotene and respiratory morbidity, all-cause and disease-specific mortalities
Respiratory morbidity was not associated with serum β-carotene levels (Table 3). Compared with the lowest tertile of serum β-carotene, the participants in the highest tertile had lower mortality of all cause (HR = 0·82, 95 % CI 0·74, 0·91). And no significant association between serum carotenoids and respiratory disease mortality was observed (HR = 0·71, 95 % CI 0·48, 1·05) in Table 4. RCS curve results are shown in Fig. 1. Similarly, sensitivity analyses were performed, and none of them affected the above results.
Association between serum β-cryptoxanthin and respiratory morbidity, all-cause and disease-specific mortalities
Table 3 shows that emphysema and chronic bronchitis morbidity were significantly associated with serum β-cryptoxanthin levels (ORemphysema = 0·67, 95 % CI 0·49, 92; ORchronic bronchitis = 0·66, 95 % CI 0·50, 0·87). Compared with the lowest tertile of serum β-cryptoxanthin, the participants in the highest tertile had lower mortality of all-cause and respiratory diseases (HR = 0·83, 95 % CI 0·75, 0·91) in Table 4. RCS curve results are shown in Fig. 1. Meanwhile, sensitivity analyses were performed, and none of them affected the above results.
Association between serum lutein/zeaxanthin and respiratory morbidity, all-cause and disease-specific mortalities
Table 3 shows that asthma morbidity was associated with serum lutein/zeaxanthin levels (Q2: OR = 0·78, 95 % CI 0·62, 0·97). Compared with the lowest tertile of serum lutein/zeaxanthin, the participants in the highest tertile had lower mortality of all-cause (HR = 0·83, 95 % CI 0·76, 0·91) and respiratory diseases (HR = 0·48, 95 % CI 0·33, 0·71) in Table 4. RCS curve results are shown in Fig. 1. Similarly, sensitivity analyses were performed, and none of them affected the above results.
Association between serum lycopene and respiratory morbidity, all-cause and disease-specific mortalities
Respiratory morbidity was not associated with serum lycopene levels (Table 3). Compared with the lowest tertile of serum lycopene, the participants in the highest tertile had lower mortality of all-cause (HR = 0·75, 95 % CI 0·68, 0·83) and respiratory diseases (HR = 0·66, 95 % CI 0·45, 0·96) in Table 4. RCS curve results are shown in Fig. 1. Meanwhile, sensitivity analyses were performed, and none of them affected the above results.
Discussion
This study examined the association of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene with the prevalence of asthma, emphysema and chronic bronchitis, and mortality of all-cause and respiratory diseases. This study found that participants with higher total carotenoids and β-cryptoxanthin levels had a lower prevalence of emphysema and chronic bronchitis, and higher total carotenoids, α-carotene, lutein/zeaxanthin and lycopene levels had lower mortality of respiratory disease(Reference Wood, Garg and Blake27,Reference Wood and Gibson28) .
Carotenoids are coloured pigments synthesised by plants, fungi or bacteria. Carotenoids are important markers of fruit and vegetable intake but are also associated with polyphenol, dietary fibre intake(Reference Eggersdorfer and Wyss29–Reference Nakano, Tanaka and Tsuruya31). Lutein/zeaxanthin may be mainly found in maize foods, while lycopene is mainly found in tomato sauce and many fast foods, and the distribution of foods will also affect their intake(Reference Böhm, Lietz and Olmedilla-Alonso32). They have potent anti-cancer properties, intercellular signalling conduction and are involved in antioxidant activities in vivo as well as immune system regulation(Reference Yeum and Russell12,Reference Milani, Basirnejad and Shahbazi13,Reference Manochkumar, Doss and El-Seedi33) . These could explain the relationship between serum carotenoids and respiratory disease morbidity and mortality observed in this study. Among them, serum carotenoids have been shown to reduce lung disease damage through antioxidant activity(Reference Dianat, Radan and Badavi34,Reference Kodama, Kishimoto and Muramatsu35) . In a study of mice with lung injury, different doses of lycopene were administered by gavage, and the results showed that lycopene had a protective effect on lung injury and could reduce lipid peroxidation and DNA damage, increased SOD, CAT and GSH activity, minimising redox processes(Reference Campos, de Oliveira Ramos and Martins36).
The study found that participants with higher total carotenoids and α-carotene levels had a lower mortality of respiratory disease. This is consistent with recent reports(Reference Wood, Garg and Blake27,Reference Jun and Root37) , but no association was found in people whose BMI was higher than 25 (P = 0·516). This may be due to the aggravation of oxidative stress caused by obesity, which makes the protective effect of serum carotenoids not obvious(Reference Pérez-Torres, Castrejón-Téllez and Soto38–Reference Grasemann and Holguin40). And no association was found in current smokers (P α-carotene = 0·525), while there is no doubt that smoking does damage to the lungs, and it will greatly affect the relationship between the association(Reference Handelman, Packer and Cross41). Participants with higher total carotenoids had a lower prevalence of emphysema, but no association was found in sensitivity analyses stratified by smoking (P current smokers = 0·141, P Never/former smokers = 0·118), and the underlying mechanism is unclear.
Participants with higher β-cryptoxanthin had a lower prevalence of emphysema and chronic bronchitis. In an experiment investigating the effects of nicotine on mice, β-cryptoxanthin (BCX) was found to inhibit nicotine-induced emphysema and lung tumourigenesis(Reference Iskandar, Liu and Smith42). The association between β-cryptoxanthin and chronic bronchitis was also observed in non-smokers, consistent with recent studies(Reference Jun and Root37), but no association was found in current smokers (P emphysema = 0·188, P chronic bronchitis = 0·162), while smoking has an indelible effect on the lungs and increases oxidative stress in the lungs, this may nullify the protective effects of carotenoids(Reference Handelman, Packer and Cross41). Interestingly, although the main results of this study did not find an association between β-carotene and respiratory disease morbidity and mortality, a positive association between carotene and asthma was found in smokers. In a clinical trial study, β-carotene was found to be positively associated with lung function(Reference Jun and Root37), and in an epidemiological study, β-carotene supplementation was also found to increase the risk of lung disease(Reference Hemilä, Virtamo and Albanes43), which is consistent with the results reported above.
This study found that participants with higher lutein/zeaxanthin levels had lower mortality of respiratory disease. In a study of forced expiratory volume in 1 s and forced vital capacity with several carotenoids, lutein/zeaxanthin was found to play an important role in respiratory health, which supports the above results(Reference Schünemann, McCann and Grant44). Similarly, this study found that the relationship between lutein/zeaxanthin and death from respiratory disease was also observed in a sensitivity analysis stratified by smoking and BMI, demonstrating the robustness of the results. Moreover, the association between lutein/zeaxanthin and respiratory disease mortality remained significant in persons older than 45 years. Participants with higher lutein/zeaxanthin had a lower prevalence of asthma (Q2: OR = 0·78, 95 % CI 0·62, 0·97)), this relationship can also be seen in the sensitivity analysis, which is consistent with recent research(Reference Jun and Root37,Reference Handelman, Packer and Cross41) .
Finally, this study found that participants with higher lycopene levels had lower mortalities of respiratory disease. In a study of ferrets exposed to tobacco carcinogens and cigarette smoke, with or without low and high doses of lycopene, lycopene was found to significantly inhibit both tobacco carcinogens and cigarette smoke-induced total accumulation of cholesterol and increases the mRNA expression of key genes related to reverse cholesterol transport (PPARα, LXRα and ATP-binding cassette transporters ABCA1 and ABCG1) in the lung(Reference Mustra Rakic, Liu and Veeramachaneni45). In a case–control study, where lutein was inversely associated with IL-6, lycopene was negatively associated with SAT, β-carotene was positively associated with a Mediterranean-style diet and COPD patients may particularly need a lycopene-rich diet, which is consistent with previous research findings(Reference Campos, de Oliveira Ramos and Martins36,Reference Kentson, Leanderson and Jacobson46) . However, no association was found between β-cryptoxanthin and respiratory disease mortality among those with a BMI of less than 25 (P = 0·066), and the underlying mechanism is unclear.
An important limitation of this study is that serum carotenoid levels were measured when participants first joined the study, which was too long since follow-up outcomes to address the effects of changes in participants’ serum carotenoid levels. Furthermore, the self-reported data are somewhat biased and not entirely correct. In addition, this study was observational. Although we have adjusted for most known confounding factors, we cannot exclude the possibility that unmeasured variables may have influenced our results. Finally, the NHANES III-linked mortality profile is constructed by the cause of death through the National Mortality Index, in which there may be errors in the classification of the cause of death. In conclusion, the results of the present study show that participants with higher serum total carotenoids and β-cryptoxanthin levels are associated with a decreased prevalence of emphysema and chronic bronchitis, and higher serum total carotenoids, α-carotene, lutein/zeaxanthin and lycopene levels had a lower mortality of respiratory disease.
Conclusions
Higher serum total carotenoids and β-cryptoxanthin levels are associated with a decreased prevalence of emphysema and chronic bronchitis, and higher serum total carotenoids, α-carotene, lutein/zeaxanthin and lycopene levels had a lower mortality of respiratory disease. These findings indicate that serum carotenoids can be considered a supplementary treatment for people with respiratory diseases.
Acknowledgements
The authors thank the participants and staff of the National Health and Nutrition Examination Survey from 1988 to 1994 for their valuable contributions.
This research was supported by funds from HMU Marshal Initiative Funding (HMUMIF-21013 to Wei Wei).
Q. R. and W. W. and X. C. conceived the study conceptualization. W. W. provided the data. R. Y. and Z. C. and X.L. did the formal analysis. R. Y. and M. L. and M. X. did the visualization. R. Y. and Y. C. and L. C. wrote the original draft. All authors provided critical revisions of the draft and approved the submitted draft. Q. R. and W. W. are the guarantor of this work and are responsible for the integrity of the data and the accuracy of the data analysis.
The authors did not have any competing interests to declare.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114523000806








