Atopic diseases, including asthma and eczema, represent a substantial public health problem in children and adolescents globally; asthma is the commonest chronic disorder of childhood(Reference García-Marcos, Asher and Pearce1). Evidence suggests that the origins of childhood asthma may lie in utero(Reference Duijts2), and several components of the maternal diet during pregnancy have been investigated in relation to atopic outcomes in children including intakes of vitamins and minerals, specific food groups (i.e. meat, fish, dairy, fruits and vegetables) and the Mediterranean diet pattern(Reference Venter, Agostoni and Arshad3,Reference Beckhaus, Garcia-Marcos and Forno4) . However, observational evidence is conflicting, and a recent European meta-analysis found no relation between maternal diet quality and childhood asthma(Reference Mensink-Bout, van Meel and de Jongste5). Recent trials of vitamin D and fish oil supplementation in pregnancy, however, have shown promise for the prevention of preschool wheeze and early childhood asthma(Reference Brustad, Bønnelykke and Chawes6), respectively, but their long-term effects are uncertain(Reference Litonjua, Carey and Laranjo7,Reference Brustad, Eliasen and Stokholm8) .
Also of interest, two birth cohort studies have implicated a higher intake of sugar in pregnancy in the aetiology of childhood asthma and atopy. In 2017, in the Avon Longitudinal Study of Parents and Children (ALSPAC), a higher maternal intake of free sugar during pregnancy was associated with an increased risk of atopy (measured by aeroallergen skin prick tests) and atopic asthma in children at 7 years of age, independently of early childhood sugar intake(Reference Bédard, Northstone and Henderson9). In 2018, in the Project Viva cohort in the USA, higher intakes of sugar-sweetened beverage and fructose intake during pregnancy and early childhood were associated with an increased risk of asthma in 7-year-old children, irrespective of childhood adiposity(Reference Wright, Rifas-Shiman and Oken10). Thus, different sources and forms of sugars may underlie these associations and the direct causal mechanisms remain unclear, partly due to varying definitions in the literature.
Despite these strong observational findings, the possibility of unmeasured or residual confounding cannot be ruled out, and randomised trial evidence supporting such a link is lacking. De novo trials of maternal dietary modification in pregnancy are time consuming and expensive, as children have to be followed up for at least 5 years in order to ascertain asthma development. However, another approach, which enables faster randomised evidence, is to follow-up previous trials, originally conducted with other outcomes in mind, and to follow-up the offspring to ascertain asthma through questionnaires or health record linkage(Reference Shaheen, Gissler and Devereux11).
We therefore followed up children from the ROLO (Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia) trial to determine whether a randomised low glycaemic index (GI) dietary intervention during pregnancy is associated with lower risk of childhood asthma and eczema. We also aimed to assess observationally whether maternal intake of sugar during pregnancy is positively associated with asthma and eczema in childhood.
Materials and methods
Study design and subject selection
This is a secondary analysis of data on 514 children from follow-up of the ROLO trial, which was a randomised control trial of a low GI diet during pregnancy. The primary aim of the trial was to reduce the recurrence of macrosomia (birthweight >4 kg) in healthy secundigravida women who previously delivered an infant with macrosomia(Reference Walsh, McGowan and Mahony12). The trial was conducted in the National Maternity Hospital in Dublin, Ireland (2007–2011). A total of 800 women who met the eligibility criteria were randomly assigned to the intervention or control group. Detailed description of the trial criteria, methodology and outcomes has been previously reported(Reference Walsh, McGowan and Mahony12,Reference McGowan, Walsh and Byrne13) . Data were collected for 759 mother–child dyads at delivery. The original trial has developed into a longitudinal cohort, with subsequent follow-ups of mother–child dyads throughout childhood(Reference Horan, Donnelly and McGowan14,Reference Callanan, Yelverton and Geraghty15) .
Ethical approval
The ROLO study and follow-ups were carried out in accordance with the Helsinki Declaration of 1975 as revised in 1983. Ethical approval for the primary ROLO trial, and the 2-year follow-up was obtained from the National Maternity Hospital Ethics Committee, Dublin, Ireland (GEN/279/12). Ethical approval for the 5- and 9–11-year follow-ups was obtained from the Ethics Committee (Medical Research) of Our Ladies Children’s Hospital Crumlin and University College Dublin, Office of Research Ethics Committee, Dublin, Ireland, respectively. The Current Controlled Trials registration number is ISRCTN54392969. Informed, written maternal consent was obtained prior to study participation, and verbal assent was obtained from the study child.
Exposure assessment
Randomised trial of a low glycaemic index dietary intervention during pregnancy
The aim of this analysis was to examine the effect of a low GI dietary intervention during pregnancy on child outcomes(Reference Walsh, McGowan and Mahony12). The quality and quantity of carbohydrates influence glycaemic responses, and the GI scale was designed as a method for quantifying the ability of different carbohydrate foods to raise blood glucose concentrations(Reference Jenkins, Wolever and Taylor16). High GI foods, such as potatoes and refined grains, are broken down rapidly in the body causing sharp peaks in blood glucose and insulin responses following food ingestion(Reference Chang, Lampe and Schwarz17). In contrast, low GI foods, such as legumes and whole grains, have a slower and smaller effect on postprandial blood glucose levels and insulin response because they are absorbed more slowly in the body and release glucose gradually into the bloodstream(Reference Chang, Lampe and Schwarz17). Therefore, the rationale of following a low GI diet in pregnancy for the purpose of the ROLO study was to regulate the amount of blood glucose supply to the developing fetus.
Women who were randomised to the intervention group attended a 2-h group education session with a research dietitian 2 weeks after randomisation. Attendees had a mean (sd) gestation of 15·7 (3·0) weeks. The research dietitian initially advised on general healthy eating in pregnancy in line with the food pyramid. Guidance was provided on the recommended daily portions of carbohydrates, fruit and vegetables, dairy foods, meat and fish and limited intake of foods high in fat or sugar. The advice lead onto the principles of a low GI diet; what it means, the concept and the rationale for adherence in pregnancy. The advice focused on carbohydrate foods (sugars and starches) and other sources of sugar (fructose in fruit, lactose in milk and yogurts). Participants were encouraged to choose as many low GI foods as possible and to exchange high GI carbohydrate foods for low GI alternatives. Participants were given information leaflets about the GI which included a list of low and high GI foods, low GI recipes, snack options, factsheets and tips (see online Supplementary Material).
The recommended diet was eucaloric and was designed to meet the guidelines for pregnant women(18). The dietary education session did not cover information on glycaemic load (GL) to prevent confusion. GL is a separate measure to GI, which provides a more accurate estimation of a food’s real-life impact on postprandial glycaemia throughout the day(Reference Bell and Sears19). GL accounts for the quality of the carbohydrate-containing food (i.e. GI) and the quantity consumed of that food (weight), to estimate how increased and prolonged glycaemia will be when ingesting a specific amount of carbohydrate-rich food(Reference Bell and Sears19). Most high GI foods will also have a high GL in a standard serving size; however, moderate GI foods can generate a high GL based on the density or if it is consumed in excess(Reference Bell and Sears19). Examples of high GL dietary patterns have been described previously(Reference Chang, Lampe and Schwarz17). The research dietitian met with intervention subjects again at 28 and 34 weeks’ gestation for brief reinforcement of the low GI diet. Compliance and acceptability of the intervention were assessed at 34 weeks’ gestation using five-point Likert scales. Women in the control group received routine antenatal care, which did not include any formal dietary advice.
Maternal sugar intake, carbohydrate intake and carbohydrate quality during pregnancy
This analysis also aimed to examine associations of sugar and carbohydrate intakes, along with the GI and GL of the woman’s diet, during pregnancy with child outcomes (regardless of intervention). Total sugar intake was of particular interest as a proxy for excess-free-fructose intake, previously postulated as a potential cause of asthma and atopy(Reference Bédard, Northstone and Henderson9,Reference Wright, Rifas-Shiman and Oken10) . It is fructose that occurs when the fructose-to-glucose ratio exceeds 1:1, as found in apples, apple juice, watermelons, mangoes and high fructose corn syrup, that is associated with fructose malabsorption and gut dysbiosis. Data on maternal diet were collected once in each trimester using 3-d food diaries from women in both trial arms. All participants were requested to record any food and beverages consumed over three consecutive days during each trimester of pregnancy, which included two weekdays and one weekend day. In brief, dietary data were entered into the dietary analysis software NetWISP (version 3·0, Tinuviel Software) by a research dietitian using household measures and average portion sizes from the UK Food Standards Agency(Reference Crawley, Patel and Mills20). The NetWISP food composition database was derived from the 6th edition of McCance and Widdowson’s Food Composition Tables(21). Mean daily intake of macronutrients and micronutrients was generated for each time point in pregnancy, as well as the GI and GL of the woman’s diet. A total of 1397 food and beverage items were used to calculate sugar and carbohydrate intakes (see online Supplementary Material). Items with zero or trace carbohydrates were excluded. GI values were determined using the 2008 International Tables of Glycaemic Index Values along with other published GI values(Reference Levis, McGowan and McAuliffe22,Reference Atkinson, Foster-Powell and Brand-Miller23) . The GL was calculated as the mathematical product of the GI of a food and its carbohydrate content in grams divided by 100. Sugar and carbohydrate intakes were adjusted for energy intake at each trimester using the residual method(Reference Willet24). Sugar and carbohydrate intakes, along with GI and GL, in each trimester were averaged to obtain mean values through pregnancy.
Outcome assessment
Of the total 759 mother–infant dyads included in the primary analysis of the ROLO trial, 369 (48·6 %), 403 (53·0 %) and 437 (57·5 %) participated in 2-year, 5-year and 9–11-year follow-up studies, respectively (see Fig. 1). All mothers who returned for follow-up when the study child was 2, 5 and 9–11 years were asked to complete a questionnaire adapted from the SLAN 2007 lifestyle habits questionnaire (Survey of Lifestyle, Attitudes and Nutrition in Ireland), which included questions on child health(Reference Harrington, Perry and Lutomski25). The question was phrased as ‘does your child have any ongoing problems, tick all that apply’; the list included whether their child had experienced asthma or eczema. At the 5- and 9–11-year follow-ups children were defined as having current doctor-diagnosed asthma if mothers said their child had ongoing problems with asthma diagnosed by a doctor. At the 2, 5 and 9–11-year follow-ups children were defined as having current doctor-diagnosed eczema if mothers said their child had ongoing problems with eczema diagnosed by a doctor. Reported asthma at 2 years of age was not included because an accurate diagnosis of asthma is difficult in children up to 5 years of age(Reference Martin, Townshend and Brodlie26). Responses were collated to analyse outcomes at ‘any time point’ in childhood to increase statistical power and separately at each follow-up.
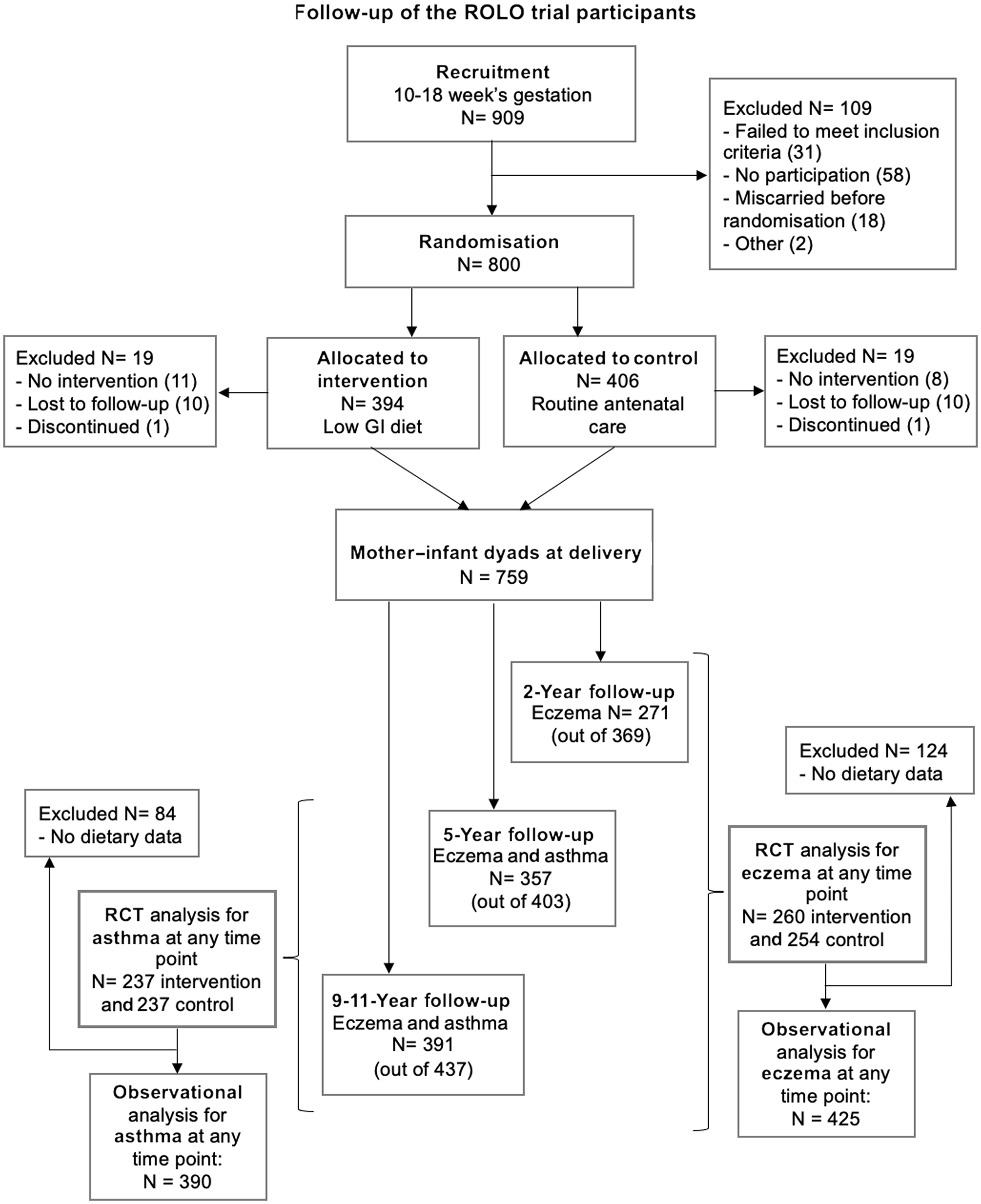
Fig. 1. Flow diagram showing loss to follow-up of ROLO trial participants and reasons for exclusion. GI, glycaemic index; RCT, randomised control trial; ROLO, Randomised cOntrol trial of LOw glycaemic index diet in pregnancy v. no dietary intervention to prevent recurrence of macrosomia.
Statistical analysis
All statistical analyses were performed using Statistical Software for Social Sciences (SPSS) version 27·0 for Mac (Macintosh). The normality of data was assessed using Kolmogorov–Smirnov tests and visually. We used independent t-tests, Mann–Whitney U tests or χ 2 tests as appropriate for univariate analysis. Logistic regression models were used to assess the effect of the trial intervention on child asthma and eczema, using a per protocol analysis approach. Of the 800 women who were randomised, those who were non-compliant, lost to follow-up or discontinued with the study prior to delivery and did not have available data on child outcomes were excluded. Therefore, in this secondary analysis, a per protocol approach was chosen to investigate an etiological question among those who completed the trial until delivery and had available data on outcomes in childhood. The intention-to-treat principle was inappropriate due to the loss of participants without outcome assessments. For analyses of the randomised intervention, we chose to compare specific baseline characteristics of interest between trial arms (intervention v. control) in participants with follow-up data, based on their known associations with the outcomes(Reference Nurmatov, Nwaru and Devereux27). Characteristics were analysed separately for each outcome and time point due to differences in sample size to mitigate the differences between the two arms. Follow-up analyses of randomised trials reduce the likelihood of unmeasured confounding; however, confounding variables that significantly differed were included in logistic regression models along with sex of child (male, yes/no) and age of child at follow-up (years) (see Table 1; online Supplementary Tables 1–3). The interaction effect of maternal education on the associations between trial arm and child outcomes was also included in the logistic regression models. We stratified by maternal education (dichotomised into those having completed education from a higher education institute and those who did not)(Reference O’Brien, Alberdi and Geraghty28). The rationale for this sub-analysis was based on previous findings that mothers who completed tertiary education responded better to the low GI dietary intervention compared with mothers with lower educational attainment, regardless of neighbourhood affluence(Reference O’Brien, Alberdi and Geraghty28). Sensitivity analyses were performed in the randomised trial analyses to test the effect of adjusting for potential mediators that differed between the two arms (birthweight and gestational age). In observational analyses, multivariate logistic regression models were used to analyse the relations of sugar and carbohydrate intake, along with GI and GL, in pregnancy to child outcomes. The selection of potential covariates to include in observational multivariable logistic regression models was informed by the literature(Reference Nurmatov, Nwaru and Devereux27) and by a directed acyclic graph, separately for asthma (see online Supplementary Fig. 1) and eczema outcomes (see online Supplementary Fig. 2). Covariates included in the models were mean total energy intake during pregnancy (kcal/day), original trial group (intervention, yes/no), maternal ethnicity (White Irish, yes/no), maternal education level (three categories), smoking in pregnancy (yes/no), age at delivery (years), sex of child (male, yes/no), age of child at follow-up (years), gestational weight gain (GWG) (three categories)(29), gestational age at delivery (days) and birthweight (kg). We analysed each dietary component in quartiles as a categorical variable using the lowest quartile as reference. We tested for linear trends across quartiles by assigning median values to each of the four categories and then including it as a continuous variable in the models (i.e. per quartile effect).
Table 1. Maternal child characteristics from the ROLO trial for those with asthma outcomes at any time point

ROLO, Randomised cOntrol trial of LOw glycaemic index diet in pregnancy v. no dietary intervention to prevent recurrence of macrosomia; HP, Hasse and Pratschke index. Results are presented as mean (sd standard deviation) for normally distributed variables, median (IQR interquartile range 25th, 75th percentile) for non-normally distributed variables and n (%) for categorical variables.
Results
Maternal and child characteristics in the Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia trial
Cohort characteristics for those with asthma outcomes at any time point (n 474) according to intervention arm are shown in Table 1. The majority of mothers were White Irish (n 438) and 51·7 % had completed tertiary level education. Of those included in this analysis, 50 % of mothers were in the intervention group and 49·8 % of the children were male. Among children with available information, 8 % had doctor-diagnosed asthma at 5 or 9–11 years, 7 % at 5 years and 7·7 % at 9–11 years.
Analyses of the randomised intervention and child outcomes
We did not find strong evidence that a low GI dietary intervention during pregnancy reduced the risk of asthma or eczema in childhood. However, a lower proportion of children whose mothers had received the intervention developed asthma overall and at each time point compared with usual care. At age 5, there was a suggestion of a reduction in asthma at 5 years of age in children whose mothers had received the intervention compared with usual care in the adjusted model (adjusted OR 0·46 (95 % CI 0·19, 1·09); P = 0·08) (Table 2). Adjustment for potential mediators (birthweight and gestational age) in sensitivity analyses yielded similar conclusions in all models (online Supplementary Table 4).
Table 2. Associations between ROLO trial intervention arms in pregnancy and child outcomes

ROLO, Randomised cOntrol trial of LOw glycaemic index diet in pregnancy v. no dietary intervention to prevent recurrence of macrosomia; Ref, Reference group.
Values determined using logistic regression.
* Asthma model: adjusted for child sex, age at follow-up.
† Eczema model: adjusted for maternal smoking in pregnancy, child sex, age at follow-up.
‡ Model adjusted for child sex, age at follow-up.
§ Models adjusted for maternal age at delivery, child sex, age at follow-up.
|| Models adjusted for child sex, age at follow-up.
In addition, there was evidence for effect modification by maternal education at 5 years of age (P interaction = 0·045). When we stratified by maternal education, the intervention was associated with a reduction in risk of asthma at 5 years of age in children born to mothers with lower educational attainment but not in those with higher educational attainment (adjusted OR 0·14 (95 % CI 0·02, 0·69); P = 0·010 and adjusted OR 1·03 (95 % CI 0·34, 3·13); P = 0·94, respectively) (Table 3).
Table 3. Associations between ROLO trial intervention arms in pregnancy and child outcomes, stratified by maternal education level

ROLO, Randomised cOntrol trial of LOw glycaemic index diet in pregnancy v. no dietary intervention to prevent recurrence of macrosomia; Ref, Reference group.
Values determined using logistic regression.
* Asthma model: adjusted for child sex, age at follow-up.
† Eczema model: adjusted for maternal smoking in pregnancy, HP index, child sex, age at follow-up.
‡ Model adjusted for HP index, child sex, age at follow-up.
§ Models adjusted for maternal age at delivery, child sex, age at follow-up.
|| Models adjusted for child sex, age at follow-up.
¶ Asthma model: adjusted for child sex, age at follow-up.
** Eczema model: adjusted for gestational weight gain, child sex, age at follow-up.
*† Model adjusted for gestational weight gain, child sex, age at follow-up.
*‡ Models adjusted for maternal age at delivery, child sex, age at follow-up.
*§ Models adjusted for gestational weight gain, child sex, age at follow-up.
Observational analyses of intakes in pregnancy and child outcomes
In the fully adjusted model, there was a positive association between intake of sugar during pregnancy and asthma at any time point (OR per quartile 1·40 (95 % CI 0·99, 1·97); P trend=0·048) (Table 4). When we analysed each time point, intake of sugar during pregnancy was positively associated only with asthma at 5 years of age (OR per quartile 1·55 (95 % CI 1·00, 2·40), P trend=0·046) (Table 5).
Table 4. Associations between maternal mean sugar intake during pregnancy and child outcomes at any time point
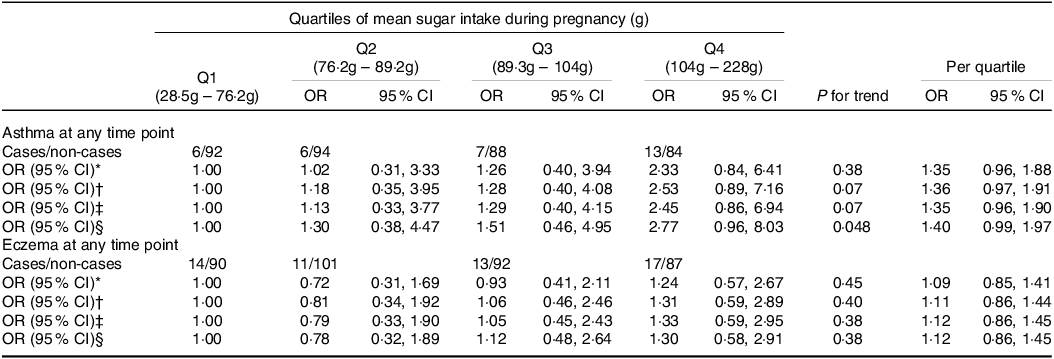
Q, quartile.
Values determined using multivariate logistic regression.
* Crude model adjusted for mean total energy intake.
† Model 1 adjusted for child sex, age at follow-up, trial group, maternal education, maternal ethnicity, mean total energy intake.
‡ Model 2 adjusted for model 1 + maternal age at delivery, maternal smoking in pregnancy.
§ Model 3 adjusted for model 2 + gestational weight gain, birthweight, gestational age at delivery.
Table 5. Associations between maternal mean sugar intake during pregnancy and child outcomes at specific time points
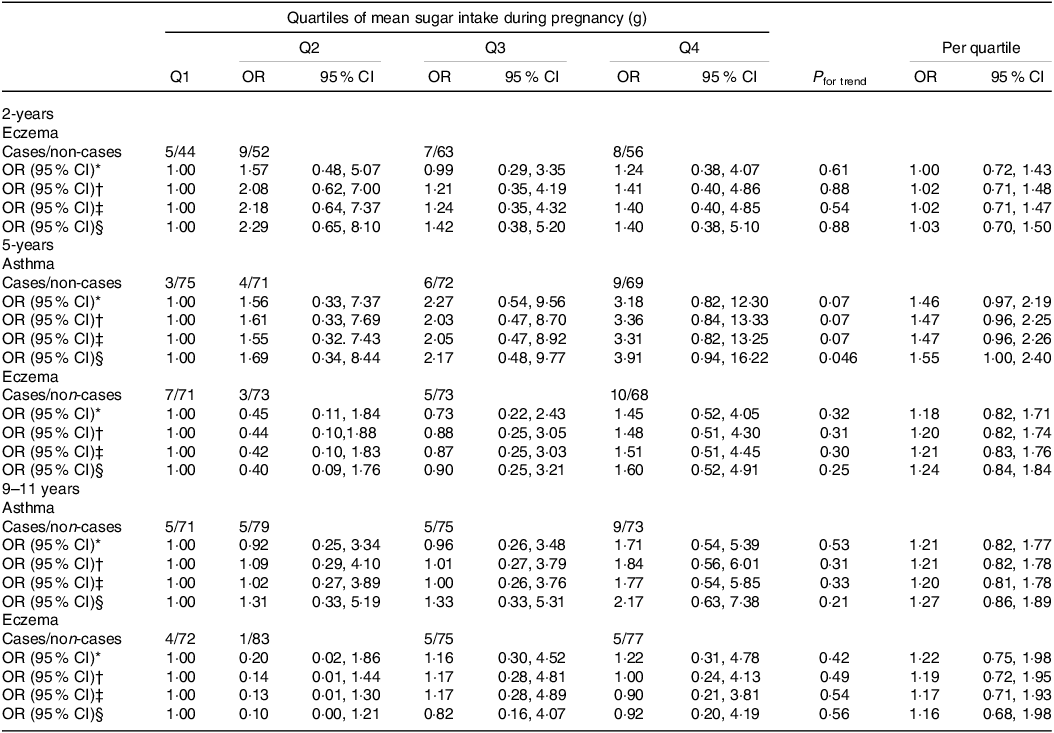
Q, quartile.
Values determined using multivariate logistic regression.
* Crude model adjusted for mean total energy intake.
† Model 1 adjusted for child sex, age at follow-up, trial group, maternal education, maternal ethnicity, mean total energy intake.
‡ Model 2 adjusted for model 1 + maternal age at delivery, maternal smoking in pregnancy.
§ Model 3 adjusted for model 2 + gestational weight gain, birthweight, gestational age at delivery.
In the fully adjusted model, intake of carbohydrate during pregnancy was positively associated with asthma at any time point in childhood (fully adjusted OR per quartile 1·59 (95 % CI 1·10, 2·30), P trend = 0·011) (online Supplementary Table 5). When we analysed follow-up at each time point, intake of carbohydrate during pregnancy was positively associated only with asthma at 9–11 years of age (fully adjusted OR per quartile 1·61 (95 % CI 1·04, 2·49), P trend = 0·024) (online Supplementary Table 6).
In the fully adjusted model, mean GI during pregnancy was positively associated with eczema at 9–11 years of age (OR per quartile 1·92 (95 % CI 0·99, 3·74), P trend = 0·027); however, mean GL during pregnancy was not associated with eczema at 9–11 years of age (OR per quartile 1·00 (95 % CI 0·57, 1·75), P trend = 0·97). While mean GL during pregnancy was positively associated with asthma at any time point (OR per quartile 1·46 (95 % CI 1·00, 2·13), P trend = 0·043), mean GI during pregnancy was not associated with asthma at any time point (OR per quartile 0·92 (95 % CI 0·63, 1·32), P trend = 0·67) (data not shown).
Discussion
Main findings
This study found a suggestion for a reduction in asthma at 5 years of age in children whose mothers had received a low GI dietary intervention during pregnancy compared with usual care. However, amongst mothers with lower educational attainment, the intervention was associated with a reduction in asthma risk in childhood. Also, in keeping with previous birth cohort findings(Reference Bédard, Northstone and Henderson9,Reference Wright, Rifas-Shiman and Oken10) , maternal intake of sugar during pregnancy was positively associated with the risk of childhood asthma in observational analyses.
Interpretation
The ROLO low GI intervention resulted in significant reductions in GI, GL and carbohydrate intake in pregnancy(Reference Walsh, McGowan and Mahony12,Reference McGowan, Walsh and Byrne13,Reference Horan, McGowan and Gibney30) , and follow-up of the offspring provided a novel opportunity to test whether previous observational associations linking higher prenatal sugar exposure to increased risk of asthma might be causal. We found a suggestion for a reduced risk of asthma in 5-year-old children born to mothers in the intervention group compared with children born to mothers in the control arm, which strengthens causal inference. However, when we carried out an a priori stratified analysis, we found that the intervention reduced childhood asthma risk amongst mothers with lower educational attainment, a strong proxy for social disadvantage(Reference Lewis, Ruiz and Goldblatt31). Pregnant women with lower educational attainment are more likely to have children with asthma(Reference Lewis, Ruiz and Goldblatt31), poor dietary intakes and to experience excess GWG(Reference O’Brien, Alberdi and McAuliffe32). Thus, the ROLO low GI dietary intervention may have had a greater impact on reducing childhood asthma risk in this vulnerable subgroup.
To our knowledge, three birth cohort studies have investigated the relation between maternal sugar intake in pregnancy and asthma and atopic outcomes in childhood(Reference Bédard, Northstone and Henderson9,Reference Wright, Rifas-Shiman and Oken10,Reference Maslova, Strøm and Olsen33) . In the ALSPAC and Project Viva cohorts, higher free sugar and fructose intakes during pregnancy were associated with increased risk of asthma in mid-childhood(Reference Bédard, Northstone and Henderson9,Reference Wright, Rifas-Shiman and Oken10) . In contrast, in the Danish National Birth Cohort, Maslova et al found no association between the consumption of sugar-sweetened beverages during pregnancy and childhood asthma(Reference Maslova, Strøm and Olsen33). Our observational associations for sugar intake in pregnancy and childhood asthma risk are in keeping with the ALSPAC and Project Viva findings. In ALSPAC, higher maternal sugar intake was also associated with an increased risk of childhood atopy, as measured by allergen skin testing(Reference Bédard, Northstone and Henderson9). In the ROLO study, we did not have objective measurements of atopy. We therefore used eczema as a proxy measure of an atopic tendency, but found no association between maternal sugar intake and childhood eczema, although GI was positively associated with eczema at 9–11 years of age.
Mechanisms
Excess dietary sugars, particularly fructose in its isolated form, are associated with elevated inflammatory markers, C-reactive protein and uric acid in mice and humans(Reference Aeberli, Gerber and Hochuli34–Reference Johnson, Nakagawa and Sanchez-Lozada36). As mentioned, excess-free-fructose is a major component of apple juice and high fructose corn syrup that is associated with upregulation of aggravated lung allergic inflammation(Reference DeChristopher and Tucker37,Reference DeChristopher, Uribarri and Tucker38) . Potential underlying mechanisms include upregulation of T-helper type 2 cells (Th2), mucus hypersecretion, increased inflammatory infiltrate and activated receptors of advanced glycation end products in the lung(Reference Kierstein, Krytska and Kierstein35,Reference Musiol, Harris and Karlina39–Reference DeChristopher, Uribarri and Tucker42) . The direct inflammatory effects of isolated fructose may be explained by adiposity-independent mechanisms(Reference Singh, Aggarwal and Singh43). Previous research suggests that fetal exposure to a high fibre diet during pregnancy may modulate Th2 immune response and reduce allergic outcomes postnatally(Reference Thorburn, McKenzie and Shen44). However, if the low GI intervention had reduced childhood asthma risk through a reduction in Th2 responses in our cohort, we would have expected to see a reduction in eczema too, and we did not. It is plausible that our mixed findings may be attributed to the consumption of low GI foods that also contain high excess-free-fructose such as apples and pears. Alternatively, there is strong evidence linking gut dysbiosis with the development of asthma(Reference Barcik, Boutin and Sokolowska45). Excess-free-fructose can trigger fructose malabsorption-related mechanisms, leading to gut dysbiosis in the gut/lung axis(Reference DeChristopher46). An overabundance of unfavourable microbiota metabolites may also trigger the receptor for advanced glycation end products, a key mediator in the pulmonary inflammatory response(Reference Oczypok, Perkins and Oury47). It is plausible that gut dysbiosis can be transferred to the fetus during pregnancy via the maternal–fetal gut microbiota axis(Reference Sajdel-Sulkowska48). Thus, maternal dietary factors could have an important role in the modulation of dysbiotic gut microbiota by increasing the production of favourable short-chain fatty acids and promoting a protective T1 phenotype in the fetal lung(Reference Alsharairi49,Reference Gray, O’Hely and Ranganathan50) .
Strengths and limitations
A strength of this study is the randomised nature of the intervention in the ROLO pregnancy trial, which reduces the likelihood of confounding which is more likely to have occurred in previous observational studies investigating this hypothesis. Consistent data collection methods for child outcomes were used at each follow-up. For the observational analyses, comprehensive dietary data at three time points in pregnancy were estimated from 3-d food diaries, which is considered a more accurate method than FFQ, and use of nine food diaries overall provides a more accurate estimate of dietary factors throughout pregnancy. Any misclassification of maternal sugar intake in pregnancy is likely to have been random with respect to childhood outcomes, which would tend to push effect estimates towards the null.
Our sample size was not large, and we are therefore likely to have been underpowered to detect modest associations. Another limitation was the substantial loss to follow-up in all three follow-up studies; however, the attrition was not materially different between the two arms of the trial and strategies have been implemented to minimise the attrition rate(Reference O’Brien, Geraghty and McAuliffe51). Our finding that the intervention reduced childhood asthma risk amongst mothers with lower educational attainment but not in those with complete tertiary education should be interpreted with caution. The wide and overlapping confidence intervals observed may indicate an unstable estimate, likely due to the low sample size. In the observational analyses, we cannot rule out the possibility that our main findings arose through unmeasured or residual confounding, although we controlled for a large number of covariates in the regression models. Given the large number of statistical tests in the secondary observational analyses, we acknowledge that some results may have arisen by chance and should therefore be interpreted with caution. However, as the hypotheses we were testing were a priori, we have not adjusted for multiple testing.
Future research and policy implications
This novel study provides stronger evidence that higher sugar intake during pregnancy is associated with an increased risk of asthma among offspring, and an intervention to reduce sugar intake in pregnancy may have potential as a primary prevention strategy. Maternity dietary guidelines focus on short-term maternal implications such as GWG and gestational diabetes mellitus(52), and there is no specific recommendation for the consumption of free sugars during pregnancy(Reference Gupta, Singh and Fernando53). Future de novo trials of restricted sugar intake during pregnancy may yield confirmatory evidence to inform new guidelines. Public health policies that educate and support pregnant women, especially those with lower educational attainment, to reduce their sugar intake may be beneficial to prevent asthma in their children.
Conclusion
To conclude, this study suggests that a low GI dietary intervention in pregnancy may reduce the risk of asthma in childhood, especially amongst those born to mothers with lower educational attainment, and further observational evidence that higher maternal sugar intake is associated with higher childhood asthma risk. This new randomised evidence supports a causal link and thus has important implications for the primary prevention of childhood asthma. Our findings may strengthen evidence-based maternity dietary guidelines related to sugar intake.
Acknowledgements
The authors would like to thank all the parents and children of the ROLO study for their continued support. Thank you also to the staff of the National Maternity Hospital for facilitating our research.
The ROLO trial and follow-ups were supported by the Health Research Board Ireland, Health Research Centre for Health and Diet Research, European Union’s Seventh Framework Programme (FP7/2007–2013), project Early Nutrition under grant agreement no. 289 346, the National Children’s Foundation, Tallaght, Dublin 24 and the National Children’s Research Centre Ireland at Children’s Health Ireland (PRPG/H/18/325). MT was supported by a grant from Barts Charity (MGU0570). The funders had no role in study design, data collection and analysis and decision to publish.
S. C. designed the study, analysed the data and drafted the initial manuscript. M. T. advised on statistical analysis and contributed to the manuscript preparation. A. D. conducted the research, collected the data and contributed to the manuscript preparation. S. O. S. and F. M. M. C. A. designed the study, advised on data analysis, contributed to the manuscript preparation and had primary responsibility for the final content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
The authors declare none.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524001612









