Vitamin A and carotenoids are nutritional components of high interest due to their numerous biological roles in health and disease(Reference Johnson1–Reference Nagao3). Relevant dietary carotenoids such as β-carotene and lycopene are associated with reduced risk of developing chronic degenerative disorders including CVD, prostate and lung cancer and macular degeneration(Reference Stahl and Sies4). Vitamin A is consumed either as preformed vitamin A (retinol or retinyl esters) from animal sources or synthesized in the human body from dietary precursor carotenoids(Reference Parker5). Carotenoids derive mainly from plant foods(Reference Holden, Eldridge and Beecher6).
Raw food diets are plant-based characterized by a high consumption of uncooked and unprocessed foods, i.e. fruits, vegetables, nuts and seeds(Reference Hoffmann, Leitzmann and Watson7–Reference Koebnick, Garcia and Dagnelie9). Raw food adherents often eat food items separately instead of mixed with other foods within the same meal; thus, raw vegetables are not eaten with salad dressing but as single foods. The intake of bread, cereals and dairy products by raw food diet adherents is negligible(Reference Koebnick, Garcia and Dagnelie9). Even though raw food diets have lately become fashionable, the benefit of long-term adherence to such diets is controversial(Reference Cunningham10). Recently, we have shown that adherence to a long-term raw food diet is associated with favourable serum LDL-cholesterol and TAG, but at the same time with elevated plasma homocysteine and low serum HDL concentrations(Reference Koebnick, Garcia and Dagnelie9).
Plant-based diets are known to provide high amounts of carotenoids due to the predominant consumption of fruits and vegetables(Reference Haddad, Berk, Kettering, Hubbard and Peters11, Reference Bederova, Kudlackova and Simoncic12). However, carotenoid status in human individuals depends on the type of carotenoid, carotenoid–carotenoid interactions, gender, smoking, oral contraceptive use in women, BMI, protein, lipid, Fe, Zn and vitamin A status and on intake of alcohol and fat(Reference van Het Hof, West, Weststrate and Hautvast13, Reference Zaripheh and Erdman14). The effects of high carotenoid intake from natural foods instead of supplements on plasma carotenoid status have rarely been studied because it is difficult to achieve a high carotenoid intake from mixed Western diets. For this reason, raw food diet adherents represent a unique group for studying the effects of high dietary levels of carotenoids.
The aim of the present study was to assess the vitamin A and carotenoid status of raw food diet adherents and to investigate which dietary factors determine plasma carotenoid and vitamin A concentrations at these high carotenoid intake levels from raw plant foods.
Methods
Study design and subjects
In the Giessen Raw Food study, a cross-sectional study in Germany, raw food diet adherents were recruited by advertisements in seminars, congresses and magazines of followers of the Natural Hygiene and raw food movement. Screening questionnaires were sent to a total of 1328 interested subjects, of whom 865 responded. Inclusion criteria were age 25–64 years, at least 70 % of the total food weight eaten as raw food, >24 months adherence to a raw food diet, non-smoking and no gastrointestinal diseases. A total of 266 interested subjects met the inclusion criteria and were invited to participate in the study. From them, 201 subjects agreed to participate and completed the study. The total number of subjects included in the data analysis was 198 because three subjects consumed vitamin and mineral supplements and were, therefore, excluded from the dataset.
The Ethics Committee of the Division of Human Medicine, University of Giessen approved of the study protocol. Before entering the study, all participants gave written informed consent.
Baseline information and dietary intake
Baseline information (age, gender), dietary habits (food preferences and avoidances, the self-reported amount of raw food consumed, the duration of raw food diet) and physical and recreational activity were appraised by a detailed self-administered questionnaire. Dietary intake was assessed before blood collection using a 7 d food record, which was validated exclusively for the present study and allowed distinction between raw and cooked food items. The procedures followed for food records are described in detail elsewhere(Reference Koebnick, Garcia and Dagnelie9). Briefly, the food record included 236 food items subdivided into twelve food groups. Typical household measures and the corresponding portion size (g) were provided for every food item; in addition, coloured photographs and detailed descriptions of portion sizes were provided for unambiguous foods. Vegetable and fruit items discriminated between cooked and uncooked foods. Detailed oral and written instructions were given on how to complete the food record. In addition, subjects kept a record on medical drugs, as well as vitamin and mineral supplements taken during the data collection period. Classification of raw food diet adherents was made according to the proportion of raw food eaten based on the calculation from the food record (70, 80, 90, 95 and 100 weight% of total food consumption) and to the restriction of animal foods (vegan, ovo-lacto vegetarian and mixed raw food diet).
Nutrient intake was calculated based on the German Food Code and Nutrition Data Base BLS II.3(15). The carotenoid intake was analysed using the BLS II.3 database, complemented with information obtained from the USDA-NCC Carotenoid Database for US Foods – 1998(Reference Holden, Eldridge and Beecher6). For the calculation of dietary retinol activity equivalents in the present study, we used the conversion factor of 12:1 (for β-carotene) or 24:0 (for other pro-vitamin A carotenoids) as recommended by the US Institute of Medicine(16).
Anthropometrical measurements and blood analyses
Body weight was measured to the nearest 0·1 kg using an electronic calibrated scale (Soehnle, Murrhardt, Germany). Height was determined to the nearest 0·1 cm. BMI was calculated as body weight (kg)/body height (m2). Fasting venous blood was drawn into both trace element free and EDTA containing vacutainers. Plasma and serum were separated from cells by centrifugation (2000 g for 15 min) within 2 min of venipuncture and stored at − 80°C until analysis.
Retinol and carotenoids were analysed by reverse phase HPLC(Reference Vuilleumier, Keller, Gysel and Hunziker17). The carotenoids α-, β-carotene and lycopene were measured in accordance with a slightly modified method of Jakob & Elmadfa(Reference Jakob and Elmadfa18, Reference Jakob and Elmadfa19). For the extraction procedure, a mixture of plasma (200 μl), ethanol (200 μl) and hexane (3 ml) was shaken for 15 min. After centrifugation (15 min, 3000 rpm), the upper phase (hexane layer; 2 ml) was drawn off and evaporated to dryness in a 37°C water bath under a gentle stream of N2. The residues were dissolved in 200 μl acetonitrile; a 50 μl volume of each extract was injected onto an RP-column (Keystone Aquasil C18, 5 μm, 150 × 4 mm) and eluted with acetonitrile–methanol (90:10, v/v) at a flow-rate of 0·8 ml/min. For all measured carotenoids intra-batch and inter-day CV were similar and did not exceed 3 and 5 %, respectively.
Statistical methods
Baseline characteristics are presented as median values with 25th and 75th percentiles due to skewed distribution. Plasma vitamin A and carotenoids had a normal distribution and are shown as means with their standard errors of the mean. All values were log transformed before further statistical analysis. Baseline characteristics and food consumption of male and female raw food diet adherents were compared using Student's t test or χ2 test where appropriate.
Vitamin A and carotenoids cut-off points were set as follows: plasma retinol concentrations of < 0·70 μmol/l indicated vitamin A deficiency and values < 1·05 μmol/l inadequate vitamin A status(Reference Gibson20, Reference Pilch21). Plasma β-carotene concentrations ≤ 0·28 μmol/l were considered as a lower reference level; ≥ 0·4 μmol/l as a level suggesting reduced risk of CHD and certain neoplasms(Reference Diplock22–Reference Gey24) and ≥ 0·88 μmol/l as a high level with a potentially favourable effect in macular degeneration prevention(Reference West, Vitale and Hallfrisch25). As a reference for lycopene concentrations, values reported for normal US populations were used (0·45 μmol/l)(Reference Ganji and Kafai26).
Partial coefficients of correlation were calculated to apprise the relationship of plasma carotenoids with food consumption and nutrient intake as well as to other plasma carotenoids, adjusting for gender, age and BMI. Based on partial correlations, stepwise multiple linear regression models were fitted with plasma retinol and carotenoids as dependent variables and the following covariates: age; BMI; gender; consumption of specific food groups (type of raw food diet) or foods (raw, cooked and total vegetables, raw and total fruits, total fruits and vegetables, total fruits and vegetables rich in β-carotene, α-carotene and lycopene, juice, dairy products, fat and oils and avocado); intake of specific nutrients (carotenoids and lycopene). Fruit and vegetables rich in β-carotene included pumpkin, carrots, sweet potatoes, broccoli, apricots, maize, green leafy vegetables, mangoes, papaya, bell peppers and kaki. Fruits and vegetables rich in α-carotene included carrots, pumpkin, squash and green beans. Fruits and vegetables rich in lycopene included tomatoes, watermelon, pink grapefruit, guava and papaya. Gender and the respective dietary carotenoid intake were always included in a basic model before further fitting in a stepwise process. The final models consisted of the following independent variables: for β-carotene of gender, total dietary β-carotene intake, the consumption of milk and dairy products and the consumption of fat and oils; for α-carotene of gender, total dietary α-carotene intake and the consumption of milk and dairy products; for lycopene of gender, total dietary lycopene intake and the consumption of fat and oils. To avoid multicollinearity, the tolerance level in all regression models was set to 0·3. Standardized regression coefficients (SRC) are given. P values < 0·05 were considered significant.
Results
Baseline characteristics, food consumption and nutrient intake
None of the 198 participants who completed the study smoked and their alcohol consumption was negligible (Table 1). Male and female raw food diet adherents were similar in age, BMI and main characteristics of their raw food diet. Males and females differed slightly but significantly with regard to the proportion of raw food eaten, with a higher proportion of raw food eaten by males than by females. Most of the participants adhered to a mixed type of raw food diet (57 %), followed by ovo-lacto vegetarian (22 %) and vegan diet (21 %). On average, 95 weight% of foods was eaten raw and 97 weight% of all foods eaten were of plant origin. Fruits, but not vegetables, were the major food groups consumed by all raw food diet adherents. The consumption of bread, cereals, rice, potatoes, legumes, dairy products, visible fats and oils was negligible. Consequently, if compared with the average German population the raw food diet was low in energy and fat (energy 11·3 MJ for men and 10·9 MJ for women; fat 98 g/d for men and 74 g/d for women(Reference Mensink and Beitz27), but high in dietary fibre (approximately 60 g/d). Carbohydrates provided more than half of the total daily energy intake, while approximately 30 % was provided by fat and approximately 10 % by protein. Gender differences were observed for food consumption as well as nutrient intake (Table 1). The main sources of fat were nuts and seeds (providing 25 % total fat intake) and fruits (20 % total fat intake). Among fruits and vegetables, avocados were the main sources of fat with a median (25th–75th percentile) of 8·6 (0/22) g/d (i.e. 14 % total fat intake).
Table 1 Baseline characteristics, food consumption and nutrient intake of raw food diet adherents (n 198) according to gender*

Pn, nth percentile; NA, not applicable; RAE, retinol activity equivalents.* For details of subjects and procedures, see Methods.
† P values were calculated using Student's t test or χ2 test.
Vitamin A and carotenoid intake was similar in males and females (Table 1) and is close to the recommended intake of vitamin A(28). Retinol intake was very low in all participants, in contrast with carotenoid intake, which was very high. The predominant carotenoid was β-carotene, followed in decreasing order by lycopene and α-carotene. A total of 16·7 mg/d carotenoids was consumed. The contribution of pro-vitamin A carotenoids to total retinol activity equivalents was 82·0 % for β-carotene and 8·6 % for α-carotene. Total carotenoid intake was similar between mixed, ovo-lacto vegetarian and vegan raw food diet adherents.
Plasma retinol and carotenoids
The majority of participants (82 %) had normal plasma vitamin A concentrations, 15 % had inadequate concentrations ( < 1·05 μmol/l) and 3 % showed a vitamin A deficiency with concentrations < 0·70 μmol/l (Table 2). Plasma β-carotene concentrations were below the lower reference values of 0·28 μmol/l in 7 % of the participants, 7 % had concentrations between 0·28–0·40 μmol/l, 23 % had concentrations between 0·40–0·88 μmol/l and 63 % had concentrations ≥ 0·88 μmol/l. Subjects with β-carotene concentrations ≥ 0·40 consumed a median of 1710 g/d (25th–75th percentiles: 1381–2167 g/d) fruits and vegetables. For lycopene, in 77 % of the subjects the plasma concentrations were below the reference values of 0·45 μmol/l. Significant gender differences in total plasma vitamin A and β-carotene concentrations were observed. Males showed higher total vitamin A concentrations than females, while β-carotene plasma concentrations were higher in females. All participants had normal total plasma protein concentrations (Table 2). Low serum albumin ( < 39 g/l) was observed in 29 % of males and 22 % of females, whereas serum retinol was similar in subjects with low albumin and subjects with normal albumin concentrations.
Table 2 Plasma concentrations of vitamin A, carotenoids and other biomarkers in raw food diet adherents (n 198)* (Mean values with their standard errors)
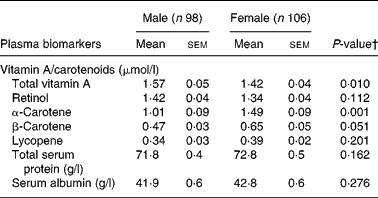
* For details of subjects and procedures, see Methods.
† P values were calculated using Student's t tests.
Associations between plasma concentrations of vitamin A and carotenoids and nutrient intake and food consumption
Plasma concentrations of carotenoids but not of retinol were significantly correlated with their respective nutrient intakes (Table 3). Plasma β-carotene, α-carotene and lycopene concentrations increased with increasing intake quintile (P for trend < 0·001 for all) independent of gender (Fig. 1). Plasma β- and α-carotene concentrations were positively correlated with the consumption of total vegetables, raw vegetables, foods rich in both carotenoids and juice, as well as with the consumption of milk and dairy products and with total consumption of cooked food. Plasma lycopene concentrations were correlated with the consumption of total vegetables, raw vegetables and lycopene-rich foods. Plasma β- and α-carotenoid concentrations were positively correlated with fat and oil consumption while plasma retinol concentration was inversely related with fat and oil intake (Table 3).
Table 3 Partial correlation coefficients between plasma retinol, plasma carotenoids, nutrient intake and food consumption of raw food diet adherents adjusted for gender, age and BMI (n 198)*

* For details of subjects and procedures, see Methods.
† Pearson coefficient of correlation significantly differed from 0 (P < 0·05).

Fig. 1 Plasma β-carotene (A), α-carotene (B) and lycopene (C) concentrations in males (●) and females (○) according to intake quintiles. The median intake of each quintile is given. For details of subjects and procedures, see Methods.
In a multiple linear regression model, plasma β-carotene concentrations were best predicted by gender (SRC 0·238, P < 0·001), total dietary β-carotene intake (SRC 0·242, P < 0·001), consumption of milk and dairy products (SRC 0·199, P = 0·003) and consumption of fats and oils (SRC 0·193, P = 0·005; adjusted R 2 of the model 0·261, P < 0·001); α-carotene concentrations were best predicted by gender (SRC 0·163, P = 0·010), total dietary α-carotene intake (SRC 0·371, P < 0·001) and consumption of milk and dairy products (SRC 0·232, P < 0·001; R 2 0·245, P < 0·001); plasma lycopene concentrations were predicted by total dietary lycopene intake (SRC 0·289, P < 0·001) and fat and oil intake (SRC 0·200, P = 0·004; R 2 0·147, P < 0·001). In summary, the main dietary determinant for all plasma concentrations of carotenoids in raw food diet adherents besides the respective nutrient intake and gender was the consumption of fat and oil. Subjects in the lowest tertiles of fat and oil consumption had significantly lower plasma β-carotene (P = 0·006), plasma α-carotene (P = 0·044) and plasma lycopene (P = 0·007) concentrations.
No relationship was observed between plasma concentrations of vitamin A or total carotenoids and the dietary intake of fat, Zn, protein, fibre, type of raw food diet or the ratio of raw:cooked vegetables. Furthermore, no relationships were found between plasma carotenoids.
Discussion
A high dietary carotenoid intake is considered as an important factor for the prevention of degenerative diseases (CVD, macular degeneration and certain cancers)(Reference Palace, Khaper, Qin and Singal29–Reference Tavani and La Vecchia31). Plant-based diets are good sources of carotenoids(Reference Rauma and Mykkanen32, Reference Rider, Calkins, Arthur and Nair33). In a dietary intervention study, circulating carotenoids increase in response to a higher intake of fruits and vegetables(Reference Yeum, Booth and Sadowski34). However, plant-based diets also may contain factors (e.g. dietary fibre) that interfere with carotenoid absorption. Raw food diets provide a unique opportunity to investigate the relationship between plasma carotenoids and carotenoid intake because of the high amount of plant foods eaten relative to animal foods.
The present study shows that plasma β-carotene concentrations ≥ 0·40 μmol/l as recommended for the prevention of CVD can be achieved by habitual adherence to a raw food diet with an exceptionally high consumption of fruits and vegetables (approximately 1700 g/d). Remarkably, the most important dietary factor predicting vitamin A and carotenoid plasma concentrations in raw food diet adherents was fat and oil consumption. The transfer of carotenoids to lipid micelles in the small intestine requires the presence of dietary fat in the small intestine(Reference Tyssandier, Reboul and Dumas35). Human studies have shown that carotenoid absorption increases when carotenoid-containing salads are eaten in combination with a full-fat dressing rather than using low-fat dressings(Reference Brown, Ferruzzi and Nguyen36). It has also been shown that the combination of a carotenoid-containing salad with avocado or avocado oil resulted in improved carotenoid absorption in normal healthy subjects(Reference Unlu, Bohn, Clinton and Schwartz37). In general, raw food diet adherents did not mix a variety of foods within one meal as a matter of principle. However, the number and type of different foods mixed in the same meal was not investigated in the present study.
Raw food diet adherents consumed amounts of fat comparable with the dietary recommendations (approximately 30 % from total energy intake, approximately 67 g/d)(28). Dietary fat intake in the present study was mainly determined by foods containing hidden fat, such as nuts, seeds and fruits. Nevertheless, the main predictor of plasma carotenoid concentrations was added fat, such as oil. These results suggest that the low intake of visible fats and oil was a limiting factor for carotenoid absorption in the current study population.
Fruit and vegetable consumption by raw food diet adherents is much higher than the average consumption of the general population in the USA (391 g/d)(Reference Bazzano, He and Ogden38) or the recommended fruit and vegetable consumption (400–800 g/d)(39–Reference Krauss, Eckel and Howard41). This reflects the very restrictive dietary regimen encompassing several factors that could interfere with carotenoid absorption, i.e. low fat intake, omission of cooked and processed foods and restrictions with regard to the combination of foods. Processing of food (grinding, fermenting, cooking) has been reported to increase bioavailability of β-carotene(Reference Rock, Lovalvo, Emenhiser, Ruffin, Flatt and Schwartz42–Reference Muller, Bub, Watzl and Rechkemmer45). The present data partly support this notion, as shown by the higher correlation between plasma carotenoids and total consumption of cooked foods v. total consumption of raw foods. However, there was not a high correlation between plasma carotenoids and cooked v. raw vegetables. Cooked foods are consumed only in low amounts and as a consequence do not have a large variability in the group of raw food dieters. Therefore, a relatively high carotenoid intake may be necessary to achieve β-carotene concentrations ≥ 0·40 μmol/l in raw food diet adherents. However, our observations also suggest that the high intake of β-carotene from raw food diets was sufficient to overcome the lack of mechanical factors potentially reducing β-carotene absorption.
The lycopene intake of the raw food diet adherents (2·8 mg/d) was comparable with the average US intake in white subjects (2·31 mg/d for males and 2·14 mg/d for females)(Reference Nebeling, Forman, Graubard and Snyder46) and higher than the German average intake for the adult general population (1·28 mg/d)(Reference Pelz, Schmidt-Faber and Heseker47). Nevertheless, plasma lycopene concentrations were below reference levels in 77 % of the participants, which suggests that raw food consumption and lack of cooking negatively affects plasma lycopene concentrations. For example, cooking tomatoes has been directly related to improvement on lycopene absorption(Reference Porrini, Riso and Testolin48, Reference Bohm and Bitsch49). However, in the present study no relationship was observed between consumption of cooked foods and plasma lycopene concentrations, possibly due to the low amount of cooked food and/or the low fat content of the diet.
Vitamin A status of raw food diet adherents was adequate in the majority of participants due to the high intake of pro-vitamin A carotenoids. Still, 15 % of participants showed an inadequate vitamin A status. In addition, plasma vitamin A concentrations are slightly lower than the values reported in the average German population (1·78 μmol/l for women and 2·04 μmol/l for men)(Reference Schneider, Eberhardt, Heseker and Kubler50). This may imply that maintaining a raw food diet on a long-term basis may progressively worsen vitamin A status. Apart from the low fat content of the diet, this may be explained by individual variations in the conversion rate of β-carotene to retinol, as reported by Wang et al. (Reference Wang, Yin, Zhao, Russell and Tang51) in Chinese subjects. The authors described that ‘poor carotenoid–vitamin A converters’ had an equivalence ratio of β-carotene:retinol of 29·0:1·0 on a molar basis and ‘normal converters’ of 4·8:1·0. For the calculation of dietary retinol activity equivalents in the present study, we used the conversion factor of 12:1 (for β-carotene) or 24:0 (for other pro-vitamin A carotenoids) as recommended by the US Institute of Medicine(16). In the literature, several factors such as dietary intake of Zn, protein and dietary fibre have been described to affect vitamin A absorption. However, in the present study none of these factors was related to plasma vitamin A concentrations.
Dietary fibre has been discussed as a factor potentially interfering with carotenoid absorption(Reference Castenmiller, West, Linssen, van het Hof and Voragen43). Furthermore, dietary fibre administration reduced carotenoid absorption in short-term trials using antioxidant supplements (β-carotene, lycopene, lutein)(Reference Riedl, Linseisen, Hoffmann and Wolfram52). However, in the present study high dietary fibre intake (approximately 60 g/d) was not correlated with carotenoid concentrations in plasma.
Gender differences in plasma α- and β-carotene concentrations have been described in several studies(Reference Olmedilla, Granado, Blanco and Rojas-Hidalgo53–Reference Brady, Mares-Perlman, Bowen and Stacewicz-Sapuntzakis56) and were confirmed in the current study in raw food diet adherents. Females showed higher plasma α- and β-carotene concentrations than males; the same has been reported in studies using β-carotene-supplemented meals(Reference Dutra-de-Oliveira, Favaro, Leonardo, Jordao Junior and Vannucchi57). No gender effects were observed for plasma lycopene levels, which is in agreement with previous studies(Reference Brady, Mares-Perlman, Bowen and Stacewicz-Sapuntzakis56).
Carotenoid–carotenoid interactions have been postulated to affect carotenoid absorption(Reference Zaripheh and Erdman14). In this study, no correlations between different plasma carotenoids were observed. A possible explanation may be a potential long-term adaptation; such interactions (lycopene–β-carotene, lutein–β-carotene) have been described in the postprandial state but could not be confirmed in medium-term studies(Reference Tyssandier, Cardinault and Caris-Veyrat58). Another plausible explanation for the lack of correlation between plasma carotenoids is the fact that, generally, raw food diet adherents did not mix a variety of foods within one meal. The consumption of fat separately from the consumption of carotenoids would limit carotenoid absorption and, therefore, explain the lack of correlation with other food groups. Therefore, raw food eaters should be encouraged to consume added fats within their meals.
One limitation of the present study is the cross-sectional design and the heterogeneity with regard to the types of raw food diets. Also, the present study did not include subjects following an average Western diet as a control. A further limitation is that we did not analyse plasma lutein and β-cryptoxanthin. However, the present study investigates a unique population with regard to the strictness of dietary regimen.
The applicability of the present findings for the general population is that an extraordinary high consumption of fruits and vegetables results in favourable carotenoid concentrations but added fats are a limiting factor for carotenoid absorption.
In conclusion, subjects adhering to a long-term, raw food diet with an exceptionally high consumption of fruits and vegetables have normal plasma vitamin A concentrations and high β-carotene concentrations as recommended for the prevention of CVD, but low plasma lycopene levels. The most important dietary factor predicting vitamin A and carotenoid plasma concentrations in raw food eaters is added fat and oil.
Acknowledgements
The study was supported by grants from the Eden Foundation, Bad Soden and the Stoll VITA Foundation, Waldshut, Germany. The authors do not have any financial or personal conflicts of interest.






