Fe deficiency is prevalent worldwide. Premenopausal women of reproductive age are at a higher risk for Fe deficiency than other sex/age groups. In the UK National Diet and Nutrition Survey (2008/2009–2011/2012), 15·5 % of women aged 19–64 years were found to have plasma ferritin levels below 15 ng/ml( 1 ). In the US National Health and Nutrition Examination Survey 1999–2006, 10·9 % of women aged 18–49 years had serum ferritin (sFer) levels below 12 ng/ml( Reference Miller 2 ). Fe deficiency is more prevalent in Japan than in other developed countries. A total of 36–45 % of women aged 20–29 years and 44–49 % of women aged 30–49 years were Fe-deficient according to the National Health and Nutrition Survey of Japan when a 15 ng/ml cut-off value for sFer was used( Reference Yokoi 3 ).
Fe-deficiency anaemia is a well-known cause of functional deterioration, resulting in various symptoms including paleness, fatigue, dyspnoea and headache( Reference Lopez, Cacoub and Macdougall 4 ). The seventeenth-century English physician Thomas Sydenham (1642–1689), who first identified the therapeutic effect of Fe, steel and chalybeate (Fe spring water) on ‘chlorosis’, also used these minerals to treat women’s hysteria and men’s hypochondria( Reference Sydenham 5 ). ‘Chlorosis’ or ‘green sickness’ corresponds to severe Fe-deficiency anaemia in modern medical terminology. Sydenham seemed to consider ‘chlorosis’ as a severe condition within a wide spectrum continuing from hysteria and hypochondria, which is close to the concept of neurotic syndrome or neurasthenia in modern medicine( Reference Sharpe 6 ). In the nineteenth century, there were several case reports (limited to women and children) of non-anaemic chlorosis (i.e. Fe deficiency without anaemia (IDNA) with signs and symptoms including fatigue and dyspnoea)( Reference Becquerel and Rodier 7 , Reference Laache 8 ).
Fatigue not associated with known causes is a very common complaint in the general population( Reference Sharpe 6 ). In UK surveys, the prevalence of fatigue was 22·5 %( Reference Skapinakis, Lewis and Meltzer 9 ) in the community (16–64 years) and 11·3 % among primary-care patients (18–45 years)( Reference Wessely, Chalder and Hirsch 10 ). About 60–80 % of clinical cases of fatigue had no identified physical cause( Reference Wessely, Chalder and Hirsch 10 , Reference Murtagh 11 ). Women complained of more fatigue than men after adjustment for psychological distress in a survey performed in Britain( Reference Pawlikowska, Chalder and Hirsch 12 ). Because women are more likely to be Fe-deficient than men, there could be an association between IDNA and fatigue.
After 1960, there were several randomised controlled trials (RCT) and observational studies regarding the relationship between IDNA and fatigue. The results of RCT on Fe and fatigue are controversial. Beutler et al.( Reference Beutler, Larsh and Gurney 13 ), Verdon et al.( Reference Verdon, Burnand and Stubi 14 ), Krayenbuehl et al.( Reference Krayenbuehl, Battegay and Breymann 15 ) and Vaucher et al.( Reference Vaucher, Druais and Waldvogel 16 ) found a significant therapeutic effect of Fe on fatigue in patients with IDNA. Morrow et al.( Reference Morrow, Dagg and Goldberg 17 ) and Waldvogel et al.( Reference Waldvogel, Pedrazzini and Vaucher 18 ) did not find a significant association between Fe therapy and fatigue improvement.
The outcomes of cross-sectional studies are also controversial. On the basis of the cross-sectional studies, Piednoir et al.( Reference Piednoir, Allou and Driss 19 ), Comin-Colet et al.( Reference Comin-Colet, Enjuanes and Gonzalez 20 ) and Sawada et al.( Reference Sawada, Konomi and Yokoi 21 ) found a positive and significant association between IDNA and fatigue. Lasocki et al.( Reference Lasocki, Chudeau and Papet 22 ) found that IDNA was associated with some increased fatigue scores. Goldenberg et al.( Reference Goldenberg, Graff and Clara 23 ) did not find an association between IDNA and fatigue. Beck et al.( Reference Beck, Conlon and Kruger 24 ) found that IDNA was significantly associated with decreased fatigue, but that this association was no longer statistically significant when factors expected to be associated with Fe status and fatigue were controlled for. A history of suboptimal Fe status was found to be a significant positive predictor of physical fatigue in their multiple regression model.
Here we aimed to resolve these controversies by performing a systematic review and meta-analysis aimed at determining whether IDNA is associated with fatigue and whether Fe therapy is effective in treating fatigue in patients with IDNA.
Methods
Literature search and study selection
A broad literature search was performed using the PubMed database. The preliminary article search was started on 13 August 2015. Articles published from 1809 to January 2016 were identified on 19 January 2016 using the following search formula: Search (((iron deficiency without anaemia) OR (nonanemic OR non-anemic OR non anaemic OR non-anaemic) OR (prelatent iron deficiency OR latent iron deficiency) OR (sideropenia OR sideropaenia OR sideropenic OR sideropaenic)) AND (fatigue OR fatigability OR tiredness)). Searching for ‘anaemia’ in the PubMed database includes searching for both ‘anaemia’ and ‘anemia’. The study selection process is shown in Fig. 1. An additional search was conducted on 14 February 2016 using the search formula: Search ((iron depletion OR iron-depleted) AND (fatigue OR fatigability OR tiredness)). However, this did not uncover an eligible article except for one duplicate of the initial search.
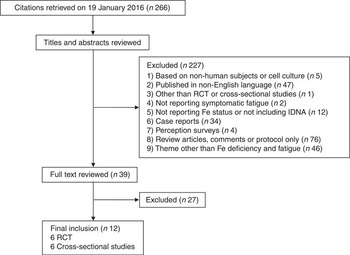
Fig. 1 Flow diagram of the systematic literature search undertaken on iron deficiency without anaemia (IDNA) and fatigue. RCT, randomised controlled trials.
Exclusion criteria as applied via a screening process included (1) studies based on non-human subjects or on cell cultures; (2) articles published in non-English languages; (3) studies other than RCT or cross-sectional studies; (4) articles that lacked reports on symptomatic fatigue; (5) articles that lacked objective parameters on Fe status or did not include study subjects with IDNA; (6) case reports; (7) perception surveys; and (8) review articles, comments or protocol-only articles and (9) articles with a theme other than Fe deficiency and fatigue. At this stage, articles that lacked an abstract in the PubMed database or that had an abstract not reporting details necessary for exclusion were included and their full-text articles obtained. Inclusion criteria were as follows: (1) cross-sectional studies on symptomatic fatigue between subjects with normal Fe status and those with IDNA or (2) RCT of Fe therapy on symptomatic fatigue in non-anaemic subjects including IDNA. Diagnostic criteria of IDNA were according to the authors of the respective articles. Two investigators independently participated in assessing the eligibility of selected studies. Disagreements were resolved by discussion between the reviewers.
Data extraction and statistical analyses
The characteristics of the subjects (age, sex, geographical area), study design (cross-sectional study or RCT; parallel or cross-over design only for RCT; type, dosage and duration of Fe preparations only for RCT; Fe status parameters; and criteria of Fe status) and measures of the effect sizes (sample sizes; means and standard deviations of fatigue parameters; differences in fatigue parameters between two groups and the corresponding P values; the P value and the regression coefficient corresponding to the specified variable for Fe deficiency in the multivariate linear regression model; the OR and its CI based on the multivariate logistic regression model; the number of parameters for multivariate regression models; the proportion of responders in fatigue treatment by an Fe preparation or placebo; or relative effectiveness in fatigue treatment between the Fe preparation and placebo for a matched comparison design) were extracted.
For cross-sectional studies, Fe status and fatigue parameters determined at the same time were extracted from reports. When the results of multivariate analyses were available, they were also used for meta-analysis.
The conversion of the test statistics into effect sizes (d) and their standard errors (σ d ) for the respective study design is shown in the online Supplementary Appendix A. All test statistics were converted into unbiased estimates of standardised mean differences or ‘effect size’ with their standard errors( Reference Cooper, Hedges and Valentine 25 ) because of the heterogeneity of symptomatic fatigue parameters( Reference Mullen 26 ) and for removal of a small sample bias from standardised mean differences( Reference Hedges 27 ). For continuous variables, conversion was carried out as described by Hedges( Reference Hedges 27 ). For dichotomous variables, corrected log OR and corrected standard error for small sample bias were calculated according to the recommendation by Cox( Reference Cox 28 ). We employed 0·607 of the scale factor in the conversion of log OR to effect size for adjusting the logistic distribution to the normal distribution originally recommended by Cox( Reference Cox 28 ), rather than division by its reciprocal number 1·65( Reference Sanchez-Meca, Marin-Martinez and Chacon-Moscoso 29 ). Although Sanchez-Meca et al. estimated the bias of the effect-size indices in the unmatched design using the Monte Carlo simulation( Reference Sanchez-Meca, Marin-Martinez and Chacon-Moscoso 29 ), they did not analyse the matched-pair design. Then, we performed a simulation study to estimate the bias of Haldane’s corrected log OR( Reference Haldane 30 ) and that of the corrected standard error by Gart & Zweifel( Reference Gart and Zweifel 31 ) recommended by Cox( Reference Cox 28 ), which were found to be negligible (online Supplementary Appendix B). For the multivariate linear regression model, conversion was carried out as described by Borenstein( Reference Borenstein 32 ) after calculating the corresponding partial correlation coefficient from P values determined by the partial F test( Reference Weisberg 33 ) (online Supplementary Appendix A).
If the overall score and its subscores were reported, the result of the overall score was taken as the representative value of the study. If multiple measures were reported in a single study, a simple average of effect sizes was calculated as a representative value. The corresponding standard error was calculated as the square root of the weighted average of squared standard errors using the degree of freedom (sample size minus 1) as weights.
Meta-analysis of effect sizes was performed using R version 3.2.2 and the meta-analysis package ‘metafor’ version 1.9–8 with DerSimonian–Laird weights in a random-effects model. The results were summarised as the effect sizes, standard errors and 95 % CI. The significance of the pooled effect size was determined by a Z test. P<0·05 was considered to be statistically significant.
The heterogeneity within the outcomes of the studies was evaluated using the I 2 statistics. Sensitivity analysis was also performed with one study removed at a time to evaluate whether a single study affected the outcomes. Potential publication bias was assessed by funnel plots displaying standard error and effect size.
Results
Study selection and characteristics
A total of 266 citations were initially retrieved by a search of the PubMed database. Finally, twelve studies were included for further analyses. The total number of participants was 1875 and the number of females was 1344 (71·7 %). In all, six RCT and six cross-sectional studies were selected. The study populations originated from the following geographical areas: France( Reference Vaucher, Druais and Waldvogel 16 , Reference Piednoir, Allou and Driss 19 , Reference Lasocki, Chudeau and Papet 22 ), Switzerland( Reference Verdon, Burnand and Stubi 14 , Reference Krayenbuehl, Battegay and Breymann 15 , Reference Waldvogel, Pedrazzini and Vaucher 18 ), Canada( Reference Goldenberg, Graff and Clara 23 ), Japan( Reference Sawada, Konomi and Yokoi 21 ), New Zealand( Reference Beck, Conlon and Kruger 24 ), Spain( Reference Comin-Colet, Enjuanes and Gonzalez 20 ), the UK( Reference Morrow, Dagg and Goldberg 17 ) and the USA( Reference Beutler, Larsh and Gurney 13 ).
The characteristics of the participants (age, country of origin, feature of the population, proportion of women and sample size), inclusion criteria of the participants, definition of Fe deficiency, mean Hb and sFer levels, study design (parallel-arm or cross-over, only for RCT) and description of Fe treatment (only for RCT) are shown in Table 1 (for the RCT) and Table 2 (for the cross-sectional studies). The fatigue scale, outcome, and corresponding effect size and standard errors, including the results for multiple measures, if present, are described in the online Supplementary Appendices C (for the RCT), D (for the cross-sectional studies using univariate analysis) and E (for the cross-sectional studies using multivariate analysis).
Table 1 Characteristics of the selected randomised controlled trials

ID, Fe deficiency; BM, bone marrow; DBPC, double-blind, placebo-controlled; sFer, serum ferritin; iv, intravenous; IBC, Fe-binding capacity.
* When no inclusion criteria were given, the proportion of subjects whose Fe status was low is shown.
† Not documented. Just written as ‘female out-patients of the University of Chicago Clinics, or were University of Chicago students’. Selection of patients: otherwise healthy women complaining of chronic fatigue.
Table 2 Characteristics of the selected cross-sectional studies (Numbers and percentages)

ID, Fe deficiency; IDNA, Fe deficiency without anaemia; CHF, chronic heart failure; sFer, serum ferritin; TS, the percentage of transferrin saturation; IBD, inflammatory bowel disease; sTfr, serum transferrin receptor; ICU, intensive care unit; CS, cardiac surgery; CRP, C-reactive protein.
* Exclusion criteria was set for Hb concentration (<85 g/l) in the article by Comin-Colet (2013).
† Median is shown because no mean value is reported.
‡ 62 % female in the total subjects.
§ No values for Hb concentration and sFer are given.
|| Although 33 % of nine ID patients were female and 21 % of ninety-eight non-ID patients were female at ICU discharge, the proportion of females were not reported on day 28 after ICU discharge.
¶ No inclusion criteria for Hb concentration is set in the article by Piednoir et al.( Reference Piednoir, Allou and Driss 19 ). Exclusion criteria were patients with a history of haematological or Fe metabolism disorders, those prescribed erythropoietin or Fe during the previous month.
All six RCT were double-blind, placebo-controlled and exclusively focused on younger adult women( Reference Beutler, Larsh and Gurney 13 – Reference Waldvogel, Pedrazzini and Vaucher 18 ). Two studies employed a cross-over design, and four used a parallel-arm design. In five trials, the study population comprised patients reporting fatigue or having symptoms mainly of fatigue (Table 1). In one Swiss study, blood donors who visited the Red Cross Transfusion Centre participated in the trial( Reference Waldvogel, Pedrazzini and Vaucher 18 ). One trial used intravenous Fe and the other five trials used oral preparations.
Two cross-sectional studies targeted young women aged about 20 years from the general population. The New Zealand study was a community-based study without subject selection by inclusion and exclusion criteria( Reference Beck, Conlon and Kruger 24 ). In this study, multivariate analysis was used to correct for confounders, because the characteristics of the IDNA and non-ID groups in the population were quite different( Reference Beck, Conlon and Kruger 24 ). In the Japanese study, the participants were screened by inclusion and exclusion criteria for intervention trials( Reference Sawada, Konomi and Yokoi 21 ). In this study, the characteristics of the IDNA and non-ID groups were well-matched and multivariate analysis was not performed( Reference Sawada, Konomi and Yokoi 21 ).
The other four studies focused on patients with chronic heart failure or inflammatory bowel disease and those who had undergone cardiac surgery or had been admitted to intensive care units (Table 2). The design of these four clinical studies was a cross-sectional comparison of IDNA and non-ID patients in longitudinal observation. In addition to sFer, all four clinical studies employed serum transferrin receptor (sTfR) and/or transferrin saturation (TS) as an Fe parameter insensitive to inflammation, a confounding factor causing elevated sFer and anaemia in chronic disease( Reference Ferguson, Skikne and Simpson 34 ), as well as fatigue( Reference Karshikoff, Sundelin and Lasselin 35 ). In the study by Piednoir et al.( Reference Piednoir, Allou and Driss 19 ), serum C-reactive protein (CRP) combined with sTfR:log ferritin ratio was also employed in the diagnosis of ID, and no significant difference in CRP was found between the IDNA and non-ID groups. In their study, multivariate analysis was not performed.
Meta-analysis and sensitivity analysis for randomised controlled trials
The selected studies used various fatigue assessment scales and evaluation methods for outcomes including continuous and dichotomous data. Therefore, all outcomes were converted to effect sizes. The results of the meta-analysis for RCT are shown in Fig. 2. The pooled effect size in the meta-analysis of the RCT was highly significant, indicating the effectiveness of Fe treatment to relieve fatigue in non-anaemic subjects, principally IDNA. By removing one study at a time, the I 2 statistics changed from 0·0 to 11·8 and the P value barely changed (from <0·0001 to 0·0022). The funnel plot for the meta-analysis on the RCT is shown in Fig. 3 and is not suggestive of publication bias.

Fig. 2 Meta-analysis of the randomised controlled trials on the therapeutic effect of iron on fatigue. ES, effect size. * The positive sign signifies that iron treatment is effective to reduce fatigue. † 1/se 2 is used as a weight. ‡ The ith study is excluded from the model-fitting.

Fig. 3 Funnel plot of the meta-analysis of the randomised controlled trials.
Meta-analysis and sensitivity analysis for cross-sectional studies using outcomes from univariate analysis
The results of cross-sectional studies on the IDNA–fatigue relationship based on outcomes from the univariate analysis are described in Fig. 4. In the meta-analysis of the cross-sectional studies, heterogeneity was evident (I 2=49·5 %). The effect size of the study by Beck et al.( Reference Beck, Conlon and Kruger 24 ) was negative, corresponding to the finding that women with IDNA reported fatigue less often than women with normal Fe status in their study population.

Fig. 4 Meta-analysis of the cross-sectional studies using outcomes from univariate analysis on the association between iron deficiency without anaemia (IDNA) and fatigue. ES, effect size; ID, iron deficiency. * The positive sign signifies that subjects in the IDNA group complain of more fatigue than those in the non-ID group. † 1/se 2 is used as a weight. ‡ The ith study is excluded from the model-fitting.
Removing the study by Beck et al.( Reference Beck, Conlon and Kruger 24 ) reduced the heterogeneity among the studies to insignificance (I 2=0·0 %), although the removal of any other study kept I 2 values above 50 %. Therefore, heterogeneity was considered to be attributable to the study by Beck et al.( Reference Beck, Conlon and Kruger 24 ). By excluding the Beck et al. study, the effect becomes significant. The funnel plot for the meta-analysis on the cross-sectional studies is shown in Fig. 5, which suggests that there could be unpublished studies in the left part of the funnel. Also, there might be two subpopulations with different effect sizes. However, the number of studies in the current data set is insufficient to test this possibility.

Fig. 5 Funnel plot of the meta-analysis of the cross-sectional studies using outcomes from univariate analysis.
Meta-analysis and sensitivity analysis for cross-sectional studies using outcomes from multivariate analysis
The severity of inflammatory diseases presumably affected the outcomes of clinical studies. Therefore, effect sizes corrected for confounders by multivariate analysis were used in our meta-analysis, if they were present. The results of our meta-analysis are shown in Fig. 6. Compared with meta-analysis using effect sizes based on univariate analysis, the overall effect size was smaller in the meta-analysis using outcomes from multivariate analyses, whereas the general tendency was similar to that obtained from the univariate analysis. Similar to the meta-analysis based on outcomes of univariate analysis, heterogeneity was evident (I 2=57·4 %). Our sensitivity analysis found that the heterogeneity was introduced by the Beck et al.( Reference Beck, Conlon and Kruger 24 ) study. The association between IDNA and fatigue became significant after excluding this study. Publication bias was not evident in the funnel plot for effect sizes based on the multivariate analysis (Fig. 7).

Fig. 6 Meta-analysis of the cross-sectional studies using outcomes from multivariate analysis on the association between iron deficiency without anaemia (IDNA) and fatigue. ES, effect size; ID, iron deficiency; CRP, C-reactive protein. * The positive sign signifies that subjects in IDNA group complain of more fatigue than those in the non-ID group. † 1/se 2 is used as a weight. ‡ The ith study is excluded from the model-fitting. § The eight covariates used were systolic blood pressure, New York Heart Association functional class, hypertension, diabetes mellitus, efficient glomerular filtration rate, time since last heart failure admission, loop diuretics and N-terminal pro-B-type natriuretic peptide. || Anaemic subjects were included in the multivariate analysis. ¶ Multivariate analysis was not performed, because there were no significant differences in CRP between the two groups. ** The three covariates were a history of suboptimal iron status, has a current medical condition and ethnicity. †† Multivariate analysis was not performed, because apparently healthy subjects were screened to exclude recurrent illness, chronic medication, anaemia, and other problems for intervention trials and characteristics were well-matched between the two groups.

Fig. 7 Funnel plot of the meta-analysis of the cross-sectional studies using outcomes from multivariate analysis.
Discussion
On the basis of a narrative review, Agarwal proposed potential non-haematological benefits of Fe supplementation that include improvements in immune competence, physical function, restless-leg syndrome and cognition( Reference Agarwal 36 ). Recently, Pratt & Khan( Reference Pratt and Khan 37 ) reported a meta-analysis based on twenty-one studies that analysed the relationship between IDNA and various physiological outcomes. Regarding fatigue, Pratt & Khan only included the study by Krayenbuehl et al.( Reference Krayenbuehl, Battegay and Breymann 15 ) and a meta-analysis was not possible. This was probably due to the strict eligibility criteria, that is, IDNA defined as sFer <16 ng/ml (<12 ng/ml if age <5 years) in the absence of anaemia. Because more relaxed criteria (i.e. IDNA defined according to the authors’ own criteria) were used in our study, twelve studies related to this issue were identified. A variety of thresholds were used for sFer and TS to detect Fe deficiency in various countries around the world( Reference Peyrin-Biroulet, Williet and Cacoub 38 ). The recent trend is to use a higher threshold for sFer than has been applied previously( Reference Yokoi 3 ), especially after Mast et al. found that 30 ng/ml of sFer gave higher sensitivity and maintained high specificity compared with 12 ng/ml of sFer( Reference Mast, Blinder and Gronowski 39 ). Thus, to include as many relevant studies as possible, it is now considered reasonable to accept the authors’ own criteria for detecting Fe deficiency, rather than strictly following the World Health Organization recommendations( 40 ).
By the meta-analysis of the six RCT, a significant treatment effect of Fe on fatigue was revealed in IDNA patients with fatigue. Because we accepted various authors’ thresholds of sFer to define IDNA, it is not possible to know the threshold effective in finding fatigue patients who benefit from Fe treatment. Large clinical trials are recommended to determine the threshold of sFer to select those fatigue patients who might benefit from Fe therapy. Alternatively, because IDNA is prevalent in women, the use of an Fe preparation or improvement in Fe status through proper diet should be considered for non-anaemic women with fatigue.
Because effect sizes for RCT are not intuitive and somewhat difficult to interpret( Reference da Costa, Rutjes and Johnston 41 ), the effect size (d) found by the meta-analysis for Fe therapy for fatigue in IDNA patients with fatigue was converted to an OR using the method of Hasselblad & Hedges( Reference Hasselblad and Hedges 42 ), that is, e dπ/√3. The effect size for Fe therapy for fatigue was 0·33 and the corresponding OR was 1·8. In the fatigue therapy for cancer patients, large placebo effects were reported( Reference Cruciani, Zhang and Manola 43 , Reference Spathis, Fife and Blackhall 44 ). Considering the relatively large placebo effect, an OR of 1·8 is rather small but clinically meaningful.
In the meta-analysis of cross-sectional studies, the overall effect size became smaller after correction for confounders by multivariate analysis. Significant covariates included CRP, Hb (<10 g/dl), active disease, current medical condition and other factors associated with active diseases or with medical condition (Fig. 6). Correction by multivariate analysis is considered necessary for observational studies unscreened by these criteria or unmatched for these variables. In the meta-analysis of the cross-sectional studies, heterogeneity was relatively large. The pooled effect size in the cross-sectional studies was positive but not significant. From the sensitivity study on the six cross-sectional studies, heterogeneity was found to be introduced by the one study based on an unscreened population from the communities. Exclusion of this study made the association between IDNA and fatigue significant, whereas the remaining five cross-sectional studies were based on a specific population (i.e. screened participants for intervention trials or patients with certain disease) (Table 2). More community-based studies will be needed to determine whether the association between IDNA and fatigue also exists in the general population. The RCT focused on a subset of patients with IDNA whose chief complaint was fatigue, whereas the cross-sectional studies surveyed the fatigue level of the subjects with IDNA or without Fe deficiency. A stronger association between Fe and fatigue should, therefore, be expected in the RCT than in the cross-sectional studies.
It is not clear how IDNA causes fatigue. One possible mechanism for this is that decreased VO2 by tissues, especially muscles, in IDNA creates cardiopulmonary stress, evoking a feeling of fatigue. Brownlie et al.( Reference Brownlie, Utermohlen and Hinton 45 ) found that Fe supplementation increased VO2max in untrained women with IDNA. Brutsaert et al.( Reference Brutsaert, Hernandez-Cordero and Rivera 46 ) found that Fe supplementation significantly improved muscle fatigability in young women with IDNA. Another possible mechanism is that Fe deficiency directly affects brain function (neither via a change of Hb nor peripheral Fe) and leads to a feeling of fatigue. Animal studies present evidence supporting this hypothesis. Rats exposed to a marginal Fe diet (20 mg/kg) through gestation and weaning exhibited abnormal auditory brainstem responses( Reference Greminger and Mayer-Pröschel 47 ). Marginally Fe-deficient rats without decreased Hb showed abnormal behaviour (i.e. increased activity and stereotypical behaviour in a light cycle), implying abnormality in dopaminergic neurons of the brain( Reference Hunt, Zito and Erjavec 48 ). Observational studies also found a significant association of IDNA with human psychology and behaviour. Sawada et al.( Reference Sawada, Konomi and Yokoi 21 ) reported that women with IDNA had higher anger and tension in addition to increased fatigue. Lozoff et al.( Reference Lozoff, Clark and Jing 49 ) reported that infants with Fe deficiency (with or without anaemia) had unfavourably altered social–emotional behaviour. Verbal learning and memory function were significantly improved by Fe supplementation in adolescent girls with IDNA( Reference Bruner, Joffe and Duggan 50 ). These studies support the idea that Fe deficiency directly affects human brain function independently of Hb levels in the blood.
Strengths and limitations
This is the first meta-analysis of RCT and cross-sectional studies focused on IDNA and fatigue. The strength of this study is that all studies published and recorded in PubMed from 1809 to 2015 were included. Compared with the existing meta-analysis on IDNA( Reference Pratt and Khan 37 ), the inclusion criteria were more relaxed and thus included more eligible studies. It would appear that this relaxation is necessary to accommodate diversity in the diagnostic criteria of IDNA. Another merit of the current study is the use of effect size, which enabled the performance of a meta-analysis by using diversified psychological tests for fatigue.
Several limitations should be acknowledged in this study. First, some participants with IDNA according to the authors’ own definitions of Fe deficiency would be considered as having normal Fe status according to the accepted IDNA definition. There is also no established diagnostic method for IDNA in patients with inflammatory diseases. There were insufficient data to determine the critical levels of sFer or TS for selecting patients with fatigue who might benefit from an Fe preparation. Second, the association found by meta-analysis of cross-sectional studies cannot infer causality. Third, although effect sizes of cross-sectional studies were corrected for possible confounders by multivariate analysis (unless study groups were screened and/or well-matched), the influence of unobserved/unobservable confounders may still exist. Fourth, there was remarkable variation in the quality of studies, especially unbalanced sample sizes. Fifth, RCT in males could not be analysed because of the absence of relevant studies, as male cases of fatigue with IDNA are rare. Sixth, most of the cross-sectional studies were conducted in patients rather than in community settings. Finally, due to diversity in fatigue assessment methods, it was only possible to perform effect size meta-analysis.
Conclusion
In summary, our meta-analyses suggest that Fe may be effective in reducing fatigue in patients with IDNA. Further studies are now necessary to identify diagnostic criteria to select fatigue patients who might benefit from Fe treatment and to estimate the prevalence of IDNA with fatigue in the general population.
Acknowledgements
This study was partly supported by Japan Society for the Promotion of Science KAKENHI for Scientific Research (C) (grant no. 25350137).
K. Y. designed the study, conducted the search, analysed the data and wrote the manuscript; K. Y. and A. K. selected the relevant literature. All authors read and approved the final content.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001349












