Food not only undergoes digestion by the mammalian gastrointestinal tract (GIT) digestive enzymes but can also be subjected to fermentation by the resident microbial population, and both dietary and endogenous (derived from the body) organic materials can be fermented( Reference Bergman 1 – Reference Nakamura, Lin and McSweeney 3 ). The degree of fermentation depends on the diet and the region of the GIT, with greater fermentation occurring in the hindgut compared with the small intestine( Reference Højberg, Canibe and Knudsen 2 , Reference Jensen and Jørgensen 4 ). The main end products of microbial fermentation in the GIT are the SCFA. SCFA have been associated with a number of beneficial effects in the GIT, including intestinal tissue proliferation, enhanced absorption of minerals and water, modulation of GIT contractility, increased numbers of beneficial bacteria and reduced numbers of pathogenic bacteria. In addition, SCFA are the main energy source for epithelial cells( Reference Bergman 1 , Reference Henningsson, Björck and Nyman 5 – Reference Vinolo, Rodrigues and Nachbar 8 ). Given the importance of SCFA, information about their production and absorption in the hindgut is a key consideration.
A number of studies in both humans and farm animals have determined SCFA concentrations in different regions of the GIT and also in the faeces, and these have been used to draw conclusions regarding the production of SCFA in the hindgut( Reference Højberg, Canibe and Knudsen 2 , Reference Weaver, Tangel and Krause 9 , Reference Whelan, Judd and Preedy 10 ). The latter approach has limitations, however, as SCFA concentrations are not a measure of SCFA production but rather are the net result of SCFA production and absorption( Reference McNeil, Cummings and James 11 – Reference Topping and Clifton 13 ). Seemingly, only approximately 5 % of the SCFA produced in the hindgut are excreted in the faeces( Reference Roy, Kien and Bouthillier 7 , Reference Topping and Clifton 13 ). The appearance of SCFA in the portal vein and in exhaled breath have also been used( Reference Topping and Clifton 13 – Reference Regmi, van Kempen and Matte 16 ) as measures related to production. However, these methods do not account for the utilisation of SCFA within the intestinal epithelium (portal blood method) or the body in general (exhaled breath). Another approach is to collect ileal digesta for a given diet and use this as a substrate for in vitro fermentation with a faecal inoculum (to simulate the action of the microbes in the hindgut)( Reference McBurney and Sauer 17 – Reference Coles, Moughan and Awati 21 ) in order to obtain an estimate of SCFA production based on the amount of DM entering the large intestine and the measure of fermentability. The latter approach has been used to estimate the available energy content of selected foods for humans( Reference Coles, Moughan and Awati 21 ). Several groups have also used this approach to estimate the SCFA production in the hindgut( Reference McBurney and Sauer 17 , Reference Anguita, Canibe and Perez 18 , Reference Christensen, Bach Knudsen and Wolstrup 22 , Reference Wang, Zhu and Li 23 ), but very few studies( Reference Anguita, Canibe and Perez 18 , Reference Christensen, Bach Knudsen and Wolstrup 22 ) have determined SCFA absorption.
The overall aim of this study was to use a combined in vivo–in vitro digestion methodology( Reference McBurney and Sauer 17 , Reference Anguita, Canibe and Perez 18 , Reference Coles, Moughan and Awati 21 , Reference Christensen, Bach Knudsen and Wolstrup 22 ) to predict both the hindgut production and the absorption of SCFA in growing pigs (an animal model for the digestion of food in humans) fed diets containing kiwifruit as the only source of dietary fibre. Kiwifruit was selected as it is a well-characterised source of dietary fibre for humans, containing pectins, hemicelluloses and cellulose( Reference Redgwell, Melton and Brasch 24 ). In contrast to previous studies( Reference Anguita, Canibe and Perez 18 , Reference Christensen, Bach Knudsen and Wolstrup 22 ), the hindgut absorption of SCFA also includes the SCFA present in terminal ileal digesta, thereby taking into account the SCFA entering the hindgut as a result of fermentation in the small intestine.
Methods
Dietary treatments
Two diets containing 133 and 266 g of green kiwifruit (Actinidia deliciosa cv. Hayward) DM/kg diet DM, as the only dietary fibre source (25 or 50 g fibre/kg DM, respectively), were formulated to meet the nutrient requirements of growing pig as prescribed by the National Research Council( 25 ) (Table 1). The diet concentrations of kiwifruit fibre were chosen to provide concentrations of total dietary fibre similar to those reported for a typical Western diet (30–70 g/kg DM)( Reference Baer, Rumpler and Miles 26 ). The daily ration was calculated as 90 g of DM/kg of metabolic body weight (BW0·75) per d and was fed to the pigs as two equal meals provided at 09.00 and 16.00 hours. Titanium dioxide was included as an indigestible marker in the diets. Kiwifruit were added to the semi-synthetic experimental diets and were freshly peeled and crushed just before each meal. Fresh water was provided ad libitum.
Table 1 Ingredients and determined nutrient compositions of the experimental diets
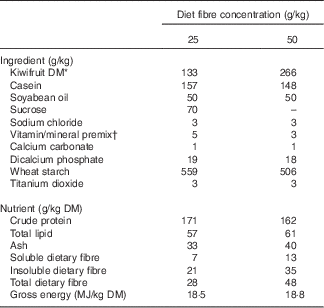
* Freshly peeled and crushed kiwifruit was added to the diets just before feeding.
† Vitamin and mineral premixes were obtained from Vitec Nutrition Ltd and supplied the following (per kg of diet as-fed): Mn, 45·0 mg; Zn, 80·0 mg; Cu, 25·0 mg; Co, 0·5 mg; Se, 0·3 mg; Fe, 100·0 mg; I, 1·0 mg; choline, 100·0 mg; vitamin A (all-trans retinylacetate), 10 000 IU (3·3 mg); vitamin D3 (cholecalciferol), 2000 IU (0·05 mg); vitamin E, 50 mg; vitamin K, 2·0 mg; thiamin, 1·0 mg; riboflavin, 3·0 mg; nicotinic acid, 15·0 mg; pantothenic acid, 20·0 mg; pyridoxine, 2·0 mg; vitamin B12, 0·01 mg; folic acid, 0·5 mg; biotin, 0·1 mg.
In vivo assay
Animals and housing
Ethics approval for the animal trial was obtained from the Animal Ethics Committee, Massey University. A total of fourteen entire male pigs (PIC Camborough 46×PICboar 356L) (41·4 (sd 2·98) kg mean body weight) were housed individually in pens (1·5×1·5 m) in a room maintained at 21 (sd 2)°C with a 10 h light–14 h dark cycle. Pigs were surgically modified by the implantation of a simple T cannula at the terminal ileum as previously described( Reference Montoya, Rutherfurd and Moughan 27 ).
Experimental design
Pigs were randomly allocated to the two kiwifruit-containing diets (seven pigs/diet), which were fed to the animals for 16 d. Faecal samples were collected directly on the 14th and 15th d following anal stimulation, and were immediately frozen at −20°C for determining total tract digestibility of organic matter (OM) and at −80°C for determining faecal SCFA concentrations. Ileal digesta were collected into a plastic bag attached to the cannula. Digesta were collected from each pig on the 15th and 16th day and over a 6- h period each day starting from 1 h after feeding. Plastic bags were changed every 30 min and the digesta were frozen immediately to minimise further digestion. From the first batch of fresh digesta, a subsample was collected into a sealed Eppendorf tube for SCFA determination in order to reduce the risk of volatilisation. The ileal digesta samples were used to determine the apparent ileal digestibility of OM, ileal SCFA concentrations and also to provide a substrate for the in vitro fermentation assay. The faecal samples were collected before collecting the ileal digesta samples in order to avoid interference with the hindgut flow of DM.
In vitro fermentation assay
Fermentation of the ileal digesta was carried out using a pig faecal inoculum as previously described( Reference Coles, Moughan and Awati 20 , Reference Coles, Moughan and Awati 21 ). Fresh faeces samples were collected from five healthy pigs (fed a commercial grower diet) into isolated containers flushed with CO2 at 37°C. In brief, the pig faecal inoculum was prepared by homogenising the faeces with 0·1 m-phosphate buffer at pH 7 (1:5, w/v) and filtering the homogenate. Inoculum (5 ml) was added to a McCartney bottle containing 5 ml phosphate buffer either alone (blank incubation) or containing 100 mg of finely ground freeze-dried ileal digesta sample collected from the pigs fed the experimental diet. There were four replicate bottles per blank or ileal digesta sample; one bottle was used to determine the SCFA after fermentation and the other three bottles were used to determine the OM fermentability. All the bottles were flushed with CO2, capped and incubated with the pig inoculum for 38 h at 37°C. After incubation, the SCFA concentrations in the material of one of the replicate bottles were determined after thoroughly mixing the contents of the bottle and transferring an aliquot (1 ml) to an Eppendorf tube, which was then centrifuged at 14 000 rpm for 15 min at 4°C. The supernatant (500 μl) was transferred to another Eppendorf tube and frozen at –20°C before analysis for SCFA. The other three bottles were placed in an autoclave (121°C for 20 min) to completely inactivate the bacteria and to remove the end products of OM fermentation (e.g. SCFA). The DM of the unfermented residue was determined in the remaining three bottles by drying them in an oven at 60°C until they reached a constant weight.
Chemical analysis
The diets, ileal digesta and faeces were analysed in duplicate for DM, OM, ash and titanium dioxide. DM, OM and ash were determined using standard procedures( 28 ), and titanium dioxide was determined following the method of Short et al.( Reference Short, Gorton and Wiseman 29 ). The diets were also analysed for crude protein, gross energy, diethyl ether extract( 28 ) and soluble, insoluble and total dietary fibre contents( Reference Prosky, Asp and Schweizer 30 ). The dried contents of the in vitro fermentation media from the three bottles were analysed for OM.
SCFA were determined in the faecal and ileal digesta samples and in the supernatants obtained from the in vitro fermentation. Thawed faecal samples were prepared for analysis by mixing them with Milli-Q-water (Millipore) (4°C) (1:3, w/v). Thawed ileal digesta and the prepared faecal samples were then centrifuged at 14 000 g for 15 min at 4°C. The supernatants were collected and analysed in duplicate for acetic, butyric, propionic and valeric acids, using a Shimadzu GC2010 Gas Chromatograph System fitted with a Zebron ZB-FFAP column (30 m×0·32 mm) (Phenomenex) as described previously( Reference Bindelle, Pieper and Montoya 31 ).
Calculations and statistical analysis
In vivo assay
The apparent ileal and faecal digestibilities and the hindgut fermentability were calculated as follows:
where TD and TF/I are the titanium dioxide contents (g/kg DM) in the diet and in the faeces or ileal digesta, respectively; OMD and OMF/I are the contents of OM (g/kg DM) in the diet and in the faeces or ileal digesta, respectively.
The concentrations of SCFA in the terminal ileal digesta and in the faeces (normalised for the food DM intake (DMI)) were calculated using the following equation:
In vitro fermentation assay
Predicted hindgut fermentability of OM and SCFA produced by fermentation were obtained after in vitro fermentation of the ileal digesta with the faecal inoculum and calculated using the following equations:
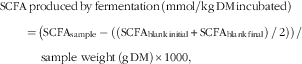 $$\eqalignno{ {\rm SCFA}&\,{\rm produced}\,{\rm by}\,{\rm fermentation}\,\left( {{\rm mmol/kg\, DM}\,{\rm incubated}} \right) \,\cr & {=}\,\left( {{\rm SCFA}_{{{\rm sample}}} -\left( {\left( {{\rm SCFA}_{{{\rm blank}\,{\rm initial}}} } \right.} \right.} \right.{\plus}\left. {\left. {\left. {{\rm SCFA}_{{{\rm blank}\,{\rm final}}} } \right)\,/\,2} \right)} \right)\,/\,\cr &\,\quad {\rm sample\ weight}\,\left( {{\rm g}\,{\rm DM}} \right){\rm {\times}1000}, $$
$$\eqalignno{ {\rm SCFA}&\,{\rm produced}\,{\rm by}\,{\rm fermentation}\,\left( {{\rm mmol/kg\, DM}\,{\rm incubated}} \right) \,\cr & {=}\,\left( {{\rm SCFA}_{{{\rm sample}}} -\left( {\left( {{\rm SCFA}_{{{\rm blank}\,{\rm initial}}} } \right.} \right.} \right.{\plus}\left. {\left. {\left. {{\rm SCFA}_{{{\rm blank}\,{\rm final}}} } \right)\,/\,2} \right)} \right)\,/\,\cr &\,\quad {\rm sample\ weight}\,\left( {{\rm g}\,{\rm DM}} \right){\rm {\times}1000}, $$
where OMb and OMa are the OM (mg) of the ileal digesta either before or after in vitro fermentation. OMblank initial, OMblank final, SCFAblank initial and SCFAblank final are the OM (mg) and the SCFA (mmol) in the blank bottle (which contained inoculum but no ileal digesta) before (initial) and after (final) in vitro fermentation, respectively( Reference Coles, Moughan and Awati 20 ).
Predicted total tract digestibility, SCFA production and absorption in the hindgut
The predicted apparent total tract digestibility (PADfaecal) of OM and the SCFA production and absorption in the hindgut were calculated based on combining results for in vivo ileal digesta flows (ileal cannulated pig) with in vitro concentrations (hindgut fermentation). The in vivo values represented digestion in the upper gut, and the in vitro data represented fermentation in the hindgut. PADfaecal of OM and predicted hindgut SCFA production were calculated as follows:
 $$\eqalignno{ & {\rm Predicted}\,{\rm hindgut}\,{\rm SCFA}\,{\rm production}\,\left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\,\cr & \qquad {{=}\,\rm SCFA}\,{\rm produced}\,{\rm by}\,{\rm fermentation} \cr & \qquad \quad \left( {{\rm mmol}/{\rm kg}\,{\rm ileal}\,{\rm digesta}\,{\rm DM}\,{\rm incubated}} \right) \cr & \qquad \quad {\times}{\rm ileal}\,{\rm DM}\,{\rm flow}\,\left( {{\rm kg}\,{\rm DM}/{\rm kg}\,{\rm DMI}} \right), $$
$$\eqalignno{ & {\rm Predicted}\,{\rm hindgut}\,{\rm SCFA}\,{\rm production}\,\left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\,\cr & \qquad {{=}\,\rm SCFA}\,{\rm produced}\,{\rm by}\,{\rm fermentation} \cr & \qquad \quad \left( {{\rm mmol}/{\rm kg}\,{\rm ileal}\,{\rm digesta}\,{\rm DM}\,{\rm incubated}} \right) \cr & \qquad \quad {\times}{\rm ileal}\,{\rm DM}\,{\rm flow}\,\left( {{\rm kg}\,{\rm DM}/{\rm kg}\,{\rm DMI}} \right), $$
where OMD (g/kg DM) is the OM content in the diet, and ileal OM flow (g/kg DMI) is the ileal flow of OM. Hindgut OM fermentability in vitro (%) was determined using the in vitro fermentation assay.
The amounts of SCFA entering the hindgut (i.e. ileal normalised SCFA concentration) and the amounts produced in the hindgut (i.e. in vitro predicted hindgut SCFA production) were used to predict the amounts of SCFA absorbed in the hindgut based on the following equation:
 $$\eqalignno{ & {\rm Amount}\,{\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm from}\,{\rm hindgut} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr &\qquad {=}\,in\,vitro\,{\rm predicted}\,{\rm hindgut} \cr & \qquad\ \ \, {\rm SCFA}\,{\rm production}\ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr & \qquad\ \ \, {\plus}{\rm ileal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr & \qquad\ \ \, {\minus} \,{\rm faecal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right), $$
$$\eqalignno{ & {\rm Amount}\,{\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm from}\,{\rm hindgut} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr &\qquad {=}\,in\,vitro\,{\rm predicted}\,{\rm hindgut} \cr & \qquad\ \ \, {\rm SCFA}\,{\rm production}\ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr & \qquad\ \ \, {\plus}{\rm ileal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)\cr & \qquad\ \ \, {\minus} \,{\rm faecal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right), $$
 $$\hskip -2pt \eqalignno{ &{\rm Extent} \ {\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm in}\,{\rm the}\,{\rm hindgut}\ \left( \% \right)\,\cr &\quad {=}\,\left( {{\rm amount}\,{\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm from}\,{\rm the}\,{\rm hindgut}} \right. \cr & \quad\quad\left( {{\rm mmol}/{\rm kg}\,{\rm DM}\,{\rm intake}} \right)/\left( {in\,vitro\,{\rm predicted}\,} \right. \cr & \quad\quad{\rm hindgut}\,{\rm SCFA}\,{\rm production } \ ( {{\rm mmol}/{\rm kg}\,{\rm DMI}} ) \cr & \quad\quad\left. {\plus }{\left. {{\rm ileal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)} \right)} \right){\times}100. $$
$$\hskip -2pt \eqalignno{ &{\rm Extent} \ {\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm in}\,{\rm the}\,{\rm hindgut}\ \left( \% \right)\,\cr &\quad {=}\,\left( {{\rm amount}\,{\rm of}\,{\rm SCFA}\,{\rm absorbed}\,{\rm from}\,{\rm the}\,{\rm hindgut}} \right. \cr & \quad\quad\left( {{\rm mmol}/{\rm kg}\,{\rm DM}\,{\rm intake}} \right)/\left( {in\,vitro\,{\rm predicted}\,} \right. \cr & \quad\quad{\rm hindgut}\,{\rm SCFA}\,{\rm production } \ ( {{\rm mmol}/{\rm kg}\,{\rm DMI}} ) \cr & \quad\quad\left. {\plus }{\left. {{\rm ileal}\,{\rm SCFA}\,{\rm concentration} \ \left( {{\rm mmol}/{\rm kg}\,{\rm DMI}} \right)} \right)} \right){\times}100. $$
Statistical analyses were performed using SAS (version 9.3, 2011; SAS Institute Inc.). A two-independent samples t test procedure was performed to test the effect of dietary kiwifruit fibre (25 and 50 g/kg DM) on both the in vivo and in vitro data. The same procedure was used to compare the determined and predicted digestibilities and fermentabilities for each kiwifruit diet. A paired t test procedure was used to compare the ileal and faecal SCFA concentrations. The normal distribution for the t test was evaluated using the ODS graphics procedure of SAS. When the variances were unequal, the P value reported was obtained using the Satterthwaite separate variance t test.
Results
The diet containing the highest concentration of kiwifruit fibre had a 1·7-fold greater content of total dietary fibre than the lower-fibre diet (Table 1). This difference, however, was reduced to only 1·2-fold in the ileal digesta (345 and 399 g/kg DM ileal digesta for pigs fed diets containing 25 and 50 g fibre/kg DM, respectively).
Determined ileal and faecal concentrations of SCFA (in vivo)
There was no difference (P>0·05) for either the ileal or the faecal concentrations of the SCFA between the two fibre-containing diets (Table 2). Similarly, there was no difference between the ileal and faecal SCFA concentrations within each diet (P>0·05), with the exception of acetic acid for the diet containing 50 g/kg of fibre. For this diet, the ileal concentration of acetic acid was 2·54-fold higher than the faecal concentration (P<0·05).
Table 2 Ileal and faecal concentrations of SCFA for ileal cannulated pigs fed diets containing different concentrations of fibre (Mean values with their pooled standard errors; n 7 per group)

* The ileal concentrations of SCFA were determined directly from the frozen samples of ileal digesta collected from the pigs.
† Faecal concentrations were determined directly from the frozen samples of faeces collected from the pigs.
Production of SCFA after in vitro fermentation and predicted hindgut SCFA production (in vivo–in vitro)
The SCFA produced by in vitro fermentation were similar for all the SCFA between the two fibre-containing diets (P>0·05; Table 3). In contrast, the predicted hindgut production of acetic, butyric and propionic acids was higher (P<0·05) for the diet containing 50 g/kg of fibre compared with the diet containing 25 g/kg of fibre (1·7-, 1·9- and 1·5-fold higher, respectively; Table 3). There was no difference (P>0·05) between diets in the case of valeric acid.
Table 3 Predicted production and absorption of SCFA in the pig hindgut (in vivo–in vitro assay) for diets containing different concentrations of fibre (Mean values with their pooled standard errors; n 7 per group)

* The hindgut production of SCFA in pigs was determined after in vitro fermentation of the ileal digesta collected from pigs fed the experimental diets with a pig faecal inoculum for 38 h at 37°C.
† n 6, an outlier was removed from the statistical analysis based on the output of SAS.
‡ The predicted hindgut production of SCFA in pigs was estimated based on the SCFA produced after in vitro incubation of pig ileal digesta with a pig faecal inoculum corrected for the ileal flow of DM.
§ The SCFA absorption in the pig hindgut was obtained after summing the SCFA entering (ileal concentrations) and produced (predicted based on an in vitro assay) in the hindgut, and then subtracting the excreted SCFA (faecal concentrations).
|| The apparent absorption in the pig hindgut was predicted based on the ratio between the predicted amount of SCFA absorbed from the hindgut and the sum of SCFA entering (ileal concentrations) and produced (predicted based on an in vitro assay) in the hindgut.
Predicted hindgut SCFA absorption (in vivo–in vitro)
The predicted amounts of acetic, butyric and propionic acids absorbed from the hindgut were higher for the diet containing the highest fibre concentration (1·8-, 1·9- and 1·5-fold higher, respectively) (P<0·05; Table 3). There was no difference (P>0·05) between diets for valeric acid. The predicted apparent absorption of the SCFA in the hindgut was similar between the two fibre-containing diets (P<0·05; Table 3), and near complete.
Determined (in vivo) and predicted (in vivo–in vitro) digestibilities
There was an effect of dietary fibre concentration on the determined apparent ileal and total tract digestibilities (P<0·05; Table 4). The diet containing the highest concentration of fibre had a lower determined digestibility of OM (3·1 % unit difference for the ileum and 2·4 % unit difference for the total tract, respectively). Similarly, the predicted apparent total tract digestibility of OM was lower for the diet containing the highest fibre concentration (2·1 % units) (P<0·05; Table 4). The predicted and determined total tract digestibilities for OM were not different (P>0·05) for the diet containing 50 g/kg of fibre but were statistically different (P<0·05) for the diet containing 25 g/kg of fibre. However, the latter difference was very small (<0·6 %).
Table 4 Determined and predicted ileal and total tract apparent digestibilities of organic matter for ileal cannulated pigs fed diets containing different concentrations of fibre (Mean values with their pooled standard errors; n 7 per group)

* Ileal digestibility was determined in pigs fed the experimental diets.
† n 6, an outlier was removed from the statistical analysis.
‡ In vivo total tract digestibility was determined directly in pigs.
§ The predicted total tract digestibility in pigs was estimated based on the ileal organic matter digestibility determined in the pig and the predicted hindgut fermentability of organic matter in the pig hindgut based on an in vitro fermentation assay using a pig faecal inoculum.
Determined (in vivo) and predicted (in vivo–in vitro) hindgut fermentabilities
There was no effect of dietary fibre concentration on either the determined or predicted hindgut fermentability of OM (P>0·05; Table 5). The determined hindgut fermentability of OM differed (P<0·05) from its predicted counterpart for the diet containing 25 g/kg of fibre (9 % units), whereas there were no differences for the diet containing 50 g/kg of fibre (P>0·05).
Table 5 Determined and predicted hindgut fermentability of organic matter for ileal cannulated pigs fed diets containing different concentrations of fibre (Mean values with their pooled standard errors; n 7 per group)

* In vivo hindgut fermentability was determined in pigs as the difference between the ileal and faecal flows of organic matter.
† n 6, an outlier was removed from the statistical analysis.
‡ In vitro hindgut fermentability in pigs was determined after in vitro fermentation of the ileal digesta with a pig faecal inoculum.
Discussion
Predicted production and absorption of SCFA in the hindgut
Owing to the difficulty in investigating the production and absorption of SCFA in the GIT directly, studies investigating SCFA production in humans and farm animals focus mainly on the faecal concentrations of SCFA( Reference Whelan, Judd and Preedy 10 , Reference Fredstrom, Lampe and Jung 32 ). The latter approach, however, has limitations because faecal SCFA concentrations reflect the overall net production and absorption of SCFA in the GIT, but provide no information about the production or the absorption of SCFA per se ( Reference McNeil, Cummings and James 11 – Reference Topping and Clifton 13 ). Other studies have been carried out to determine SCFA absorption by quantifying the appearance of SCFA in portal blood and in exhaled breath( Reference Topping and Clifton 13 – Reference Regmi, van Kempen and Matte 16 ). However, significant amounts of SCFA absorbed from the GIT are utilised by the epithelial cells, and therefore may not appear in the bloodstream( Reference Bergman 1 , Reference Roy, Kien and Bouthillier 7 , Reference Von Engelhardt, Rönnau and Rechkemmer 33 ); thus, the latter approaches are also limited. In the present study, a combined in vivo–in vitro methodology was used to predict the production and absorption of SCFA in the hindgut using diets containing different concentrations of kiwifruit as a model dietary fibre. The latter approach used the growing pig as a model to derive estimates of upper tract digestion and an in vitro fermentation assay, where pig ileal digesta were incubated with a pig faecal inoculum, to model hindgut fermentation. By combining the two (in vivo and in vitro) sets of data, the production and absorption of SCFA in the hindgut can be predicted( Reference Christensen, Bach Knudsen and Wolstrup 22 ). In contrast to previous studies, the predicted amount of SCFA absorbed from the hindgut also considered the SCFA entering the hindgut (based on determined ileal concentrations) (Fig. 1). The latter explains the higher values of SCFA absorbed than that produced in the hindgut. It is important to note that the present combined in vivo–in vitro approach does not account for the SCFA produced and absorbed in the upper GIT (‘?’ in Fig. 1), but the contribution of the stomach and small intestine to SCFA production and absorption across the entire GIT is expected to be relatively low. In addition, this approach does not consider the SCFA potentially produced by the fermentation of endogenous material entering the large intestine directly from colonic tissues.

Fig. 1 Principle of the combined in vivo–in vitro methodology to determine the hindgut production and absorption of SCFA. The ‘?’ represents the SCFA absorbed in the small intestine, which were not determined in this study.
The similar content of total dietary fibre in the ileal digesta of both kiwifruit fibre diets explains the similar hindgut SCFA concentrations after in vitro fermentation. The predicted production of total SCFA in the hindgut of pigs fed diets containing 25 and 50 g/kg DM of kiwifruit fibre was 472 and 777 mmol/kg DMI, respectively. Christensen et al.( Reference Christensen, Bach Knudsen and Wolstrup 22 ) also, using a combined in vivo–in vitro approach, reported similar predicted production of total SCFA in the hindgut of ileal cannulated pigs to the values reported in our study with wheat flour, wheat bran and oat bran as fibre sources (369–850 mmol/kg DMI). The wider range in the predicted hindgut production of SCFA in the study of Christensen et al.( Reference Christensen, Bach Knudsen and Wolstrup 22 ) may be explained by a higher and wider range in dietary fibre content of the diets (63–110 g/kg DM of dietary fibre) and the type of fibre used.
In the present study, the normalised faecal concentrations of SCFA represented only 0·5–1·6 % of the predicted hindgut SCFA production when examined across both fibre-containing diets and all SCFA. Such low values are not surprising, as faecal SCFA output is the net result of SCFA production and absorption in the hindgut, and therefore is a measure of the unabsorbed SCFA rather than SCFA production per se.
It is noteworthy that based on faecal SCFA concentrations it can be concluded that dietary fibre concentration had no effect on SCFA production and absorption. However, based on the combined in vivo–in vitro approach, it is apparent, perhaps not unexpectedly, that higher dietary fibre concentrations led to a greater production of SCFA in the simulated hindgut fermentation. Clearly, using faecal SCFA concentrations to describe SCFA production can be misleading, and the present study supports the findings of McNeil et al.( Reference McNeil, Cummings and James 11 ), Cummings & Macfarlane( Reference Cummings and Macfarlane 12 ) and Topping & Clifton( Reference Topping and Clifton 13 ), all of whom have cautioned against the use of faecal SCFA flows and concentrations for describing SCFA production in the hindgut.
Dietary fibre comprises a mixture of complex NSP (e.g. hemicellulose and cellulose), lignin, resistant starch, oligosaccharides and resistant maltodextrins that differ among foods( Reference Jones 34 ). The relative amounts of SCFA produced by fermentation are influenced by the type of dietary fibre being fermented( Reference Henningsson, Björck and Nyman 5 , Reference Cummings and Macfarlane 12 , Reference Macfarlane and Macfarlane 35 ), and it is not appropriate to extrapolate the results of the present study to other fibre sources. However, using the approach described in the present study, it is possible to predict the production and absorption of SCFA in the hindgut of pigs after consumption of fibre sources other than kiwifruit and such studies are warranted. This approach can also be applied to humans by collecting ileal digesta from the growing pig (an accepted animal model for upper gut digestion in humans), which can be fermented in vitro with a human faecal inoculum( Reference Coles, Moughan and Awati 20 , Reference Coles, Moughan and Awati 21 ). The combined in vivo–in vitro methodology can also be extended to other species of animals (e.g. poultry).
The predicted extent of SCFA absorption in the hindgut, based on the amounts of SCFA entering the hindgut (estimated from the SCFA in the ileal digesta) and the SCFA produced in the hindgut, was high (mean absorption across all SCFA and dietary treatments was 99·8 %) as has been reported previously (95–99 %)( Reference Roy, Kien and Bouthillier 7 , Reference Topping and Clifton 13 ). Based on the SCFA content of the ileal digesta, it would appear that as much as 3 % of the SCFA absorbed from the hindgut was derived from fermentation in the small intestine. Given that it is likely that a high proportion of the SCFA produced in the small intestine was also absorbed in the small intestine, it would appear that microbial fermentation in the small intestine may be more quantitatively significant than is often recognised. Indeed, a recent study has shown that the corrected upper gut fermentation of the soluble fraction of kiwifruit fibre was 80 %( Reference Montoya, Rutherfurd and Moughan 27 ).
Predicted total tract digestibility and hindgut fermentability
When the fibre-containing diets were compared, both the predicted and determined hindgut fermentabilities of OM showed the same trend in results (i.e. no difference in hindgut fermentability between the two fibre-containing diets). Similarly, both the predicted and determined values for total tract digestibility of OM led to the same conclusions (i.e. the diet containing the lowest fibre concentration had higher total tract digestibility). In addition, and in general, the predicted and determined total tract digestibilities of OM were very similar quantitatively, supporting the combined in vivo–in vitro approach( Reference Coles, Moughan and Awati 21 ). Although in the present study the combined in vivo–in vitro method was used to predict the total tract digestibility of OM, this technique can also be used to predict the digestibility of other compounds (e.g. NSP)( Reference Anguita, Canibe and Perez 18 , Reference Christensen, Bach Knudsen and Wolstrup 22 ).
In conclusion, the combined in vivo–in vitro methodology is a useful approach for predicting the production and absorption of SCFA in the hindgut. The ileal and faecal concentrations of SCFA per se do not reflect the SCFA production and absorption in the hindgut and may lead to misleading conclusions. Considerable quantities of SCFA are produced and absorbed in the hindgut when pigs are fed diets enriched with kiwifruit.
Acknowledgements
The authors acknowledge Stuart Saigeman and Trent D. Olson for running the animal trials and ZESPRI International Ltd for supplying the kiwifruit.
This study was funded by ZESPRI International Ltd. ZESPRI International Ltd had no role in the design, analysis or writing of this manuscript.
C. A. M., S. M. R. and P. J. M. were responsible for planning the study. C. A. M. was responsible for conducting the experiments. In addition, C. A. M. carried out the statistical analysis and prepared the first draft of the manuscript that was revised by S. M. R. and P. J. M. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest to declare.









