Diabetes and its complications represent major and increasing challenges to healthcare systems worldwide. According to the International Diabetes Federation in 2017, 425 million adults have diabetes in the World from whom one in two adults remains undiagnosed. In 2045, this number will rise to 629 million people.
One of the most common consequences of diabetes is the damage of the peripheral nervous system.(Reference Skalli, Muller and Pradines1,Reference Shehab, Al-Jarallah and Mojiminiyi2) It is estimated that 50 % of diabetic patients end up having diabetic neuropathy (DN).(Reference Skalli, Muller and Pradines1,Reference Chaychi, Mackenzie and Bilotta3) DN is a microvascular complication leading to nerve damage leading to high morbidity and mortality.(Reference Shehab, Al-Jarallah and Mojiminiyi2) While the exact mechanism of DN is still unclear; male gender, increasing age, BMI, height and disease duration were identified as potential risk factors for DN.(Reference Alamdari, Mozafari and Tafakhori4,Reference He, Hu and Zeng5) Further, several studies have suggested that vitamin D deficiency could be an independent risk factor for DN.(Reference Shehab, Al-Jarallah and Mojiminiyi2,Reference He, Hu and Zeng5–Reference Fan, Zhang and Zhu8) Vitamin D is a steroid that functions as a hormone in the human body. It has also a role in glucose homoeostasis and sensitivity to insulin.(Reference El Hajj, Walrand and Helou9) In addition, recent work has shown that patients diagnosed with vitamin D deficiency (serum level of 25-hydroxyvitamin D below 20 ng/ml) had a higher prevalence of DN than those who were diagnosed with insufficiency (20–30 ng/ml) or sufficiency (30–60 ng/ml).(Reference Fan, Zhang and Zhu8,Reference Jung, Jung and Kim10,Reference Ozuguz, Oruc and Ulu11) Moreover, many studies suggest that the use of vitamin D supplementation could improve the symptoms of DN.(Reference Shehab, Al-Jarallah and Mojiminiyi2,Reference Greenhagen, Frykberg and Wukich12–Reference Ghadiri-Anari, Mozafari and Gholami17)
Despite several studies, there appears to be no consensus among researchers with respect to the relation between 25-hydroxyvitamin D and DN. This may be due to differences in sample size, study design, participant ethnicity and cut-off values used in the published work. Moreover, to our knowledge, there are very few published meta-analyses about this relationship which only included between six and thirteen studies each that were either all cross-sectional or all case–control.(Reference Lv, Zhao, Gong, Fang, Wang, Fu, Yan and Wang18–Reference Zhang, Zhao and Tu20) The present systematic review and meta-analysis (MA) therefore aims at clarifying the association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus.
Methods
Search strategy
A specific search strategy has been elaborated to locate the maximum number of relevant studies using the following electronic databases: Medline, EMBASE, Web of Science, Cochrane Library, CINHAL, EBSCO and Google Scholar, from inception to 01 March 2020. The following Boolean terms were used [diabetic AND neuropathy AND (‘’vitamin D’’ OR 25(OH)D OR ‘’25(OH) vitamin D’’ OR ‘’25-hydroxy vitamin D’’ OR ‘’25-hydroxyvitamin D’’)]. No language or date limitations were imposed.
Criteria for study selection
All study types were accepted for inclusion but two: case reports and reviews. Only patients having type II diabetes were included. Studies reporting comparisons between healthy, diabetic patients without DN or diabetic patients with DN were accepted for inclusion. Whether by clinical exam, validated tool or nerve studies, diabetic neuropathy assessment should be explicitly reported for a study to be included.
Quality study appraisal
The quality of the included English studies was evaluated by the Joanna Briggs Institute critical appraisal tool for cross-sectional and case–control studies.(21) The quality assessment of the included studies written in Chinese language was reported from the study of Qu et al. (Reference Qu, Wang and Tang19)
Outcome definition
The primary outcome was set to be the serum level of vitamin D. Units were converted and reported in ng/ml. The secondary outcomes were defined as the prevalence of vitamin D insufficiency and the prevalence of vitamin D deficiency.
Data collection and extraction
This review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. An excel sheet was used to record all extracted data. Relevant data such as sample and patient characteristics, diabetes duration, types of comparison, BMI, fasting plasma glucose, HbA1c, tests used to detect and/or assess DN and laboratory methods for measuring vitamin D were recorded.
Data analysis
Statistical analysis was conducted using the software StatsDirect (Cambridge, UK). Two methods were used for mean comparison. First, an ANOVA was computed to look for significant differences between groups of comparison (diabetic patients with DN, without DN and in healthy subjects) when applicable. Then, an effect size MA using standardised mean difference (SMD) was conducted including those studies that reported their standard deviation values along with their mean values. An odds ratio MA was conducted to look for significant proportion differences related to the prevalence of vitamin D insufficiency and/or deficiency. Heterogeneity was assessed by the I2 inconsistency test; random-effects estimate was reported whenever I2 value exceeded 50 %. A P value <0·05 was considered as significant.
Results
Search results
The electronic search yielded ninety-four hit records, where six were duplicates. Forty-eight abstracts were excluded after initial checking. The remaining forty articles had their full manuscripts available for details. Sixteen were excluded for the following reasons: four studies reported outcomes of vitamin D supplementation to treat DN, six reviews, four lacked primary outcomes and two were irrelevant. Reference list checking of the twenty-four studies yielded another three relevant studies that fulfilled inclusion eligibility. In total, twenty-seven studies(Reference Skalli, Muller and Pradines1–Reference Fan, Zhang and Zhu8,Reference Jung, Jung and Kim10,Reference Ozuguz, Oruc and Ulu11,Reference Bilir, Tulubas and Bilir13,Reference Shillo, Selvarajah and Greig22–Reference Zhang, Xu and Yin35) met the inclusion criteria. Fig. 1 shows details of the search.
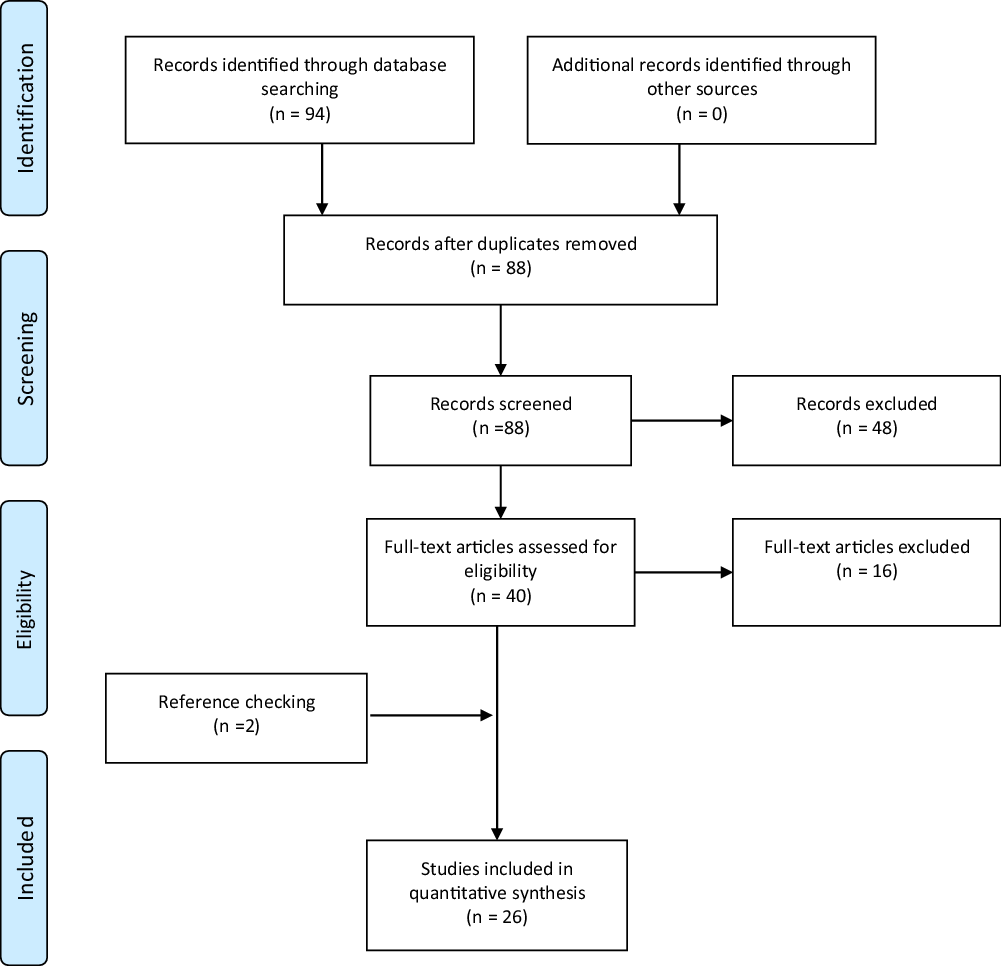
Fig. 1. PRISMA flow diagram.
Study and pooled sample characteristic results
The total pooled sample included 6277 participants. All studies but one(Reference Jung, Jung and Kim10) reported the subgroup sample number where 2218 patients were diabetic with DN, 2959 were diabetic without DN and 406 healthy subjects. The mean age of the total sample was 53·7 ± 9·2 years. The mean diabetes duration was 10·2 ± 3·5 years. Table 1 shows the characteristics of the included studies.
Table 1. Characteristics of included studies
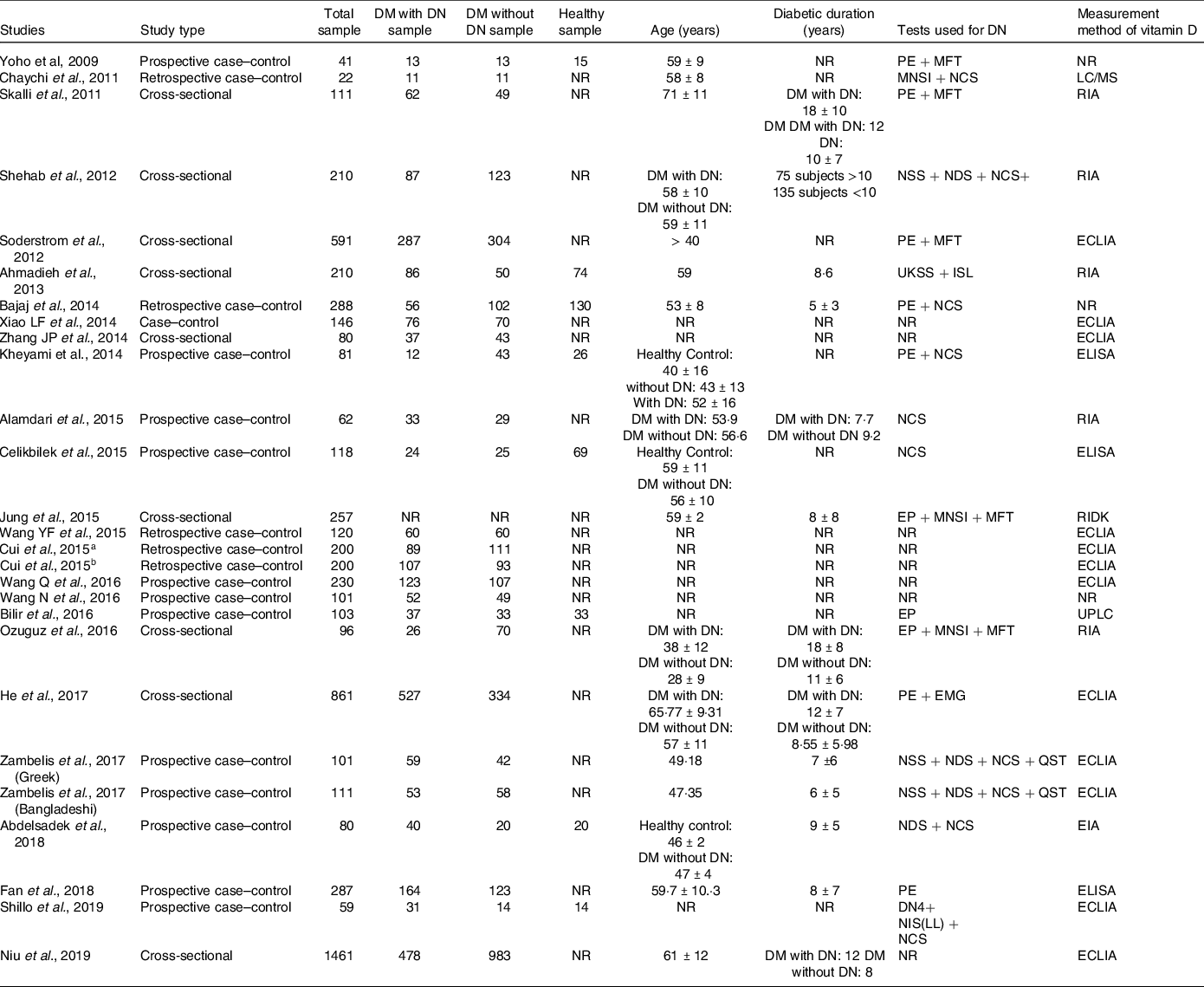
DM, diabetes mellitus; DN, diabetic neuropathy; DN4, Douleur neuropathique-4; DNES, diabetic neuropathy examination score; EIA, enzyme immunoassays; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; EP, electrophysiological; LC/MS, liquid chromatography-mass spectrometry; MFT, monofilament test; MNSI, Michigan neuropathy screening instrument; NCS, nerve conduction studies; NDS, neuropathy disability score; NIS (LL), neuropathy impairment score lower limb; NR, not reported; NSS, neuropathy symptom score; PE, physical examination; QNE, quantitative neurological examination; QST, quantitative sensory test; RIA, radioimmunoassay; RIDK, radio immunologic determination kit; UKSS, UK screening score; UPLC, ultra-performance liquid chromatography.
Quality appraisal
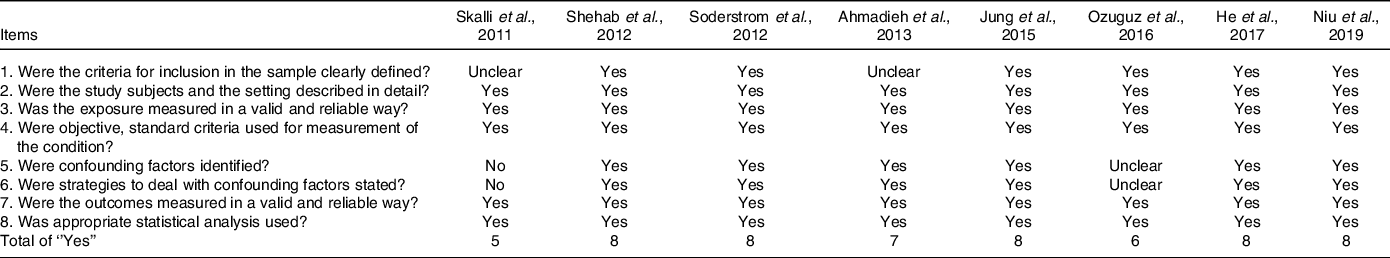
Scores of the Joanna Briggs Institute tool for cross-sectional studies ranged between 5 and 8, out of a maximum value of 8 (Table 2).
Table 2. Joanna Briggs institute critical appraisal checklist for cross-sectional studies
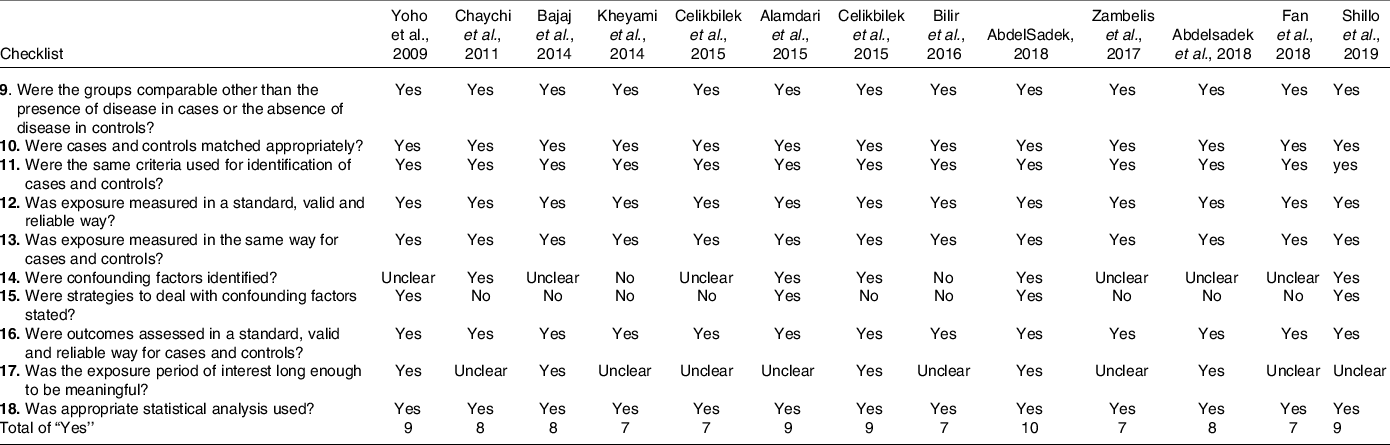
Scores of the Joanna Briggs Institute tool for case–control studies ranged between 7 and 10, out of a maximum value of 10 (Table 3).
Table 3. Joanna Briggs institute critical appraisal checklist for case–control studies
The quality of the Asian studies written in Chinese were not assessed in this review. However, those seven studies(Reference Wang, Guan and Wang27–Reference Cui, Sun and Chang30,Reference Wang, Yang and Song33,Reference Xiao, Li and Meng34,Reference Zhang, Xu and Yin35) were considered as having a moderate quality according to the Newcastle-Ottawa Scale.(Reference Qu, Wang and Tang19)
Outcomes
Table 4 summarises the outcomes of each included studies.
Table 4. Primary and secondary outcomes
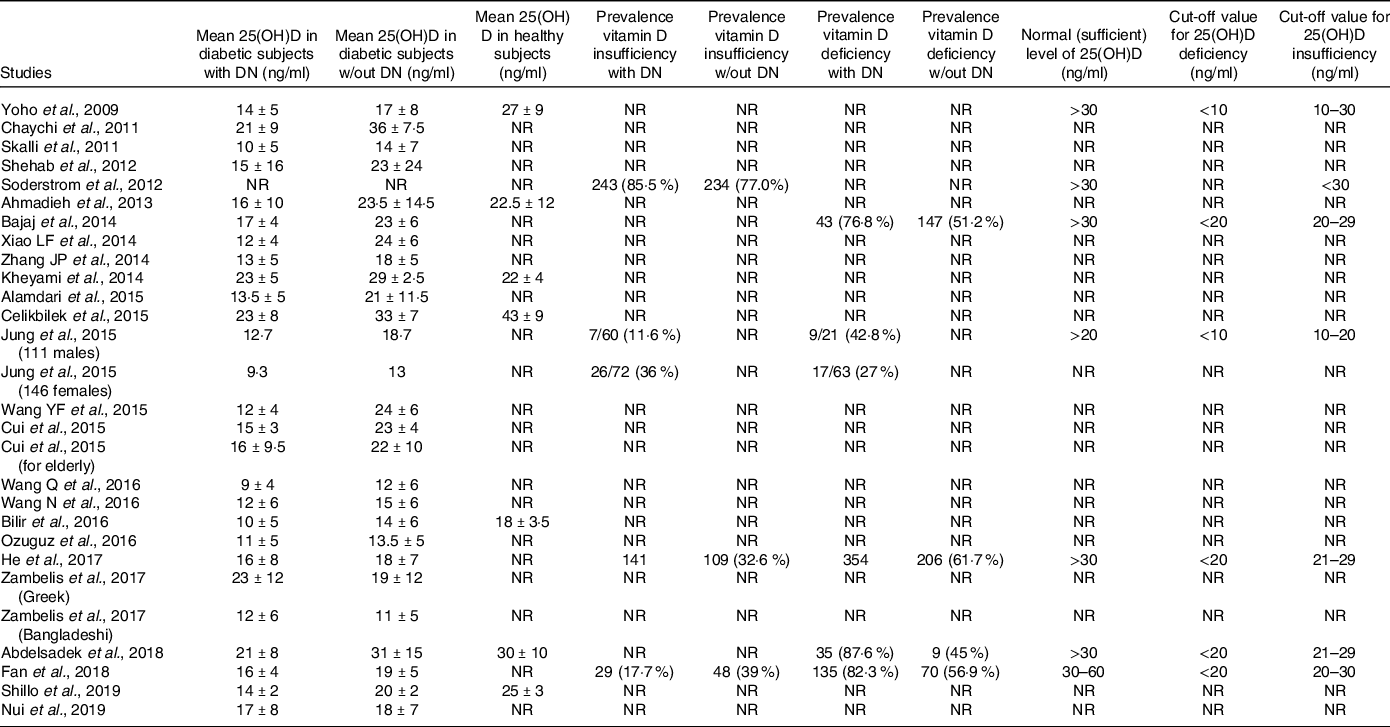
Mean serum vitamin D level
Twenty-five studies reported this outcome. Serum vitamin D values were 15·1 ± 4·2 ng/ml, 20·5 ± 6·4 ng/ml and 27·6 ± 8 ng/ml for diabetic patients with DN, without DN and in healthy subjects, respectively.
A two-way ANOVA analysis showed high significance between diabetic patients with and without DN (P < 0·0001) where those having DN showed significantly lesser values of serum vitamin D. When compared with healthy people, patients with or without DN had significantly lesser values of serum vitamin D (P < 0·0001, for both comparisons).
Similarly, when an effect size MA was conducted including twenty-five studies, it yielded a standardised mean difference of −0·92 (95 % CI −1·18, −0·65, I2 = 93·3 %, P < 0·0001), significantly lesser serum Vitamin D values in diabetic patients with DN (Fig. 2).
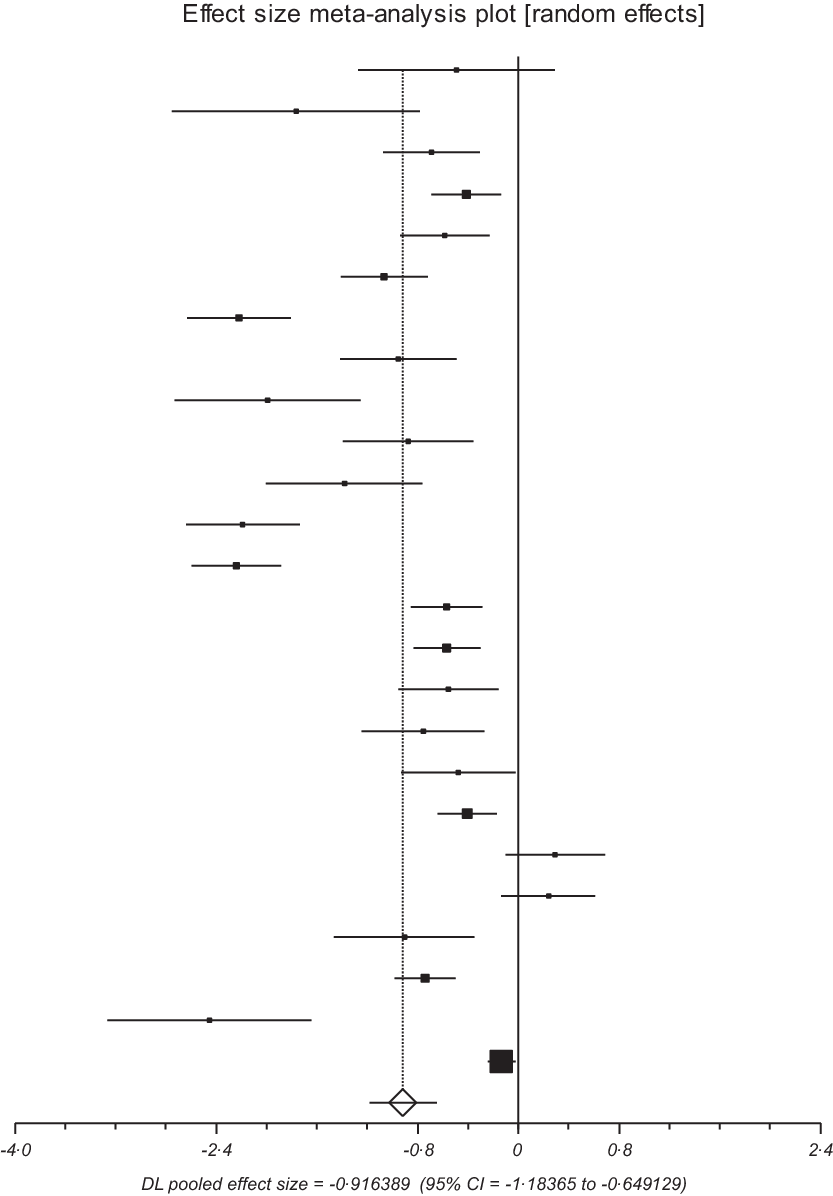
Fig. 2. Forest plot of main finding.
Subgroup analysis based on race showed the following values: SMD of fifteen Caucasians studies was −0·81 (95 % CI −1·13, −0·49, I2 = 85·4 %, P < 0·0001) and that of ten Asian studies was −1·04 (95 % CI −1·45, −0·59, I2 = 96·6 %, P < 0·0001). Based on the study design, seven cross-sectional yielded an SMD of −0·48 (95 % CI −0·69, −0·26, I2 = 75·8 %, P < 0·0001), and eighteen case–control yielded an SMD of −1·1 (95 % CI −1·47, −0·72, I2 = 93 %, P < 0·0001).
Prevalence of vitamin D insufficiency
Three studies(Reference He, Hu and Zeng5,Reference Soderstrom, Johnson and Diaz6,Reference Fan, Zhang and Zhu8) including 1996 diabetic patients with and without DN yielded a pooled OR of 0·84 (95 % CI 0·69, 1·04, I2 = 86·4 %, P = 0·1). Non-significance was found for both models.
Prevalence of vitamin D deficiency
Four studies(Reference He, Hu and Zeng5,Reference Fan, Zhang and Zhu8,Reference Bajaj, Singh and Dwivedi25,Reference Abdelsadek, Saghier and Raheem32) including 1405 diabetic patients with and without DN yielded pooled OR of 1·84 (95 % CI 1·46, 2·33, P < 0·0001) and 2·9 (95 % CI 1·10, 7·52, P = 0·03) when using fixed-effects and random-effects models, respectively. For both models, the I2 = 91·4 %.
Prevalence of vitamin D insufficiency or deficiency
When combining insufficiency with deficiency with a total of 2076 diabetic patients with and without DN subjects, five studies yielded an OR of 2·8 (95 % CI 1·39, 5·79, I2 = 82·9 %, P = 0·004).
Gender-based outcomes
Only Jung et al. (Reference Jung, Jung and Kim10) reported outcomes based on sex. The mean levels of 25(OH)D were significantly lower in patients with DN than in those without DN (12·7 ng/ml v. 18·7 ng/ml, P = 0·007) and (9·3 ng/ml v. 13 ng/ml, P = 0·002) in men and women, respectively.
Similarly, the prevalence of vitamin D deficiency was significantly higher in men and women with DN than in those without DN.
Discussion
The aim of the present MA was to explore the association between diabetic neuropathy and vitamin D levels in patients with type 2 diabetes. Diabetic neuropathy is one of the many complications that develop with diabetes and among the major causes of death in this population.(Reference Zhang, Zhao and Tu20) Thus, it is crucial to identify its risk factors. The present MA included twenty-six studies with a total sample size of 6277 participants; mean age of 53·7 (sd = 9·2) and mean diabetes duration of 10·2 years (sd = 3·5). The findings indicated a statistical significance between serum levels of vitamin D and diabetic neuropathy.
Main findings
Results showed that patients with neuropathy had significantly lower levels of serum vitamin D compared with those without neuropathy. Moreover, patients with diabetic neuropathy had 1·84 and 2·87 higher odds of being deficient in vitamin D using both fixed and random effect models, respectively. Subgroup analyses of vitamin D serum level showed similar significance; results were not affected by race or study design. The prevalence of vitamin D insufficiency among diabetic neuropathy patients was assessed in 1996 patients but with non-significant differences. When combining both outcomes, deficiency and insufficiency, patients with diabetic neuropathy had 2·8 higher odds of being deficient/insufficient in vitamin D.
Interpretation of the results
The findings are in line with previously published meta-analyses that have proven the association between vitamin D deficiency and diabetic neuropathy.(Reference Lv, Zhao, Gong, Fang, Wang, Fu, Yan and Wang18) However, our MA differs considerably given that: a) twenty-six studies were included (v. 6 to 13)(Reference Lv, Zhao, Gong, Fang, Wang, Fu, Yan and Wang18–Reference Zhang, Zhao and Tu20), b) our sample size was considerably larger (6277 v. sample sizes below 3000) and c) our weighted SMD was the highest, which gives higher evidence towards an association between vitamin D and DN. Though a correlation could not be stated, the association seem to be very high and warrants future large-sampled prospective trials.
Regarding vitamin D insufficiency, it is important to note that there is a lack of consensus regarding the threshold in the literature. As such, to address this ambiguity pooled insufficiency, data analyses were conducted to examine its association with diabetic neuropathy. The low statistical significance may be due to the lack of consensus for cut-off values for defining vitamin D levels. Indeed, cut-off points to define vitamin D deficiency, insufficiency, sufficiency and intoxication vary considerably among studies. For example, vitamin D deficiency may be set at levels below 20 ng/ml or 10 ng/ml (e.g.(Reference Jung, Jung and Kim10,Reference Mozos and Marginean36) ) and insufficiency at 20–29 ng/ml or below 24 ng/ml depending on the studies.(Reference Lee and Chen14,Reference Diaz, Mainous and Carek37) Some authors suggested the importance of the parathyroid hormone (PTH) as a functional measure for assessing the ‘adequacy’ for serum vitamin D.(Reference Thacher and Clarke38) Taking into consideration that an optimum level of 25(OH) D is required to suppress PTH activity, results from cross-sectional examinations revealed a value of 30 ng/ml vitamin D as adequate. However, this was only a representation of an average value of the population and did not reflect the variation of vitamin D adequacy for individuals.(Reference Thacher and Clarke38) This suggests that more studies are needed to determine official cut-offs that can be used globally to determine Vitamin D deficiency, insufficiency and sufficiency.
The predominance of vitamin D deficiency and insufficiency was assessed among diabetic neuropathy patients using five studies with a sample size of 2076. The purpose of assessing both deficiency and insufficiency was to capture the gap between the values of deficiency and insufficiency found among most studies. Again, our results point out to the necessity of more reliable cut-off values to differentiate between deficiency, insufficiency and sufficiency. Two very recent studies were located at the completion of our review; Darraj et al. (Reference Darraj, Badedi and Poore39) reported a vitamin D deficit prevalence of 60·8 % among patients with diabetes, and Senyigit(Reference Senyigit40) demonstrated that diabetic patients with DN had significant lower levels than those without the neuropathy.
Vitamin D role in diabetes and diabetic neuropathy
Previous work has shown that diabetic neuropathy is associated with decreased nerve growth factor (NGF) expression in human diabetic nerves and that exogenous NGF can reverse some of the pathological changes in diabetic nerves.(Reference Pittenger and Vinik41–Reference Kim, Cho and Ahn43) In parallel, vitamin D is known to induce NGF synthesis in human cell lines.(Reference Bell44) Therefore, vitamin D may protect diabetic patients from neuropathy through its enhancement of NGF synthesis. In fact, in an experiment on streptozotocin-diabetic rats, a vitamin D3 derivative induced NGF synthesis and prevented neurotrophic deficits.(Reference Riaz, Malcangio and Miller45)
In addition, vitamin D supplementation was previously shown to result in a significant improvement in neuropathic pain management in diabetic patients(Reference Chaychi, Mackenzie and Bilotta3) as well as a reduction in neuropathy symptom scores.(Reference Shehab, Al-Jarallah and Mojiminiyi2) Similarly, a number of clinical trials have linked vitamin D supplementation with the improvement of both pain and neuropathy-specific quality of life in diabetic patients concluding that vitamin D is an independent risk factor for the presence and severity of diabetic neuropathy.(Reference Alamdari, Mozafari and Tafakhori4)
Recent published work has also suggested that vitamin D deficiency may play a role in the pathogenesis of small-fibre neuropathy particularly affecting nociceptor fibres.(Reference Shillo, Selvarajah and Greig22) Other studies proposed that the association between vitamin D deficiency and neuropathy stems from the pathogenesis of type 2 diabetes through β-cell function, insulin secretion and plasma calcium levels. In that context, vitamin D supplementation was shown to cause an increase in serum Ca, a reduction in serum free fatty acids, higher insulin secretion as well as better glucose tolerance.(Reference Shehab, Al-Jarallah and Mojiminiyi2) These findings and mechanisms suggest the importance of vitamin D levels when assessing diabetic neuropathy as well as the effect of vitamin D on the control of insulin in diabetic patients.
Limitations
The present MA does have some potential limitations. First, severe heterogeneity was observed between included articles, and sensitivity analysis found that one article made a great influence on this MA. Second, some included articles were small sampled; however, the pooled sample of 6277 participants could be considered as a fair representative of the population. Larger prospective comparative studies are needed to support our findings. Third, the definition of DPN was not uniform due to a variety of different measurements of DPN and that might have impacted our results. Fourth, different methods were used to assess peripheral neuropathy. Fifth, grey literature was not searched with the possibility of missing relevant unpublished articles, conference abstracts or public reports. Sixth, other potential confounding factors that were not investigated and reported in the included studies (season variations at the time of measurement, levels of sun exposure and populations of different ethnicities) could have introduced bias to the true association between vitamin D level and DPN. Though we conducted two subgroup analyses, one based on race and the other based on study design, heterogeneity was still too high. Selection bias could be one cause of such heterogeneity, despite the fact that quality scores ranged from good to excellent.
Conclusion
In conclusion, the available literature exhibits that vitamin D deficiency could be used as a predictor of diabetic peripheral neuropathy in older adults. However, despite the validation of the studies used in this MA, more randomised controlled trials and prospective studies as well as studies testing the optimum serum vitamin D for normal functioning should be conducted. These additional studies are necessary to understand the mechanism directly linking vitamin D to diabetic neuropathy and to set recommendations for vitamin D supplementation in order to prevent or slow down the development of DPN in diabetic patients.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author contributions
The study was designed by KY. Each author equally contributed to the literature search and data extraction. Data analysis was completed by KY. All authors contributed to the interpretation of the results and the manuscript draft which then was approved by all.









