In modern Western societies the lifestyle is characterized by a highly caloric diet, rich in fat, refined carbohydrates and animal protein, combined with low physical activity, resulting in an overall energy imbalance. Facing the fact that such inappropriate dietary habits are contributing to health risks, including obesity, diabetes, CVD, arterial hypertension and cancer, optimal nutrition that focuses on optimizing the quality of daily diet by ‘innovative foods’ that favour the maintenance of health may help to decrease current and future health care costs.
Within the current discussion on functional foods, scientific research on the physiological effects of fructans is of importance for consumers when food manufacturers and retailers introduce new products with possible ‘health benefits’. In the bakery industry, inulin or fructo-oligosaccharides from chicory (Cichorium intubus) roots or Jerusalem artichoke (Helianthus tuberosus) tubers are often used as fat replacers to improve the acceptability of low-fat products by the consumer.
Interest in these fructans for human nutrition also stems from their prebiotic properties. Prebiotics are defined as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of potentially health-promoting bacteria in the colon such as bifidobacteria or lactobacilliReference Gibson and Roberfroid1, Reference Van Loo2. Moreover, prebiotics are claimed to provide, by selective modification of the composition of the intestinal microflora, enhanced intestinal and systemic function, and reduction of risk of diseaseReference Roberfroid3–Reference Gibson, Probert, Van Loo, Rastall and Roberfroid6.
Yet most of the research on prebiotic activity in man has been limited to inulin-type fructans of chicory rootsReference Gibson, Beatty, Wang and Cummings7–Reference Menne, Guggenbuhl and Roberfroid10, whereas inulin or fructo-oligosaccharides derived from other natural food plants, such as Jerusalem artichoke, have received little attention. Interest in their tubers for food purposes is derived from the special combinations of its prebiotic inulin and fructo-oligosaccharides, natural fructose, minerals, essential amino acids, vitamins and flavonoids.
At the present stage of knowledge, different kinds of inulin-type fructans are equally active as prebiotics in manReference Gibson, Beatty, Wang and Cummings7, Reference Menne, Guggenbuhl and Roberfroid10, Reference Tuohy, Finlay, Wynne and Gibson11 but it cannot be excluded that these substrates incorporated into processed foods can influence the microflora in a different way.
Evidence exists that heat treatment of inulin-type fructans under conditions used for baking leads to changes of the fructan-structure and the formation of more low-molecular-weight degradation productsReference Boehm, Kaiser, Trebstein and Henle12. The consequences of such heat-induced degradation reactions on the prebiotic properties of the fructans in vivo are rarely studiedReference Tuohy, Kolida, Lustenberger and Gibson13.
Taking all the above aspects into account, we were interested in determining the effects of both chicory inulin (CH) and Jerusalem artichoke inulin (JA) in snack bars on intestinal microbiota of healthy human subjects and to evaluate possible gastrointestinal side-effects associated with the consumption of these products. We compared the structure of inulin in raw materials and in processed snack bars, as possible changes during processing may occur which influence the properties of the fructans.
Materials and methods
Subjects and study design
A randomized, double-blind, placebo-controlled study with parallel groups that included forty-five healthy volunteers (students of the Veterinary Faculty, University of Leipzig, Germany) who maintained their usual lifestyle was carried out. All subjects (thirty-five women and ten men) aged 23·5 (sd 2·3) years completed the study. The BMI of the women was 22·4 (sd 2·7) kg/m2 and of the men 23·4 (sd 1·4) kg/m2. Volunteers did not take any antibiotics or laxatives up to 6 months before the start of the study, nor did they take any during the study. Exclusion criteria were severe chronic disease, gastrointestinal disease, abdominal discomfort, constipation, severe food allergy, abnormal dietary habits (e.g. vegetarian, high fibres or soya diet, nutritional supplementation, excessive consumption of alcohol), pregnancy and non-compliance with stool sampling. The study was carried out with a 1-week run-in period followed by a 3-week intervention period. During the run-in period, the volunteers were asked to consume their usual meals but excluding inulin-rich products like onions, leeks, bananas, artichokes, asparagus; inulin-fortified foods as well as fermented dairy products. All participants received a list of prohibited foods. At the end of the run-in period baseline levels of faecal bacterial populations were determined.
After the run-in period the participants were randomly assigned into three groups of fifteen subjects who consumed: (1) snack bars without supplementation of inulin (placebo), (2) snack bars with CH or (3) snack bars with JA. Subjects ingested one snack bar per day (between 07·00 and 08·00 hours) in the first week of the intervention period (adaptation phase to prevent adverse gastrointestinal side-effects), followed by consumption of two snack bars per day (between 07·00 and 08·00 hours and 19·00 and 20·00 hours) in the second and third week of the intervention period. The participants were instructed to maintain their dietary habits and physical activity pattern during the study. All participants were fully informed of the aims and course of the study and gave their written consent. The study protocol was approved by the Research Ethics Committee of the University of Leipzig, Germany.
Characterization of fructans before (raw material) and after bakery processing (snack bars)
The CH (Fibruline® Instant) was supplied by Cosucra, Brussels, Belgium.
Fibruline® Instant is a white powder obtained from chicory roots through conventional manufacturing techniques, including extraction with water, purification, isolation and drying. The product contains 88 % inulin, maximum 10 % free sugars (glucose, fructose and sucrose) and maximum 0·3 % minerals. JA was obtained in the form of syrup from Liven GmbH, Dabendorf, Germany. For production of the syrup, Jerusalem artichoke tubers were washed, hackled and squashed. The resulting slurry was filtered and the syrupy filtrate was evaporated to dryness. The finished product contains the following components: 55 % inulin, 0·5 % glucose, 2 % fructose, 14 % sucrose, 25 % water, 3 % minerals. The data were given by the manufacturer, except the inulin content, which was determined in our laboratory. For quantitative determination of the inulin content, a combination of two commercial test kits was used, which were supplied by r-biopharm (Darmstadt, Germany) and Megazyme (Bray, Ireland). The method is described in detail elsewhereReference Boehm, Kaiser, Trebstein and Henle12.
To map inulin profiles and to determine the degree of polymerization (dp), high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD; Metrohm, Filderstadt, Germany) was used as described previously by Boehm et al. Reference Boehm, Kaiser, Trebstein and Henle12. For qualitative and quantitative analysis, 0·5 g of each inulin was dissolved in 50 ml bidistilled water and subjected to analysis as described earlier. Snack bars were extracted with hot bidistilled water (80°C) for 15 min, and inulin was quantified in the extract.
Preparation of snack bars
Snack bars were produced by Dr Quendt Backwaren GmbH (Dresden, Germany) for the present study. Three batches of snack bars were prepared (two batches containing approximately 7·7 g fructans/bar, differing in the supplemented kind of inulin-type fructans: CH or JA, and the placebo snack bars, which did not contain any supplemented fructans).
These bars (weight 36 g) consisted of purely vegetable ingredients, which naturally did not contain any cholesterol or gluten. Snack bars without supplementation of inulin (placebo) contained a cereal mixture and a binder to approximately equal shares. The cereal composition contained ready-to-eat cereal pieces like rice crisps (20 %), roasted soyabean seeds (12 %), cornflakes (12 %) and buckwheat (3 %). The cereals were mixed with slightly heated binder consisting of several juice concentrates (apple, aronia, apricots, pineapple), dried fruits (cranberries, apricots, pineapple) and a thickening agent. The fluffy mass was rolled out evenly and dried at 135°C for 18 min. After cooling, the dough was cut in bars of similar size. Snack bars with JA contained syrup of the tubers of Jerusalem artichoke in substitution to the equal amount of juice concentrates. Snack bars with CH consisted of Fibruline® Instant, which was also a component of the binder.
Collection and processing of stool samples
Fresh stool samples were collected at the end of the run-in period (on day 7) and weekly during the intervention period (days 14, 21 and 28). Volunteers were asked to bring the samples as soon as possible after defecation. Stool samples were processed within 2 h of collection. For whole-cell fluorescent in situ hybridization, 1 g faecal aliquots were fixed with paraformaldehyde as described previouslyReference Kruse, Kleessen and Blaut14. For analysing SCFA, 0·2 g of specimen was prepared as described by Kruse et al. Reference Kruse, Kleessen and Blaut14.
Bacterial enumeration using fluorescent in situ hybridization combined with conventional microbiological culture techniques
Whole-cell fluorescent in situ hybridization was performed on silanized microscope slidesReference Kleessen, Hartmann and Blaut15 with indocarbocyanin Cy3-labelled 16S/23S rRNA-targeted oligonucleotide probes: (1) an equimolar mixture of five Bacteria-directed probes EUB 338 (S-D-Bact-0338-a-A-18), EUB 785 (S-D-Bact-0785-a-A-19), EUB 927 (S-D-Bact-0927-a-A-17), EUB 1055 (S-D-Bact-1055-a-A-20), EUB 1088 (S-D-Bact-1088-a-A-20)Reference Amann, Ludwig and Schleifer16, Reference Kleessen, Noack and Blaut17, referred to as EUB mix, to detect all bacteria; (2) Erec 482 (S-*-Erec-0482-a-A-19) specific for most of the clostridia and eubacteria belonging to the Clostridium coccoides/Eubacterium rectale groupReference Franks, Harmsen, Raangs, Jansen, Shut and Welling18; (3) Bac 303 (S-*-Bacto-0303-a-A-17) to detect the Bacteroides/Prevotella clusterReference Manz, Amann, Ludwig, Vancanneyt and Schleifer19 combined with a set of two probes for species of the Bacteroides fragilis group Bfra 602 (S-*-0602-a-A-19) and the species Bacteroides distasonis Bdis 656 (S-*-Bdis-0656-a-A-18)Reference Franks, Harmsen, Raangs, Jansen, Shut and Welling18; (4) Fprau 645 (S-*-Fprau-0645-a-A-23) for the Faecalibacterium prausnitzii groupReference Suau, Rochet, Sghir, Gramet, Brewayes, Sutren, Rigottier-Gois and Dore20; (5) the bifidobacterial probe Bif 164 (S-G-Bif-0164-a-A-18)Reference Langendijk, Schut, Jansen, Raangs, Kamphuis, Wilkinson and Welling21; (6) Ato 291 (S-*-Ato-0291-a-A-17) to detect species of the Atopobium cluster Reference Harmsen, Wildeboer-Veloo, Grijpstra, Knol, Degener and Welling22; (7) Chis150 (S-*-Chis-0150-a-A-23) to detect bacteria of the Clostridium histolyticum group; (8) Clit135 (S-*-Clit-0135-a-A-19) to detect clostridia belonging to the Clostridium lituseburense groupReference Franks, Harmsen, Raangs, Jansen, Shut and Welling18. After hybridization bacterial cell enumeration was performed using an epifluorescence microscope (Axioskop, Carl Zeiss, Germany). The detection limit of the method was determined to be log10 5/g stool. Therefore, the cultural approach was used for the detection of some less abundant microbial groups/species in human faeces which might be affected by fructan-type inulin. Enterobacteriaceae, lactobacilli, enterococci and Clostridium perfringens and yeasts were determined using selective media and incubation conditions that have been described previouslyReference Kleessen, Bunke, Tovar, Noack and Sawatzki23, Reference Kleessen, Elsayed, Loehren, Schroedl and Krueger24.
SCFA
SCFA were extracted as described previously by Kruse et al. Reference Kruse, Kleessen and Blaut14. Samples of 1 μl were injected into a gas chromatograph (GC 3900; Varian Deutschland GmbH, Germany) equipped with a flame-ionization detector and a capillary column. Helium was used as carrier gas at a column flow rate of 1·5 ml/min with a split ratio of 1 : 10. The temperature of both detector and injector was 230°C. The concentrations of SCFA were calculated based on peak areas according to the internal standard method using isocapronic acid as an internal standard.
Assessment of bowel habit and gastrointestinal symptoms
During the whole study period, volunteers kept a daily chart and recorded subjective intestinal characteristics, stool frequency and stool consistency, and the time of snack bar consumption. Gastrointestinal complaints concerning repeated flatulence, abdominal bloating, abdominal pain, bowel rumbling as well as bowel cramps could be selected as being absent (0), mild (1), moderate (2) or severe (3). Stool consistency was also scored from 1 (hard) to 4 (watery).
Statistical analysis
Statistical analyses were conducted with the Statistical Package for Social Science, version 11·5 (SPSS Inc., Chicago, IL, USA). Normal distribution of analytical variables was checked with the Shapiro–Wilk test. The general linear model repeated measure procedure was used to test between treatment groups and within group differences in the repeated continuous variables. If there were significant overall effects, ANOVA with the Bonferroni test was used for between treatment group analyses and the paired samples t test for within group analysis. The Pearson Chi-square test was used to assess the significance of differences between the numbers (percentage) of subjects harbouring specific bacteria. The occurrence and the intensity of gastrointestinal symptoms were scored on the basis of the 7 d observations both in the run-in period and weekly during the intervention period. Groups were compared statistically using the non-parametric Kruskal–Wallis and Mann–Whitney U test. Time comparison was done with the Friedman and Wilcoxon signed-rank testReference Wilcoxon and Wilcox25. Before statistical analysis of the bacteriological results, the cell counts were transformed to log10 numbers. Differences were considered to be significant at P < 0·05.
Results
Fructans before and after processing of snack bars
HPAEC-PAD patterns of CH and JA are shown in Fig. 1. For semi-quantitative characterization, the inulin profile was separated into defined dp ranges and the relative peak area was calculated after integrationReference Boehm, Kaiser, Trebstein and Henle12. With dp values mainly between 5 and 12, CH and JA belong to the short-chain inulin-type fructans. Inulin from chicory comprises approximately 46 % fructose chains with dp 5–12, 24 % fructo-oligosaccharides with dp below 5, and the residual fructose units show a dp higher than 12. Only 2 % of the inulin is found in the higher molecular weight fraction above a dp of 30. Inulin from syrup of Jerusalem artichoke contains approximately 40 % fructose chains with dp below 5, 49 % with dp between 5 and 12 and only 11 % chains with dp above 12. The inulin content was analysed in both inulin samples based on fructose quantification after enzymatic hydrolysis. The inulin content of JA is lower (54·8 g/100 g) than that of CH (88·0 g/100 g). The contents of inulin of the produced snack bars, also determined with the method used for CH and JA, are comparable. We found an amount of 20·8 g inulin (JA) or 21·0 g inulin (CH), respectively, per 100 g of the snack bar. Relating to a snack bar with a weight of 36 g, this corresponds to an amount of 7·5 g inulin per bar and is in good agreement with the expected amount (7·7 g CH/JA). No inulin was found in the placebo snack bar.

Fig. 1 High-performance anion-exchange chromatography with pulsed amperometric detection of (a) chicory inulin (CH); (b) Jerusalem artichoke inulin (JA); (c) snack bar with CH; (d) snack bar with JA. dp, degree of polymerization; I, amperage.
Faecal microflora
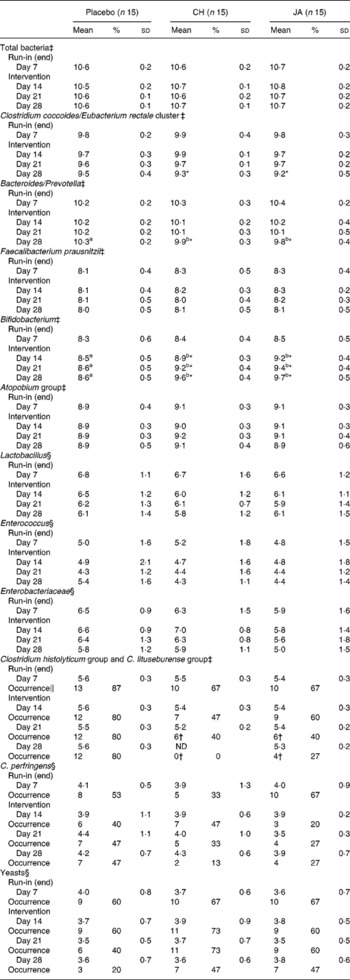
Baseline data of faecal microbial populations at the end of the run-in period showed that no significant differences existed among volunteers due to normal biological fluctuation in the composition of the faecal microflora (Table 1).
Table 1 Faecal bacteria (log10 counts/g wet weight) in faeces of healthy volunteers before and after consuming snack bars containing chicory inulin (CH) or Jerusalem artichoke inulin (JA) v. placebo
ND, not detected.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Mean values were significantly different from those of the run-in period: *P < 0·05.
Mean values were significantly different from those of the placebo group: †P < 0·05.
‡ Bacterial counts were assessed by fluorescent in situ hybridization.
§ Bacterial counts (colony forming units) were assessed by conventional culture methods.
‖ The occurrence of organisms is expressed as the number of subjects colonized with percentage. Microbial groups without given occurrence were detected in all subjects per group (n 15; 100 %).
After consuming CH or JA compared with placebo, total bacterial numbers were constant throughout the study, but there were differences with respect to counts and frequency of certain bacterial groups (Table 1). No time effects on the different bacterial counts were observed in the placebo group. In subjects consuming CH or JA, a steady increase in bifidobacteria by approximately 1·2 log in 3 weeks was observed and there were significant differences in bifidobacterial numbers compared to the placebo group at all sampling times of the intervention period (P < 0·05).
On the final sampling day (intervention, day 28), in contrast, subjects consuming CH or JA showed lower numbers of Bacteroides/Prevotella than the placebo group (P < 0·05). At the same time, potential pathogenic bacteria of the Clostridium histolyticum and C. lituseburense group were less frequently isolated (P < 0·05) in JA or were beyond the detection limit in CH subjects.
Compared to the placebo group, subjects who had consumed CH or JA showed approximately 0·6 log less numbers of the Clostridium coccoides/Eubacterium rectale group at the end of the intervention period (day 28) than at the end of the run-in phase (day 7). Counts of Enterobacteriacea were higher in subjects consuming CH than in those consuming JA only at the end of the first week of intervention (day 14). No differences among groups were noted in counts and frequency of Faecalibacterium prausnitzii, the Atopobium group, Lactobacillus, Enterococcus, C. perfringens and yeasts (Table 1).
Overall, the chosen method allowed us to assign up to 62 % of the total bacteria detected with the mixture of five bacteria-directed probes to distinct bacterial groups.
SCFA
Parallel to microbiological studies faecal samples were analysed for SCFA. Faecal concentrations of total SCFA (μmol/g wet weight) did not differ significantly between treatment groups: for example 138·8 (sd 39·4) in the placebo group, 142·4 (sd 43·7) in the CH group and 135·2 (sd 50·7) in the JA group at the end of the intervention period. The same also applied to faecal concentrations of the individual SCFA, i.e. acetic, propionic, n-butyric and of the sum of iso-C4-C6 fatty acids (data not shown). The molar proportion of acetic, propionic, n-butyric and of the sum of iso-C4-C6 fatty acids varied from 64 to 70 %, 14 to 16 %, 11 to 13 % and 4 to 6 %, respectively, for all treatments at the end of the intervention period (P>0·05).
Bowel habit and gastrointestinal symptoms
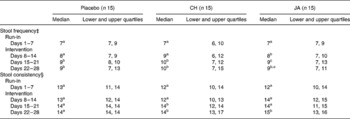
Consumption of snack bars resulted in a slight increase in stool frequency to approximately nine or ten bowel movements per week, independent of CH, JA or placebo consumption (Table 2). Stool consistency, however, was only slightly affected in subjects consuming two snack bars with CH or JA per day in the second and/or third week of intervention (P < 0·05). The main reported daily stool forms were rated between cylindrical formed to soft (Table 2).
Table 2 Stool frequency and stool consistency of healthy volunteers before and after consuming snack bars containing chicory inulin (CH) or Jerusalem artichoke inulin (JA) v. placebo
a,b,c Median values within a column with unlike superscript letters were significantly different (P < 0·05).
‡ Stool frequency expressed as number of stools per 7 d.
§ Stool consistency expressed as sum of scores per 7 d. Daily scores: 1, hard pellets; 2, cylindric formed; 3, soft, pudding-like; 4, watery.
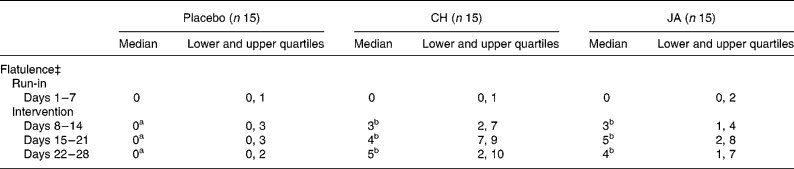
Gastrointestinal complaints concerning flatulence were significant higher (P < 0·05) during consumption of CH or JA compared with placebo (Table 3). This intestinal characteristic, however, was usually mild and sometimes moderate and in no case led to discontinuation of the study (Table 4). The other subjective gastrointestinal parameters were not significantly affected by treatment (Table 3), although few subjects rarely reported mild discomfort after consuming CH or JA.
Table 3 Subjects reporting gastrointestinal complaints after consuming snack bars containing chicory inulin (CH) or Jerusalem artichoke inulin (JA) v. placebo

a,b Values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4 Severity of the reported symptom ‘flatulence’ in healthy volunteers before and after consuming snack bars containing chicory inulin (CH) or Jerusalem artichoke inulin (JA) v. placebo
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
‡ ‘Flatulence’ symptom intensity was expressed as sum of scores per 7 d. Ranking: 0, absent; 1–7 mild; 8–14 moderate; 15–21 severe.
Discussion
The increasing consumer awareness that diet and health are linked is stimulating the development of novel products by the food industry. However, little information exists on how food processing, such as thermal treatment of the raw materials of inulin-type fructans in bakery products, may affect their prebiotic properties in vivo. Moreover, only a few studies have addressed the effects of the chemistry and structure of fructans on the composition and the microbial activity of the gastrointestinal microflora during its continued administration.
The results of the present study show that CH and JA in bakery products, namely in our snack bars, resulted in marked changes in faecal microbiota measured by conventional culture techniques and fluorescent in situ hybridization with 16S/23S rRNA-targeted oligonucleotide probes. To our knowledge, this was the first study investigating prebiotic properties of the above fructans after bakery processing in vivo in a 3-week double-blinded placebo-controlled intervention trial.
It is noteworthy that both CH and JA had similar structures before and after processing of the snack bars. HPAEC-PAD analysis indicates that the samples used (CH, JA) had similar inulin profiles (Fig. 1(a, b)). The HPAEC-PAD patterns obtained after separation of extracts from inulin-containing snack bars (Fig. 1(c, d)) showed no explicit differences compared to the inulin profiles of the raw materials CH and JA (Fig. 1(a, b)). Thus, the production process does not lead to a change of the inulin profile and the content of inulin. This is probably due to the fact that during the production process the snack bars are heated at relatively low temperatures. Dry heating of inulin from chicory for up to 60 min at temperatures between 135 and 195°C resulted in a significant degradation of the fructan ranging from 20 to 100 %Reference Boehm, Kaiser, Trebstein and Henle12. In vitro fermentation experiments with highly heated inulin from Jerusalem artichoke showed an improved prebiotic effect for the heated samples compared to the unheated samplesReference Boehm, Kleessen and Henle26. We suppose that di-d-fructose dianhydrides found during heating from fructo-oligosaccharides may be responsible for this phenomenon, as di-d-fructose dianhydrides were found to be prebiotic in former studiesReference Orban, Patterson, Sutton and Richards27, Reference Minamida, Shiga, Sujaya, Sone, Yokota, Hara, Asano and Tomita28. This hypothesis, however, must be proven in further studies. Thus, baking products containing higher-heated inulin should be investigated regarding the degradation products of inulin and their prebiotic effects.
In view of the finding that CH and JA did not differ in structure after bakery processing we expected similar effects of these fructans on the intestinal microbiota of healthy human subjects consuming snack bars containing CH or JA. One important finding of the present study concerns the increase in faecal bifidobacteria in volunteers consuming CH or JA compared with placebo. Such observation is well in line with the hypothesis that the prebiotic acts as a selective substrate on which the bifidobacteria growReference Gibson and Roberfroid1. In the present study the increase in bifidobacteria was detected after only 1 week of consumption of one snack bar providing the lower dose of approximately 7·7 g fructan/d. The present observation is consistent with previous microbiological results obtained by other trials in man. Bouhnik et al. Reference Bouhnik, Vahedi, Achour, Attar, Salfati, Porchart, Marteau, Flourié, Bornet and Rambaud9, Reference Bouhnik, Raskine, Simoneau, Vicaut, Neut, Flourié, Brouns and Bornet29 have observed that numbers of bifidobacteria in volunteers increased after a 7 d feeding period of short-chain fructans. RaoReference Rao30 demonstrated that a low dose of oligofructose (5 g/d) resulted in an increase in faecal bifidobacteria by approximately 1·0 log after 11 d. Contrary to other studies, however, which have demonstrated that inulin consumption in the second week or as long as the prebiotic was eaten did not further increase numbers of bifidobacteriaReference Menne, Guggenbuhl and Roberfroid10, Reference Tuohy, Finlay, Wynne and Gibson11, the present results support the hypothesis that after an initial adaptation period, a higher dosage of fructans (two snack bars per day providing the dosage of approximately 14·5 g fructan/d) may lead to increased faecal numbers of bifidobacteria (Table 1). The increase in bifidobacteria in the intestine of the volunteers consuming CH or JA may contribute to the health of the host. Among the major bacterial groups of colonic bacteria, Bifidobacterium are usually thought to be apathogenicReference Tuohy, Rouzaud, Bruck and Gibson31. These organisms may increase resistance to disease by reducing the growth of pathogenic and putrefactive bacteria by lowering pH, competing directly for substrates and/or attachment sites on the epithelial mucus layer, producing inhibitory molecules and stimulating the enteric immune systemReference Tuohy, Rouzaud, Bruck and Gibson31–Reference Picard, Fioramonti, Francois, Robinson, Neant and Matuchansky34.
It is noteworthy that the increase in bifidobacteria in the present study was paralleled by a decrease of various potential pathogenic bacterial groups, such as Bacteroides/Prevotella in number and the Clostridium histolyticum/C. lituseburense group in frequency. The latter effects reached significance (P < 0·05) after the 3-week intervention period in the faeces (Table 1), suggesting the need for a certain time of adaptation. Other, mainly short-time, nutrition trials using inulin-type fructans often failed to show changes in the composition of the gut microflora towards reducing the number of species which are considered to be harmful to the hostReference Kleessen, Sykura, Zunft and Blaut8, Reference Menne, Guggenbuhl and Roberfroid10, Reference Tuohy, Kolida, Lustenberger and Gibson13.
It should be noted that several of the fluorescent in situ hybridization probes employed in the present study target quite large and diverse groups of bacteria. Therefore the study does not exclude the possibility that inulin-driven changes might also occur in some components of these groups. Moreover, it cannot be excluded that other known or unknown bacteria, which were not detected with the set of probes used in the present study, may be affected by CH and/or JA.
In view of the changes in the microbial counts after CH or JA consumption, we expected alteration in indexes of microbial metabolism such as luminal SCFA. The present results show, however, that the faecal output of SCFA was not significantly affected by either CH or JA intake. This may be because the concentrations of SCFA in the faeces do not reflect the differences in their rate of production by the colonic flora. Moreover, SCFA concentrations are markedly affected by their distribution volume and their absorption rate (up to 95 % in human intestine)Reference Alles, Hautfast, Nagengast, Hartemink, Van Laere and Jansen35, Reference Wong, de Souza, Kendall, Eman and Jenkins36. Only a small proportion of all SCFA produced in the large bowel is excreted in the faeces. Therefore, faecal data are of low physiological relevance. For this reason new areas of interest in this field should be the direct effects of SCFA on immune cells and immune function (e.g. cytokine production). Mechanisms of mucosal protection in relation to alteration of levels of SCFA should be addressed.
Several studies in man have shown that fermentation of inulin-type fructans may stimulate bowel movements and may normalize stool frequency, especially in slightly constipated subjectsReference Roberfroid3, Reference Gibson, Beatty, Wang and Cummings7, Reference Kleessen, Sykura, Zunft and Blaut8. In the present study the volunteers were variable in their response to CH or JA. The data indicate a slight laxative effect of CH or JA in healthy volunteers (Table 2). In accordance with other studies, indicating efficient fermentation of fructans in the colonReference Kruse, Kleessen and Blaut14,Reference Pedersen, Sandström and van Amselvoort37,Reference Sabotka, Bratowa, Slemrova, Manak, Vizda and Zadak38, this positive effect was sometimes accompanied by a slight abdominal discomfort. Mainly flatulence was reported by a few volunteers, who reported mild and sometimes moderate discomfort after consuming CH or JA (Table 4).
The formation of hydrogen, which is a metabolic end-product of bacterial fermentation in the colon, is most probably the major cause of this symptom. However, it has to be emphasized that bifidobacteria, the numbers of which are increased significantly with inulin, are not considered to be producers of hydrogen. For this reason, the relationship between specific intestinal bacteria and gas production as a result of colonic fermentation remains to be clarified.
It should be mentioned that the present study extends earlier studies conducted in man and human flora-associated ratsReference Kleessen, Sykura, Zunft and Blaut8, Reference Kleessen, Hartmann and Blaut15 using a stepwise adaptation on the doses of inulin-type fructans. The strategy of adaptation was effective in producing changes in the microbiota, namely by increasing bifidobacteria, that persisted during the intervention period and resulted in a decrease of potentially harmful bacteria at the end of the intervention. Further studies are needed to clarify whether long-term consumption of CH or JA may lead to further changes or to stabilization of the altered microbiota and whether alterations in colonic function and microflora may be detectable in volunteers after the dietary intervention has ended.
In summary, the data indicate that JA in bakery products resulted in similar effects on microbiota and bowel habit compared with CH, the so-called gold-standard for the demonstration of a prebiotic effect. The prebiotic nature of the raw materials was maintained in the processed snack bars.
Acknowledgements
We are especially grateful to our volunteers for their cooperation. The expert technical assistance of Gabriele Dobeleit and Gerald Vallentin for SCFA analysis is acknowledged. We thank Dr Quendt GmbH, Dresden, Germany for preparation of the snack bars. This study was partly supported by a BMBF InnoRegio BioMeT-Project.







