Fe deficiency is the most common human nutritional deficiency in the world. It is caused not only by low intake but more often by poor bioavailability from the diet, due to Fe interaction with other dietary components(Reference Baynes and Bothwell1). Dietary fibres have been shown to impair the absorption of minerals in the small intestine because of their binding and/or sequestering effect(Reference Bosscher, Van Caillie-Bertrand and Deelstra2, Reference Fuchs, Farris and DeWier3).
Pectin is primarily a polymer of α-(1 → 4)-glycosidic-linked d-galacturonic acid residues that are usually esterified to various degrees with methanol(Reference Van Soest4). Dietary fibres with a high content of carboxyl groups, such as pectin, have increased cation-binding ability, because the carboxyl group is deprotonated when pH is close to neutrality and can interact electrostatically with mineral cations. At the pH of the small intestine, about 6·5–7·0, carboxyl groups are deprotonated. In solution, the carboxyl groups of the unesterified units of pectin can bind cations such as Ca, Mg and Fe(Reference BeMiller, Fishman and Jen5–Reference Debon and Tester7), supporting the assumption that pectin in the diet would reduce mineral bioavailability. However, if the binding of mineral cations by pectin depends on the carboxyl groups, this binding appears to be reversible and would be affected by pH, ionic strength and temperature. Very few studies have reported on the bioavailability of Fe bound to pectin.
In the present study, we have investigated the availability of Fe bound to pectin.
Materials and methods
Pectin
Commercial citrus pectin (Classic AM201) was purchased from Herbstreith & Fox GmbH (Neuenburg, Germany). The degree of esterification of pectin and the Fe content in pectin are 72 % and 7·2 μg/g, respectively. The Fe content in pectin was measured by flame atomic absorption spectrophotometry (AA 6400F; Shimadzu, Kyoto, Japan) after wet ashing in HNO3–HClO4 (3:1). To determine Fe levels, the standard addition method was used.
In vitro study
Determination of iron released from pectin by ultrafiltration
The influence of pH and ion length on the release of Fe bound to pectin was examined by ultrafiltration.
Briefly, 10 ml of pectin solution (1 g/100 ml) were introduced into the chamber of a batch-type stirred ultrafiltration cell (Model 8010; Amicon, Beverly, MA, USA) fitted with a disc membrane (YC01; Amicon) with a molecular weight cut-off of 500. The pectin solution was filtered under pressure (about 3·5 kg/m3) of N2 gas until about 20 % of the original volume remained in the cell. The filtrate was collected. The chamber was covered with a polyethylene tube (IGM, outer diameter × inner diameter, 4 mm × 2 mm; HAGITEC Company Limited, Yotsukaido, Chiba, Japan), and the pectin solution in the chamber was maintained at 37°C by circulating hot water (37°C) in the tube.
Before starting the in vitro study, we confirmed that galacturonic acid was not detected in the solution filtered by ultrafiltration. Total Fe content in the filtrate was measured by flame atomic absorption spectrophotometry (AA 6400F; Shimadzu) after wet ashing in HNO3–HClO4 (3:1). Ferrous iron (FeII) content in the filtrate was determined by the bathophenanthroline method. Ferrous iron+ferric iron (FeII+FeIII) content in the filtrate was determined by the bathophenanthroline method after reducing with hydroxylamine hydrochloride solution (4 %, w/v). FeIII content was determined using the difference between FeII and FeII+FeIII measurements. Non-ionic Fe was determined using the difference between FeII+FeIII and total Fe.
Effect of pH on the release of iron bound to pectin
Briefly, 1 g of pectin was dissolved in 150 mm-NaCl solution to give 10 ml and adjusted to pH 2·0, 3·0, 5·0, 7·0 and 9·0 with 0·1 m-HCl or 0·1 m-NaCl. The pectin solution was ultrafiltrated, and 8 ml of the filtrate were collected.
Effect of ionic strength on the release of iron bound to pectin
In brief, 1 g of pectin was dissolved in distilled water to give 10 ml and adjusted to pH 2·0 or 5·0. The pectin solution was ultrafiltrated, and 8 ml of the filtrate were collected.
Effect of pectin as a reducing agent
Briefly, 0·5 g of pectin were dissolved in 275 mm-NaCl solution to give 40 ml and adjusted to pH 2·0 with 0·1 m-HCl. The pectin solution was increased to 50 ml using distilled deionised water. The final concentration of NaCl was 150 mm. Then, 10 ml of pectin solution were ultrafiltrated, and 8 ml were collected. After filtrating, 8 ml of 150 mm-NaCl solution containing ferric chloride (Fe of 200 mg/l) were added to the residue and the solution was ultrafiltrated. Then, 8 ml of the filtrate were collected. In addition, 0·5 ml of ferric iron solution only (Fe of 200 mg/l) was dissolved in 275 mm-NaCl solution to give 40 ml and adjusted to pH 2·0 with 0·1 m-HCl. The ferric iron solution was increased to 50 ml using distilled deionised water. Ferric iron solution (10 ml) was ultrafiltrated, and 8 ml of the filtrate were collected.
In vivo study (animal experiment)
The present study was approved by the Laboratory Animal Care Committee of Ehime University. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University. The Fe content in the experimental diets was determined by flame atomic absorption spectrophotometry (AA 6400F; Shimadzu) after wet ashing in HNO3–HClO4 (3:1).
Experiment 1
Animals and diets
Wistar male rats weighing about 80 g (Japan SLC, Hamamatsu, Japan) were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (light on, 07.00–19.00 hours). The rats were acclimatised by feeding a commercial solid diet (MF; Oriental Yeast, Osaka, Japan) for 3 d. After acclimatisation, rats were randomly divided into five groups (n 6) and were allowed free access to distilled deionised water and one of the following diets for 3 weeks: Fe-deficient diet (FeD); diet containing ferrous iron at 3, 6 or 12 mg/kg diet (FeII-3, FeII-6 or FeII-12 diets); diet containing pectin at 50 g/kg diet (Table 1)(8). For each rat, body weight and food intake were recorded daily in the morning before the food was replaced. Ferrous sulphate (FeSO4.7H2O) was used as the source of ferrous iron. The amount of Fe/kg diet of the FeD, FeII-3, FeII-6, FeII-12 and the pectin diet was 1·7, 4·5, 7·6, 13·4 and 5·1 mg, respectively.
Table 1 Composition of the experimental diets
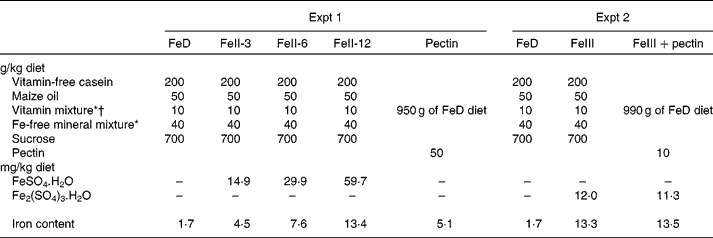
FeD, Fe-deficient diet; FeII-3, Fe at 3 mg/kg diet; FeII-6, Fe at 6 mg/kg diet; FeII-12, Fe at 12 mg/kg diet.
* Based on AIN-76(8).
† The vitamin mixture used in the present study contained 20 g choline chloride.
Experiment 2
Animals and diets
Wistar male rats weighing about 80 g (Tokushima Jikken-Dobutsu Kenkyusho, Tokushima, Japan) were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (light on, 07.00–19.00 hours). The rats were acclimatised by feeding a commercial solid diet (MF; Oriental Yeast) for 3 d. After acclimatisation, rats were randomly divided into three groups (n 6) and were allowed free access to distilled deionised water and one of the following diets for 21 d: FeD; FeIII; FeIII+pectin diet (Table 1). FeSO4.7H2O and ferric sulphate (Fe2(SO4)3.nH2O) were used as the sources of ferrous iron and ferric iron, respectively. For each rat, body weight and food intake were recorded daily in the morning before the food was replaced. The amount of Fe/kg diet of the FeD, FeIII and FeIII+pectin diets was 1·7, 13·3 and 13·5 mg, respectively.
Chemical analysis
Hb concentration was measured by the cyanmethaemoglobin method using a colorimetric haemoglobin assay kit (Hemoglobin-Test; Wako Pure Chemical Industries Limited, Osaka, Japan). Blood was obtained from the tail tip. To calculate total Hb content in the blood, the mass of blood was assumed to be 67 g/kg body mass, and Hb was assumed to contain 3·35 mg Fe/g(Reference Miller9). Hb regeneration efficiency was calculated according to the method of Mahoney & Hendricks(Reference Mahoney, Hendricks and Kies10).
Statistical analysis
All values in the in vivo studies (animal experiments) are given as means with their standard errors (n 6), and a P value of less than 0·05 was considered significant using the Tukey–Kramer honestly significant difference test using JMP® 6 (SAS Institute Japan, Tokyo, Japan).
Results
In vitro study
Effect of pH on the release of iron bound to pectin
The release of Fe from pectin was at a maximum at pH 2·0; however, the amount of released Fe was about one-third of the total Fe contained in pectin. The release of Fe from pectin was linearly decreased as the pH value increased (r − 0·991, P < 0·008 for ferrous iron and r − 0·984, P < 0·016 for ferric iron; Fig. 1). Most of the Fe released from pectin at pH 2·0 was ferrous iron. Most of the Fe released from pectin at pH 9·0 was non-ionic Fe.
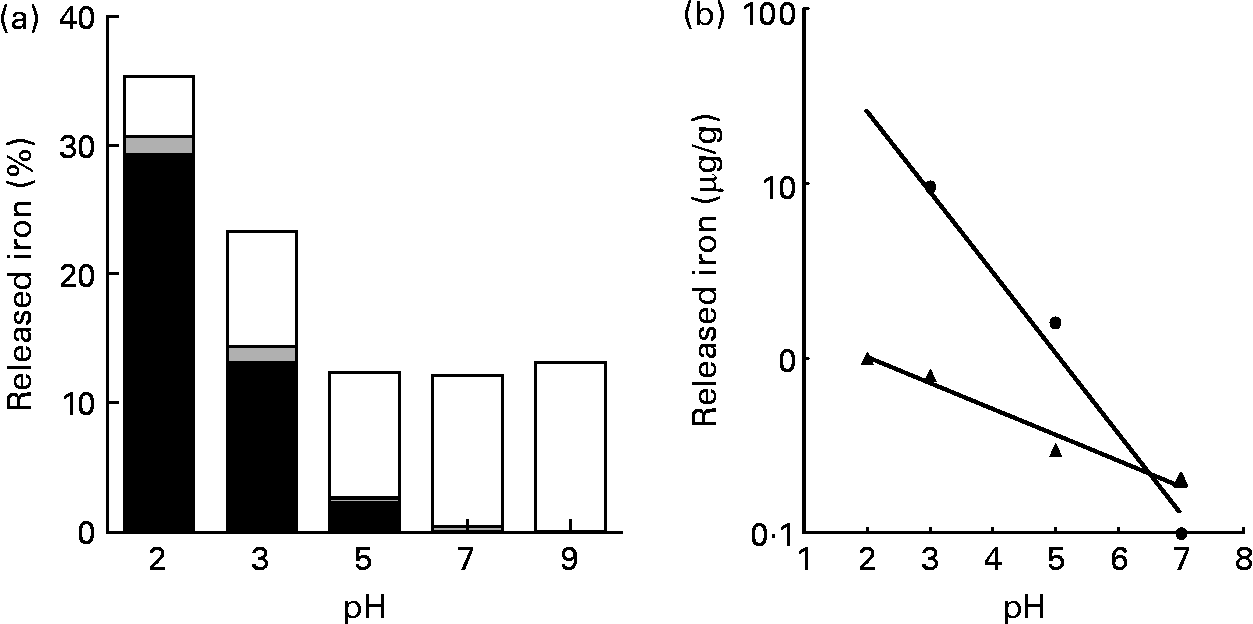
Fig. 1 Effects of pH on the release of iron bound to pectin: (a) percentage of the released ferrous (■), ferric (![]() ) and non-ionic (□) iron from pectin, with solutions at indicated pH values; (b) relationship between released ferrous iron (Fe2+; ●) from pectin and pH (y = − 0·462x+2·34; r − 0·991; P < 0·0079) and between released ferric iron (Fe3+; ▲) from pectin and pH (y = − 0·149x+0·302; r − 0·984; P < 0·0157). Values are means of five obervations.
) and non-ionic (□) iron from pectin, with solutions at indicated pH values; (b) relationship between released ferrous iron (Fe2+; ●) from pectin and pH (y = − 0·462x+2·34; r − 0·991; P < 0·0079) and between released ferric iron (Fe3+; ▲) from pectin and pH (y = − 0·149x+0·302; r − 0·984; P < 0·0157). Values are means of five obervations.
Effect of ionic strength on the release of iron bound to pectin
At pH 2·0, most of the Fe released from pectin was ferrous iron. The amount of Fe released from pectin at pH 2·0 increased about two- and fourfold in 150 and 500 mm-NaCl solutions, respectively, when compared with the solution without NaCl (Fig. 2). When the concentration of NaCl solution was increased from 500 to 1000 mm, the amount of Fe isolated from pectin did not increase. At pH 5·0, the release of Fe from pectin was hardly detectable.
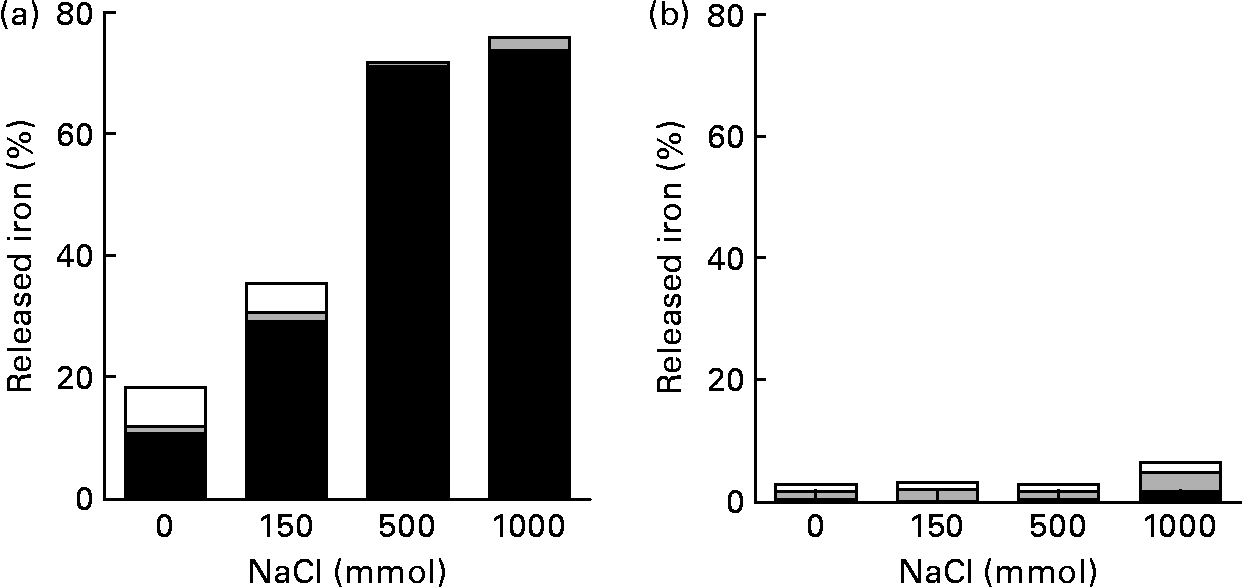
Fig. 2 Effects of ionic strength and pH on the release of iron bound to pectin: percentage of the released ferrous (■), ferric (![]() ) and non-ionic (□) iron from pectin, with solutions at indicated ionic strengths, (a) at pH 2·0 and (b) at pH 5·0. Values are means of five observations.
) and non-ionic (□) iron from pectin, with solutions at indicated ionic strengths, (a) at pH 2·0 and (b) at pH 5·0. Values are means of five observations.
Effect of pectin as a reducing agent
Most of the Fe isolated from the solution without pectin was ferric iron (Fig. 3). However, most of the Fe isolated from the solution containing pectin was ferrous iron.
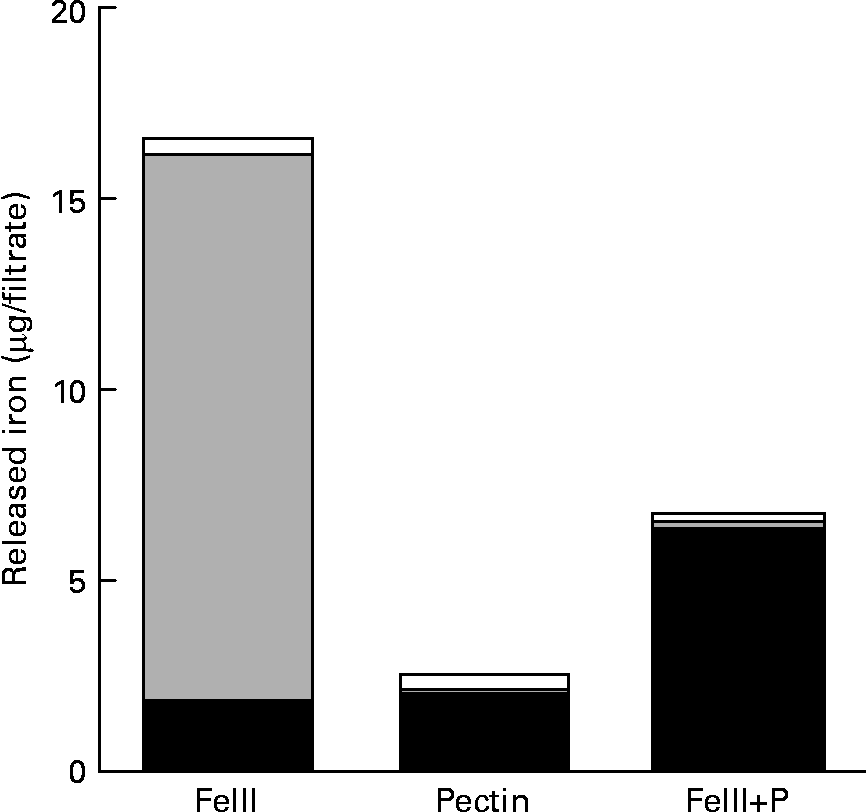
Fig. 3 Reducing effect of pectin to ferric iron: amount of the released ferrous (■), ferric (![]() ) and non-ionic (□) iron in 150 mm-NaCl solution at pH 2·0 with or without pectin. Values are means of five observations.
) and non-ionic (□) iron in 150 mm-NaCl solution at pH 2·0 with or without pectin. Values are means of five observations.
In vivo study (animal experiment)
Experiment 1
Body-weight gain in rats fed the FeII-6 and FeII-12 diets was significantly higher compared with rats fed the FeD diet. Body-weight gain in rats fed the FeII-3 and pectin diets was not significantly different from rats fed the FeD diet (Table 2). Hb concentration in rats fed the FeII-6, FeII-12 and pectin diets was significantly higher compared with rats fed the FeD diet. Hb concentration in rats fed the FeII-3 diets was not significantly different from rats fed the FeD diet. Hb gain and Hb regeneration efficiencies in rats fed the FeII-3, FeII-6, FeII-12 and pectin diets were significantly higher compared with rats fed the FeD diet. Hb gain and Hb regeneration efficiency increased as the Fe intake increased (r 0·928, P < 0·0001 and r 0·556, P < 0·003).
Table 2 Effect of iron source on body-weight gain, final Hb concentration, Hb gain and Hb regeneration efficiency in rats fed the experimental diet for 21 d
(Mean values with their standard errors)
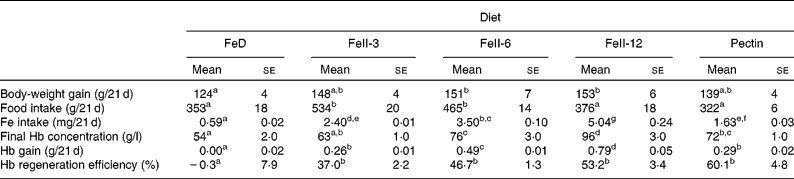
FeD, Fe-deficient diet; FeII-3, Fe at 3 mg/kg diet; FeII-6, Fe at 6 mg/kg diet; FeII-12, Fe at 12 mg/kg diet.
a,b,c,d,e,f,g Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Experiment 2
Body-weight gain in rats fed the FeIII and FeIII+pectin diets was significantly higher compared with rats fed the FeD diet (Table 3). Fe intake was not significantly different between rats fed the FeIII and FeIII+pectin diets; however, Hb concentration, Hb gain and Hb regeneration efficiency in rats fed the FeIII+pectin diet were significantly higher compared with rats fed the FeIII diet.
Table 3 Effect of pectin on body-weight gain, final Hb concentration, Hb gain and Hb regeneration efficiency in rats fed a diet containing ferric sulphate as the iron source for 21 d
(Mean values with their standard errors)
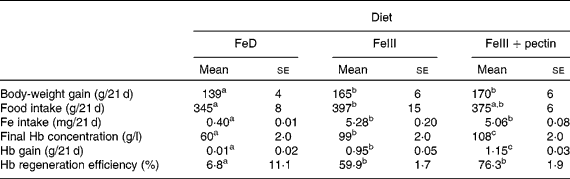
FeD, Fe-deficient diet.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
Pectin contains methylated carboxyl groups. When pectin is dispersed in water, some of the carboxyl groups ionise. Pectin is a polycarboxylic acid with a pK a value of about 3·5(Reference Sila, Van Buggenhout and Duvetter11). The pK a value is known to depend on the temperature of the solution. Therefore, in the present in vitro study, the temperature of the pectin solution was kept at 37°C. At pH values higher than 3·5, pectin is a negatively charged polysaccharide in its ionised form, which can interact with positively charged Fe. The dissociation of pectin is reversible, depending on pH conditions. The amount of Fe released from 1 % pectin solution (w/v) at pH 2·0 was about three times higher than the amount of Fe released at pH 5·0, 7·0 and 9·0. About 90 % of Fe released from pectin at pH 2·0 was ionic Fe. Most of the Fe released from 1 % pectin solution (w/v) at pH values higher than 5 was non-ionic Fe. Our results suggest that Fe and pectin would form an ionic-bound or electric-bound complex with free carboxyl groups in the pectin molecules. Most of the Fe released from 1 % pectin solution (w/v) at pH 2·0 was ferrous iron, suggesting that most of the Fe bound to pectin is ferrous iron; ferric iron bound to pectin might be reduced to ferrous iron by pectin.
Fe absorption occurs predominantly in the duodenum and upper jejunum(Reference Muir and Hopfer12). Gastric acid lowers the pH in the proximal duodenum, which enhances the solubility and uptake of Fe. Gastric acid is an important luminal factor in the absorption of non-haem Fe. Depending on the acidity of the stomach, Fe bound to pectin might be partially released before passing into the small intestine. However, ingestion of food causes a transitory pH rise(Reference Ovesen, Bendtsen and Tage-Jenesen13). The mean pH of the digesta in the upper small intestine in rats fed a commercial pellet was about 6·0(Reference Shiga, Sakai and Horii14). At or above pH 4, Fe was not released from dietary fibres such as maize bran and soya hull(Reference Laszlo15).
In addition to the effect of pH, ionic strength is also a critical parameter in the release of Fe from pectin. The pK a value rises as ionic strength increases.
The amount of Fe released from pectin at pH 2·0 increased as the ionic strength of the pectin solution increased; however, at pH 5·0, Fe release was not increased, suggesting that the release of Fe from pectin is affected by pK a and pH. Solutions with KCl concentrations as high as 150 mm released less than 10 % of Fe from maize bran and soya hull(Reference Laszlo15).
The fate of pectin in the large intestine is also extremely important with regard to the bioavailability of Fe bound to pectin. Pectin is almost completely fermented in the caecum of rats, resulting in the release of Fe from pectin. In ileally fistulated rats, ferrous and ferric iron infused in the caecum was similarly utilised(Reference Ebihara, Okano and Miyada16, Reference Ebihara and Okano17). Fe absorption from the large intestine is less efficient compared with the duodenum, but it is significant, especially, during Fe deficiency(Reference Yeung, Glahn and Welch18). Studies of gastrectomised rats have shown that dietary fructo-oligosaccharides prevented anaemia, but this effect was diminished by caecectomy(Reference Sakai, Ohta and Shiga19), suggesting that Fe absorption takes place to some extent in the colon. Acidic fermentation in the large intestine stimulates absorption of Ca and Mg in the large intestine of the rat(Reference Demigné and Rémésy20). Therefore, increased Fe absorption in the presence of pectin could depend on the decrease in pH due to fermentation of pectin in the large intestine.
Ferrous iron is absorbed much more efficiently than ferric iron. Under physiological conditions, it is thought that inorganic forms of Fe need to be reduced to the ferrous form to be effective. When ferric iron was added to the filtrate of pectin solution, most of the Fe in the filtrate was ferrous iron, suggesting that pectin has reducing potential. In Expt 2 of the present in vivo study, the final Hb concentration, Hb gain and Hb regeneration efficiency in rats fed the FeIII+pectin diet were significantly higher compared with rats fed the FeIII diet. Therefore, the higher bioavailability of ferric iron in rats fed the FeIII+pectin diet compared with those fed the FeIII diet would partially depend on an increased Fe absorption in the upper small intestine by the reduction of ferric iron to ferrous iron by pectin.
In our other study, the amount of Fe released from pectin was not proportional to the amount of Fe bound to pectin, suggesting that the amount of Fe released from pectin depends on the type and source of pectin. In comparison with rats fed the FeD diets containing various pectin concentrations, the Hb gain increased as Fe intake from pectin increased (r 0·962, P < 0·0001), but it did not increase as the Fe bound to pectin increased (T Miyada and K Ebihara, unpublished results). The degree of esterification, molecular weight and/or mode of distribution of free carboxylic groups along the polymer chain strongly affect the strength of binding of minerals to pectin(Reference Kohn, Furda and Kopec6, Reference Kim and Atallah21, Reference Kim, Atallah and Amarsiwardena22).
In conclusion, the release of Fe from pectin was increased at lower pH and higher ionic strength. The Fe bound to pectin is utilised by rats.
Acknowledgements
The present study was supported, in part, by the Iijima Memorial Foundation. The authors declare that there are no conflicts of interests to disclose. T. M., A. N. and K. E. conducted the research; T. M. and A. N. analysed the data; T. M. and K. E. managed the study and drafted the manuscript; K. E. had the primary responsibility for the final content. All authors read and approved the final manuscript.








