I is a trace element necessary for the synthesis of thyroid hormones, triiodothyronine and thyroxine, which are critical for human growth and development( Reference Halpern, Boyages and Maberly 1 , Reference Kapil 2 ). I is particularly important during pregnancy for brain and neurological development( Reference Zimmermann 3 ). Severe I deficiency during pregnancy can cause cretinism, a condition of mental and physical impairment( Reference Halpern, Boyages and Maberly 1 ). Even mild-to-moderate I deficiency during pregnancy can impair cognitive development in the offspring( Reference Bath, Steer and Golding 4 ). Of particular public health concern in the UK and Ireland is the prevalence of mild-to-moderate I deficiency in women of childbearing age( Reference Nawoor, Burns and Smith 5 – Reference Lampropoulou, Lean and Combet 7 ).
Adequate dietary I intake is required before conception and during pregnancy to meet the requirement for increased maternal thyroid hormone synthesis, I renal clearance and I transfer to the fetus( Reference Glinoer 8 , Reference Bath and Rayman 9 ). Much debate surrounds the UK dietary recommendations for I, which have not been updated in more than 20 years, and, despite the WHO recommending an incremental intake of 250 µg/d during pregnancy, in the UK, the dietary recommendation for both pregnant women and women of childbearing age remains at 140 µg/d( 10 , 11 ).
There are a number of dietary factors that may increase the risk of I deficiency in women of childbearing age: consumption of seafood and dairy products is lower compared with other population groups; milk consumption is declining; and there is limited consumption of I-containing supplements( Reference Bates, Lennox and Prentice 12 – Reference Bath, Sleeth and McKenna 14 ). Organic milk is increasing in popularity and as it typically contains 30 % less I than conventional cow’s milk, and those who replace conventional cow’s milk with organic milk are likely to have a reduced I intake( Reference Bath, Button and Rayman 15 ). The most recent National Diet and Nutrition Survey reported that women aged 19–64 years have a median I intake of 130 (interquartile range 48–283) µg/d and 10 % have a daily I intake below the lower reference nutrient intake (LRNI)( Reference Bates, Lennox and Prentice 12 ). In the UK and Ireland, there is currently no national strategy to address I deficiency. Along with other European countries, an iodised salt programme as recommended by the WHO in order to increase I intake and to eliminate I deficiency has not been implemented( 11 ). As a consequence, the availability of iodised salt in the UK is low and is unlikely to contribute significantly to I intake( Reference Bath, Button and Rayman 16 ).
It has been suggested that a low level of knowledge surrounding I is a major factor contributing to the risk of I deficiency( Reference Jooste, Upson and Charlton 17 – Reference Combet, Bouga and Pan 22 ). Studies of pregnant women in Australia reported that more than half of the participants could not identify any adverse health problem related to I deficiency, and only 19 % were aware that they needed I supplements( Reference El-Mani, Charlton and Flood 23 , Reference Martin, Savage and Mitchell 24 ). Furthermore, a recent UK study reported that the majority of pregnant women were aware of the general diet and lifestyle recommendations during pregnancy but knowledge of I nutrition was low( Reference Combet, Bouga and Pan 22 ). Improving public knowledge and awareness of I nutrition may play an important role in reducing the prevalence of I deficiency.
To our knowledge, there are no public education initiatives aimed at improving I knowledge in the UK or Ireland. Although the level of I knowledge in pregnant women and mothers of children aged 0–36 months has been previously reportedReference Combet, Bouga and Pan ( 22 ) , I knowledge in women of childbearing age is currently unknown. Women of childbearing age, pregnant women and those planning a pregnancy in the UK are not routinely provided with specific dietary I information, and the availability of scientifically accurate I nutrition information is limited to one fact sheet produced by the British Dietetic Association( Reference Bath and Rayman 9 , Reference Bath and Rayman 25 ).
The present study, therefore, aimed to investigate I knowledge and awareness among women of childbearing age in the UK and Ireland, and to determine whether a relationship exists between I knowledge and dietary I intake.
Methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by Ulster University, School of Biomedical Sciences, Ethics Filter Committee. Data were collected anonymously, and participants were advised that by completing the questionnaire they were giving consent for their data to be used for the present study. On the basis of an estimated 10 % of women failing to meet the LRNI( Reference Bates, Lennox and Prentice 12 ) (α=0·10, β=0·8), the sample size calculation estimated a requirement of a minimum of n 448 subjects.
Females of childbearing age (18–45 years) were invited to complete a twenty-nine-item questionnaire (online Supplementary Material), which was administered online using SurveyMonkey® from 23 February to 16 March 2015. The questionnaire was designed to assess I nutrition knowledge and dietary I intake. Participants were also asked to provide general demographic information on age, ethnicity, education and employment status. Information was also collected on the diagnosis of a thyroid condition, smoking status, pregnancy and lactation. The questionnaire was pre-tested and modified to ensure there was no ambiguity in the questions and to identify any potential problems the user might experience when completing the survey.
The study was advertised via emails to Ulster University staff and students and externally through social media.
Assessment of iodine knowledge
Questions to assess I knowledge were adapted from previous I knowledge studies( Reference Jooste, Upson and Charlton 17 , Reference Kim, Jeong and Seok 21 , Reference El-Mani, Charlton and Flood 23 , Reference Charlton, Yeatman and Lucas 26 ). Participants were asked five questions in this section on their knowledge and awareness of (a) the nutrient I (see question 11 in the online Supplementary Material), (b) the food sources of I (question 12), (c) health problem(s) associated with I deficiency (question 13), (d) the most at-risk population group(s) (question 14) and (e) I deficiency as a current health concern in the UK and Ireland (question 15). The questionnaire also asked whether participants had ever received information on I and collected information on these resources.
On the basis of the Jooste et al. ( Reference Jooste, Upson and Charlton 17 ) questionnaire, scores for the four individual questions assessing I knowledge (see questions 12–15 above) were combined to calculate a total I knowledge score ranging from 0 to 8. The maximum scores available for each question were as follows: question 12 (1), question 14 (1) and question 15 (1). There was a total of five correct answers for question 13, and for each correct answer (goitre, mental retardation, malformations in pregnancy (birth defects), impaired physical development during childhood and blindness) selected, participants were awarded 1 point (for a total of five). Total I knowledge scores were categorised into four levels as follows: (a) score 0, (b) score 1–3, (c) score 4–6 and (d) score 7–8, with a score of 0 indicating poor I knowledge and 7–8 indicating excellent I knowledge. For multiple-choice questions, random order of answers was utilised.
Assessment of iodine intake
The questionnaire incorporated the previously validated Glasgow Iodine FFQ to estimate intake of I-containing foods over the previous 6 months( Reference Combet and Lean 27 ). The Glasgow Iodine FFQ was modified to include intake of eggs owing to evidence that eggs can contribute to the total dietary I intake( Reference Bath, Sleeth and McKenna 14 ). Food sources of I were grouped into nine categories: milk, eggs, oily fish, white fish, other seafood, yogurts, cheese, cheese-based dishes, and milk- or cream-based puddings. Participants were asked to record how frequently they consumed each food group (per day, per week or per month). The I content of all foods included in the questionnaire was sourced from the UK food composition database, and information on typical portion size was obtained( 28 ). These data were then combined to estimate the total daily I intake (µg/d) and the intake from the following food groups: milk, eggs, fish and other dairy products. Participants were also asked about their use of I containing supplements (i.e. frequency of consumption, dose, brand, duration).
Statistical analysis
Statistical analyses were conducted using Statistical Package for Social Sciences, version 22. Descriptive statistics were conducted for all outcome variables. Participants were categorised into five age groups: (a) 18–25 years, (b) 26–30 years, (c) 31–35 years, (d) 36–40 years and (e) 41–45 years. Participants were also categorised according to I intake: (a) below the LRNI (70 µg/d), (b) between the LRNI and the reference nutrient intake (RNI) (70·1–139·9 µg/d) and (c) meeting the RNI (≥140 µg/d)( 10 ). Normality was assessed using the Kolmogorov–Smirnov test. To investigate the effect of demographic characteristics on total I knowledge scores, χ 2, Mann–Whitney U tests and Kruskal–Wallis tests were used for non-parametric data, and one-way ANOVA analyses were conducted on parametric data. Data on total I intake and the contribution of food groups to I intake were not normally distributed after transformation. Subsequently, the non-parametric Kruskal–Wallis and χ 2 tests were used to study the relationships between demographic characteristics and total I knowledge on I intakes. To investigate whether I supplementation affected I knowledge, χ 2 and one-way ANOVA analyses were conducted. Spearman’s correlation analysis was used to assess the relationship between total dietary I intake and scores of I knowledge. Results for non-parametric data are presented as median values and interquartile ranges. Results for parametric data are presented as mean values and standard deviations. A P-value of <0·05 was considered statistically significant throughout.
Results
Table 1 outlines the participants’ demographic characteristics. A total of 520 females completed the questionnaire, of whom 9 % were smokers, 4 % were currently pregnant and 2 % were currently breast-feeding. A total of 5 % reported diagnosis of a medical condition affecting the thyroid gland.
Table 1 Participant characteristics (n 520) and the relationship between participant characteristics and total iodine knowledge scores (0=poor knowledge, 8=excellent knowledge) (Mean values and standard deviations)

GCSE, General Certificate of Secondary Education; A-Level, Advanced Level; BTEC, Business and Technology Education Council.
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* P value for comparison between groups from independent t test, Mann–Whitney U test, ANOVA or Kruskal–Wallis test.
Iodine knowledge
The mean total I knowledge score was 2·1 (sd 0·8) out of a maximum score of 8 (Table 1). The majority of participants had a mean I knowledge score between 1 and 3 (56 %). Table 1 outlines total I knowledge scores according to demographic characteristics. Those who previously received I information (9 %) had a significantly greater awareness of I, I deficiency and better knowledge of the dietary sources of I and the health problems associated with I deficiency than those who had never received information related to I (all P<0·05). Many of the current study participants who had obtained I nutrition information did so during time spent in formal education (75 %). Among other demographic characteristics, only age was associated with significant differences in total I knowledge scores (Table 1).
In general, participant awareness of I was low; only 43 % of the participants were aware of the nutrient I. Those with a higher level of education had a significantly greater awareness of the nutrient I (P=0·020) (data not shown). Knowledge of pregnancy as being the most important stage of the lifecycle for I was also low (Table 2). Age and employment status significantly affected participant knowledge, in that younger participants and those who were in either full or part-time employment or were currently studying were more likely to correctly identify pregnancy as the most important stage of the lifecycle for I nutrition in comparison with older participants (P<0·001) and those who were unemployed, self-employed or retired (P=0·008) (data not shown).
Table 2 Knowledge of the stage of lifecycle when iodine is most important as identified by participants (Numbers and percentages)

n, Number of participants; %, percentage of participants.
* Denotes correct answer.
Almost half (41 %) of the participants ‘did not know’ or could not correctly identify any health problem associated with I deficiency. Goitre was recognised as a health problem associated with I deficiency in one-fifth of participant responses (19 %). Knowledge on the health problems resulting from I deficiency was lowest in Northern Ireland (n 355) in comparison with those from the Republic of Ireland (n 116) and other UK regions (n 49) (P=0·016) (data not shown). Knowledge of the health problems associated with I deficiency was not significantly related to any other demographic characteristic.
As shown in Table 3, 39 % of the participants identified fish and seafood as the richest source of I and only 9 % identified milk and dairy products. Younger participants (18–25 years) had significantly better knowledge of the dietary sources of I than older participants (41–45 years) (P<0·001) (data not shown). Knowledge of the dietary sources of I was not significantly different among women who were pregnant or breast-feeding. Less than one-third (27 %) of participants were aware that I deficiency is a current public health concern in the UK and Ireland. Those who had been diagnosed with a thyroid condition (n 25) had a significantly greater awareness of I deficiency as a current health problem in the UK and Ireland than those who did not have a thyroid condition (n 481) (P<0·001) (data not shown).
Table 3 Participant identification of the food groups richest in iodine in the UK and Ireland (Numbers and percentages)

n, Number of participants; %, percentage of participants.
* Denotes correct answers.
Dietary iodine intake
Complete dietary I intake data were available for 502 participants. The median daily I intake was estimated as 152 (interquartile range 98–213) µg/d. A total of 46 % of the participants failed to meet the RNI of 140 µg of I/d (Fig. 1). I intake was not significantly different between age groups (P=0·080) (data not shown). A significantly greater percentage of unemployed and self-employed participants failed to meet the RNI compared with those who were in full-time or part-time employment or studying (83 v. 45 %; P=0·008) (data not shown). There was no association between total daily I intake, being currently pregnant or breast-feeding, level of education, smoking status, diagnosis of a thyroid condition or having previously received I information (all P>0·05) (data not shown).
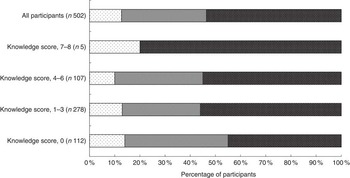
Fig. 1 Percentage of respondents with daily iodine intake below the lower reference nutrient intake (LRNI) (≤70 µg/d), between the LRNI and the reference nutrient intake (RNI) (70·1–139·9 µg/d) and meeting the RNI (≥140 µg/d)(
10
). ![]() , <LRNI;
, <LRNI; ![]() , LRNI–RNI;
, LRNI–RNI; ![]() , >RNI.
, >RNI.
Milk and dairy products made the largest contribution to I intake in this cohort as detailed in Table 4. Older participants (31–45 years) had a significantly higher I intake from milk than younger participants (18–31 years) (P=0·025) (data not shown). The majority of participants never consumed organic milk (74 %), 20 % consumed organic milk sometimes and 6 % consumed organic milk often or always. Iodised salt was not widely consumed; only 7 % stated that they purchased iodised salt for use at home. Participants who stated that they consumed iodised salt (n 37) were asked to state which iodised salt brand they consumed, and in total twelve salt brands were listed. Contact was made with the salt manufacturers, and none of the salts listed by participants were found to have been fortified with I. One manufacturer reported that their salt contained trace amounts of I, and this means that I was present in the salt but not at a level that can be accurately measured. Only 4 % of the women surveyed reported consuming a supplement containing I. Although supplement consumption was significantly greater in pregnant or breast-feeding women than non-pregnant and non-breastfeeding women (P=0·010), only 12 % of pregnant or breast-feeding women reported consuming an I-containing supplement (data not shown). On the basis of manufacturers’ label information, ten of the thirteen dietary supplements consumed by participants contained I. The I content of these supplements ranged from 100 to 200 µg per recommended daily dosage, and the average I content was 132 µg per recommended daily dosage.
Table 4 The contribution of food groups to total daily iodine intake in women of childbearing age (Medians and 25th–75th percentiles)

Relationship between iodine knowledge and iodine intake
There was a weak positive association between total daily I intake and total I knowledge score as outlined in Table 5. Of those participants with a daily I intake above the LRNI, significantly more participants were aware of the nutrient I compared with those with an intake below the LRNI (P=0·043) (data not shown). A higher daily I intake from fish and dairy products was positively associated with awareness of I and knowledge of the health problems associated with I deficiency (P<0·050) (data not shown). Women consuming supplements (n 18) containing I had a significantly better awareness of the nutrient I, knowledge of I and of the health problems associated with I deficiency compared with those not consuming a supplement (P<0·020) (data not shown).
Table 5 Associations between daily iodine intake and iodine knowledge and awareness

* Assessed by Kruskal–Wallis test.
† Assessed by Spearman’s correlation analysis.
Those who reported consuming organic milk ‘often’ (n 21) or ‘always’ (n 9) had a significantly lower awareness of the nutrient I than those who ‘sometimes’ or ‘never’ consumed organic milk (P=0·039) (data not shown). Consumption of organic milk was negatively associated with total I knowledge; significantly more women who reported consuming organic milk ‘often’ or ‘always’ had a total I knowledge score of 0 (P=0·008) (data not shown).
Discussion
Despite growing concern regarding the prevalence of I deficiency among women of childbearing age in the UK and Ireland, the present study has shown that there is a lack of I knowledge among this population group. Women of childbearing age were shown to have limited knowledge of the dietary sources of I, the problems arising from deficiency and the population group most at risk of deficiency. The present findings also indicate that women of childbearing age are relatively unaware of I deficiency as a current health problem in the UK and Ireland. Similar levels of I knowledge have been reported in previous studies in other countries, and it has been suggested that a low level of knowledge surrounding I is a major factor contributing to the risk of I deficiency( Reference Jooste, Upson and Charlton 17 – Reference Combet, Bouga and Pan 22 ). As demonstrated by the present study, I knowledge was positively associated with I intake. Therefore, a poor knowledge of dietary I sources helps explain why almost half of the participants had a daily I intake below the RNI. Similar to the latest National Diet and Nutrition Survey findings, 13 % of the participants in the current study failed to meet the LRNI( Reference Bates, Lennox and Prentice 12 ). Factors such as socio-economic status and food availability may also in part explain why dietary recommendations were not met. These findings also suggest that it may be difficult for women who become pregnant to ensure an adequate daily I intake, given that their knowledge of the dietary I sources is poor.
There have been several measures implemented worldwide to eradicate I deficiency with the primary method being salt iodisation. Policy makers in the UK and Ireland have been reluctant to implement an iodised salt policy, and therefore viable alternatives to iodised salt are important for increasing I status and preventing deficiency. The current study confirms that iodised salt is not widely consumed, and is therefore unlikely to contribute significantly to I intake, consistent with previous studies( Reference Bath, Button and Rayman 16 , Reference Bath, Steer and Emmett 29 ). I-containing supplements were not widely consumed by this cohort, and there was large variability in the I content of nutritional supplements. The results of this survey also indicate that there is a lack of awareness about which nutritional supplements and salt brands contain I. It is noteworthy that approximately one-fifth of the participants were consumers of organic milk, although only ‘sometimes’, as recent research has found that organic milk typically contains 30 % less I than conventional milk( Reference Bath, Button and Rayman 15 , Reference Payling, Juniper and Drake 30 ). Organic milk consumers in the present study had a significantly lower I intake than conventional milk consumers. In general, women of childbearing age should be made more aware of the dietary sources of I. Those who avoid milk and dairy products may be at risk of consuming insufficient I as conventional cow’s milk makes the largest contribution to dietary I intakes in many countries including the UK and Ireland( Reference Bates, Lennox and Prentice 12 , 31 ). Observational evidence from several countries has consistently reported milk consumption to be positively associated with I intake and status( Reference Perrine, Herrick and Serdula 32 – Reference Fernando, Cavedon and Nacamulli 34 ). It should also be communicated to pregnant women and women of childbearing age that organic milk has a lower I concentration and they should be made aware of other dietary I sources.
There is a clear need for greater public awareness of the importance of I nutrition for health. Previous studies have investigated how women of childbearing age would prefer to have I nutrition education, and reported that information delivered via health practitioners or mass media was the preferred option( Reference Charlton, Yeatman and Houweling 19 ). Mass media strategies are integral to increasing public awareness and knowledge and should be considered for increasing I knowledge( Reference Mehran, Nazeri and Delshad 35 ). There is also a need to update healthcare professionals, particularly those involved in the care of women planning a pregnancy, on the importance of I as previous research has demonstrated that midwives and other healthcare professionals have a low level of I knowledge( Reference Williamson, Lean and Combet 36 – Reference El-Mani, Mullan and Charlton 38 ). To address I deficiency sufficiently, healthcare professionals will be instrumental as they are in a position to advocate for policy change both within their own practices and in the external policy environment. I knowledge was still relatively low for those who had previously received I information, and it could therefore be recommended that changes to the curriculum and informing educators on the importance of I nutrition may be beneficial for improving knowledge and awareness of I. To target teenage girls and younger women before pregnancy may be the most valuable public health strategy given that maternal I stores are essentially utilised during the first trimester( Reference Glinoer 8 ).
There are a number of considerations to be taken into account when interpreting the present study’s findings. When compared with UK population data for females, there was a lower smoking prevalence and a higher prevalence of thyroid disorders in this cohort of women of childbearing age( Reference Vanderpump, Tunbridge and French 39 , 40 ). Furthermore, this was largely a Northern Irish population (68 %), and compared with national averages for the UK a higher proportion of the women who participated in this study had a university degree owing to recruitment primarily within a university setting. Considering that this cohort had attained higher levels of education it is likely that I knowledge in the general population may be lower( 41 , 42 ). There were some differences in I knowledge and awareness according to employment status and highest level of education attained, which may be indicative of socio-economic status. Future studies should investigate the potential barriers to I knowledge and status in women of childbearing age with lower socio-economic status. This research could potentially be used to inform policy in the context of increasing I knowledge, dietary intake, and thus status.
This study was limited at assessing the I knowledge of pregnant and breast-feeding women as there were a few pregnant and breast-feeding participants. However, given their low level of knowledge previously reported( Reference Combet, Bouga and Pan 22 , Reference Martin, Savage and Mitchell 24 , Reference Lucas, Charlton and Brown 37 , Reference Axford, Charlton and Yeatman 43 ), educational intervention among pregnant and breast-feeding women should be considered. A number of these studies were conducted in Australia and New Zealand, and these studies( Reference Martin, Savage and Mitchell 24 , Reference Lucas, Charlton and Brown 37 , Reference Axford, Charlton and Yeatman 43 ) were conducted at a time when mandatory bread fortification with iodised salt had been introduced; however, I knowledge was low. Australia and New Zealand are also industrialised countries at a similar stage of economic development as the UK and are countries that share similar political and legal systems, diets and cultures as the UK, which increases the comparability of studies between countries.
Estimating dietary I intake presents many challenges, and when using FFQ to estimate dietary intake there are inherent limitations such as precision and reporting bias to be taken into account( Reference Byers 44 , Reference Kristal, Peters and Potter 45 ). It is also recognised that FFQ often overestimate the intake of ‘healthy’ foods and underestimate the intake of more ‘unhealthy’ foods( Reference Fallaize, Forster and Macready 46 ). In the context of this study, estimated I intakes from the FFQ may be overestimated and I intakes may be lower than reported. The FFQ used as part of this study has previously been validated in women of childbearing age( Reference Combet and Lean 27 ) and was the best available FFQ for assessing I intake. The large sample size in the present study may increase the validity of I intake estimations from the FFQ. The FFQ used was I specific and was validated for estimating I intake at a group level, making it suitable for the current study. However, it was not designed to estimate I intake at the individual level and, as such, there is a possibility that some participants may have been misclassified (<RNI) on the basis of their I intake. A further limitation of this study is that dietary I intake was only captured on one occasion. There is also concern regarding the over-interpretation of distribution data both for urinary iodine concentration (UIC) and dietary intakes. A new approach has been suggested whereby UIC are extrapolated to dietary I intake, which is then adjusted for intra-individual variation and interpreted according to estimated average requirement( Reference Zimmermann and Andersson 47 ). To corroborate the findings reported here, biomarkers of I intake should be measured including UIC and thyroglobulin, a promising biomarker of I status( Reference Vejberg, Knudsen and Perrild 48 ), to further investigate the association between I knowledge and I intake in women of childbearing age.
Conclusion
This study is the first to investigate I nutrition knowledge among women of childbearing age in the UK and Ireland. A low level of I knowledge was apparent and this was reflected in the low dietary I intake and the infrequency of consumption of I-containing supplements. As part of a larger public health policy to eradicate I deficiency, educational intervention should be considered. Among women of childbearing age, targeted public health campaigns are warranted to increase I nutrition knowledge and intake.
Acknowledgements
The authors wish to thank all the study participants.
S. M. O. is in receipt of a postgraduate studentship from the Department of Agriculture, Environment and Rural Affairs (DAERA), which funded this research. DAERA had no role in the design, analysis or writing of this article.
A. J. Y., L. K. P., M. S. M. and J. J. S. were responsible for the study design. K. M. F., L. K. P., A. J. Y. and S. M. O. designed the questionnaire and obtained ethical approval. S. M. O. and K. M. F. were responsible for data collection. S. M. O. conducted statistical analysis with guidance from L. K. P. S. M. O. prepared the initial manuscript, which was edited by L. K. P., A. J. Y., M. S. M. and J. J. S. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516003925









