Lycopene, the predominant carotenoid in tomatoes and tomato products responsible for the red colour, has attracted considerable attention as epidemiological evidence continues to suggest that it may provide protection against a number of cancers and other degenerative diseases (Giovannucci, Reference Giovannucci1999; Nguyen & Schwartz, Reference Nguyen and Schwartz2000; Khachik et al. Reference Khachik, Carvalho, Bernstein, Muir, Zhao and Katz2002). The observation that serum and tissue lycopene consists of between 50 and 90 % cis-lycopene isomers, whereas tomatoes and tomato-based products contain mainly all-trans-lycopene, has led to the hypothesis that cis-isomers of lycopene are produced during food processing, cooking, or digestion or are more bioavailable (Erdman, Reference Erdman2005). Improved absorption of lycopene cis-isomers is hypothesised to result from greater solubility in mixed micelles and a lower tendency to aggregate (Stahl & Sies, Reference Stahl and Sies1992; Boileau et al. Reference Boileau, Merchen, Wasson, Atkinson and Erdman1999), a hypothesis supported by rodent studies (Boileau et al. Reference Boileau, Merchen, Wasson, Atkinson and Erdman1999; Korytko et al. Reference Korytko, Rodvold, Crowell, Stacewicz-Sapuntzakis, Diwadkar-Navsariwala, Bowen, Schalch and Levine2003). Although most commercially processed tomato products such as juice, paste, soup and ketchup contain a relatively low percentage of cis-isomers ( < 10 %), aggressive food processing or cooking has been shown to increase cis-isomer content (Shi & Le Maguer, Reference Shi and Le Maguer2000). The greatest increase in cis-isomer formation was observed when tomato products were heated in the presence of oil (Stahl & Sies, Reference Stahl and Sies1992; Schierle et al. Reference Schierle, Bretzel, Bühler, Faccin, Hess, Steiner and Schüep1997; van het Hof et al. Reference van het Hof, Gartner, West and Tijburg1998). Thus, research conducted so far suggests that lycopene cis-isomer formation is usually not observed at the mild temperatures commonly employed in most commercial food processing but is enhanced by the presence of oil at medium or higher processing and cooking temperatures.
The presence of elevated levels of lycopene cis-isomers in human biological samples compared with fresh tomatoes may also have biological significance, but this remains speculative (Clinton et al. Reference Clinton, Emenhiser, Schwartz, Bostwick, Williams, Moore and Erdman1996). Cis-isomers may show unique tissue biodistribution patterns or perhaps distribute differently within cellular compartments to influence cell functions and responses to physiological or toxic stimuli in the external or intracellular environment. Thus, it is hypothesised that modification of the cis- and trans-lycopene-isomer pattern of foods may allow investigators to achieve targeted changes in absorption and distribution of specific carotenoid isomers in order to influence biological effects and health outcomes.
The objective of the present study was to develop two similar tomato sauces with high and low cis-lycopene-isomer content by different processing techniques. In addition, our goal was to compare postprandial absorption patterns of lycopene isomers in human subjects after a single-meal consumption of these sauces to address the question whether there is a preferential uptake of lycopene from the cis-isomer-rich product and whether further in vivo isomerisation takes place. Furthermore, we tested the feasibility of shortening the time- and labour-intensive 10 h postprandial blood collection with eight samples to a four-sample collection following test-meal consumption. Lycopene absorption was assessed by analysing the plasma-triacylglycerol (TAG)-rich lipoprotein (TRL) fraction of plasma, reflecting newly absorbed carotenoids as a measure of bioavailability, as compared with a large number of human studies typically investigating lycopene bioavailability based on plasma response (McEligot et al. Reference McEligot, Rock, Shanks, Flatt, Newman, Faerber and Pierce1999; Rao, Reference Rao2004; Frohlich et al. Reference Frohlich, Kaufmann, Bitsch and Bohm2006).
Experimental methods
Subjects
Twelve healthy, non-pregnant, non-smoking subjects (six male and six female; age 19–43 (median 29) years) were recruited for the study. Eligibility was based on a screening test for normal blood lipid profiles (cholesterol, TAG) and a health and lifestyle questionnaire. Exclusion criteria were hyperlipidaemia, any history of chronic gastrointestinal disease, use of medications affecting lipid metabolism, regular use of carotenoid-containing supplements, and frequent alcohol consumption. The study was approved by the Biomedical Sciences Institutional Review Board of The Ohio State University (OSU) and written informed consent was obtained.
Tomato sauce processing
High-lycopene-variety tomatoes, FG99-218 (Department of Horticulture and Crop Science, OSU, Wooster, OH, USA), were used to produce tomato sauces. FG99-218 was homozygous for the dark green (dg) gene and for the old-gold crimson (og c) gene. Plants were grown with 30 cm within-row spacing and 1·5 m between-row spacing at OSU North Central Agricultural Research Station (near Fremont, OH, USA), following standard practices (Precheur, Reference Precheur2000). Following harvest, tomatoes were immediately processed (104°C, 30 min) into canned tomato juice. Afterwards, tomato juice was concentrated in the pilot plant scale rotary evaporator, at mild temperature (60°C) and under vacuum. After adding small amounts of an Italian seasoning mix (dried oregano, marjoram, basil and black pepper, prepared at the OSU pilot plant), 7 % high-fructose maize syrup (both added to improve taste and palatability), and 10 % maize oil to the concentrate on the basis of weight, the mixture was heated to 75°C (about 15–20 min) in an agitated steam-jacketed vessel and was hot-filled into small cans (type 300 × 303). After sealing and cooling in an upside-down position, this product was stored to be served as hot-filled tomato sauce rich in all-trans-lycopene (sauce A; 95 % all-trans-lycopene). The second sauce, rich in lycopene cis-isomers (sauce B; 55 % all-trans-lycopene), was obtained by applying the same procedure to the same tomato juice concentrate followed by further processing in a steriotort at 127°C for 40 min.
Test meals
Test meals consisted of tomato sauce (150 g) served with spaghetti (200 g) along with a slice (25 g) of fat-free white wheat bread to absorb the tomato sauce leftover on the plate, and 240 ml bottled water. The energy and macronutrient content of all meals were determined by Food Processor™ version 8.1 (ESHA Reach, Salem, OR, USA). The test meals provided 2·19 MJ energy, with 29 % of the energy coming from fat, with the remainder being almost entirely carbohydrates. Both tomato sauces were kept in the refrigerator until served to the subjects. They were served at room temperature and added over warm, not hot spaghetti, in order not to cause any possible changes in the carotenoid composition.
Experimental design
Subjects presented at the clinic on two separate occasions to consume the two tomato sauce meals and were instructed to avoid foods rich in lycopene (especially tomatoes and tomato-containing food products such as pizza, ketchup and lasagne) for 2 weeks as a washout period before each visit. Tomato sauces were served in a randomised cross-over design, each subject acting as his/her own control. Subjects arrived at the OSU General Clinical Research Center after overnight fasting for 12 h. Following a baseline blood draw (0 h), test meals were served at 08.00 hours and consumed under supervision within 20 min. Consecutive blood samples were collected at 2, 3, 4, 5, 6, 8 and 9.5 h following test-meal consumption. At 4.5 h, subjects consumed a standardised lunch (120 ml reduced-fat cream of mushroom soup, 15 g fat-free saltines, light chunk tuna (56 g) sandwich in two slices of fat-free bread (52 g), two vanilla sandwich cookies (26 g), one bottle of diet soda (240 ml), one bottle of water (240 ml)) low in carotenoids and fat, providing 1·92 MJ energy from 77 g carbohydrate, 22 g protein and 7 g fat. No other foods and beverages except water (ad libitum) were allowed during the 10 h clinical visit.
Blood sampling and analysis
Blood samples (12 ml) drawn from a forearm vein into EDTA tubes were immediately centrifuged for plasma separation at 1250 g for 10 min at 4°C (Bamon/IEC Division, Needham, MA, USA). Measurements of plasma cholesterol and TAG levels were based on a spectrophotometric enzymatic method (Beckman-Coulter, 2006), using a Synchron LX 20 system (Beckman Coulter Inc., Fullerton, CA, USA). TRL fractions were isolated from plasma by ultracentrifugation at 155 000 g for 30 min at 20°C (Beckman L8-M ultracentrifuge, SW 50·1 swinging bucket rotor) following a method described by van Vliet et al. (Reference van Vliet, Schreurs and van den Berg1995).
Carotenoid extractions and quantification
Carotenoids were extracted from tomato sauces (Ferruzzi et al. Reference Ferruzzi, Sander, Rock and Schwartz1998) and from the plasma-TRL (Ferruzzi et al. Reference Ferruzzi, Nguyen, Sander, Rock and Schwartz2001) fraction with acetone–hexane as described previously. All procedures were conducted under red light. Standards of lycopene and β-carotene were purchased from Sigma Chemicals (St Louis, MO, USA). Stock solutions of carotenoids were prepared in hexane and their concentrations were determined spectrophotometrically, using their specific absorption coefficients (Weast, Reference Weast1973). All-trans standards were used for the quantification of both trans- and cis-isomers. Analysis of all-trans-lycopene and all-trans-β-carotene standards indicated no degradation during simulated extraction and on-column during HPLC analysis. Identification of cis-isomers was based on the comparison with previously reported UV-visible spectrophotometry and electrochemical methods (Ferruzzi et al. Reference Ferruzzi, Sander, Rock and Schwartz1998, Reference Ferruzzi, Nguyen, Sander, Rock and Schwartz2001; Hadley et al. Reference Hadley, Clinton and Schwartz2003). Carotenoids were analysed using a Hewlett Packard 1050 HPLC system (Foster City, CA, USA) connected to an electrochemical detector (5600 Coularray™; ESA, Chelmsford, MA, USA) with cell potentials set from 200 to 620 mV in 60 mV increments. A C30 column (YMC™, 150 × 4·6 mm, 5 μm; Waters, Milford, MA, USA) was used for the analysis of carotenoids in conjunction with a gradient method (Ferruzzi et al. Reference Ferruzzi, Nguyen, Sander, Rock and Schwartz2001).
Statistical analyses
Postprandial absorption for each carotenoid in the plasma-TRL fraction, expressed as baseline-corrected area under the concentration v. time curve (AUC) over 9.5 h after test-meal consumption, was calculated by trapezoidal approximation using Igor Pro 4.06 software (Wave Metrics, Lake Oswego, OR, USA). Statistical analyses were performed using SAS JMP version 5.0 (SAS Institute, Cary, NC, USA). ANOVA was carried out with multiple covariates (sex, age, BMI, plasma TAG, plasma cholesterol and baseline values) to compare AUC responses between the two tomato sauces. Adjustment of AUC data was made by dividing through the total amount of lycopene consumed. Log-transformation (decadic) of the AUC data was used to improve the statistical modelling and residuals were examined for model compliance. Normality of residuals was checked using the Shapiro–Wilk test.
Baseline-corrected AUC (nmol × h/l) values, peak concentrations (Cmax; nmol/l, baseline corrected), fractional absorption and carotenoid content (mg/150 g serving size) of the test meals were expressed as mean values with their standard errors. P values < 0·05 were considered significant (two-sided). Pearson correlation was calculated and linear regression analyses were performed to study the relationship between AUC4–6 and AUC0–9.5 values. Fractional lycopene absorption from the test meals was estimated according to O'Neill & Thurnham (1998):
assuming t \textstyle{1\over 2} of chylomicrons to be 0.192 h, plasma volume (ml) being 927+31·47 × body weight in kg, mass being the molecular weight of lycopene in g/mol, and oral dose being the amount lycopene consumed in g.
Results
All recruited subjects (six males, six females; Table 1) completed the study and consumed both test meals. Age, BMI, plasma cholesterol and TAG levels were not significantly different between men and women. Fasting plasma TAG and cholesterol levels were all within the normal reported range ( < 2·3 mmol/l for TAG, and < 5·2 mmol/l for cholesterol (National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, & Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002). Effects of sex, age, BMI and plasma cholesterol values were found not to affect carotenoid absorption significantly, while plasma TAG values had a significant effect on carotenoid absorption when these variables were used in the model as covariate, i.e. higher TAG concentrations were significantly associated with higher carotenoid absorption.
Table 1 Subject characteristics at the onset of the study (Mean values with their standard errors)
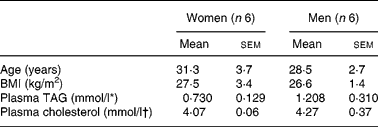
* Conversion factor to mg/l is × 885·7.
† Conversion factor to mg/l is × 386·7.
Total lycopene (sum of all-trans- and cis-isomers identified as 5-cis-, 9-cis-, 13-cis-, and other unidentified cis-isomers) and β-carotene (sum of all-trans- and the 9-cis-isomer) content of both sauces per 150 g serving size are given in Table 2. The additional heat treatment (steriotort) of sauce A elevated the percentage of lycopene cis-isomers approximately 9-fold in sauce B, resulting in about 45 % cis-isomers of total lycopene. Higher temperatures were also tested; however, setting temperature and time above the chosen parameters of 127°C and 40 min resulted in an unacceptable brown-coloured product in preliminary trials, which was of a burnt taste. Because the two tomato sauces were processed in two different batches, DM content was slightly different, Brix values for sauce A and B being 20·8 (sem 0·09) and 19·6 (sem 0·07) (n 10), respectively. As the total lycopene and β-carotene contents of the two sauces were not exactly the same in the final product, plasma-TRL AUC responses between the two sauces were compared on both a lycopene-dose-adjusted and non-adjusted basis (Table 3).
Table 2 Carotenoid profile of the test meals† (Mean values with their standard errors)
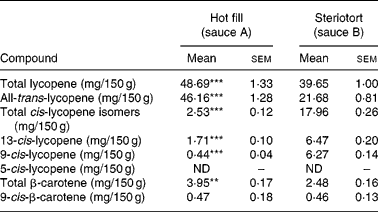
ND, not detectable.
Mean value was significantly different from that for sauce B: *P < 0·05, **P < 0·01, ***P < 0·001 (Fisher F test).
† Values are based on serving size (n 6).
Table 3 Distribution of lycopene isomers in plasma triacylglycerol-rich lipoprotein fractions following the consumption of tomato sauces in human subjects† (Mean values with their standard errors)
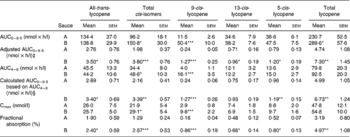
AUC, area under the curve; Cmax, peak concentration.
Mean value was significantly different from that for sauce A: *P < 0·05, **P < 0·005, ***P < 0·0005 (Fisher F test; tests based on log-transformed data).
† All values are based on n 12. All values are baseline corrected. Test meals consisted of 150 g tomato sauce, served together with 200 g spaghetti and 25 g white wheat bread.
‡ Adjustment was done by dividing through the amount of total lycopene served in the test meal.
§ Values are dose adjusted for the total amount of lycopene consumed. The method is based on only four blood samples drawn at 0, 4, 5 and 6 h following test-meal consumption. No significant differences were detected compared with the measured values (adjusted AUC0–9·5).
In addition to the all-trans form, several cis-isomers of lycopene as identified by Hadley et al. (Reference Hadley, Clinton and Schwartz2003) were detected in the plasma-TRL fraction obtained after test-meal consumption (Table 3). Thus, results are expressed as all-trans-lycopene, total lycopene cis-isomers including 5-cis-, 9-cis- and 13-cis-isomers, and total lycopene. In addition, low amounts of all-trans-β-carotene along with its 9-cis-isomer were detected. The oral β-carotene dose consumed with the tomato sauce was relatively low, and subjects were only on a lycopene-free diet and not on a β-carotene-free diet during the washout period. Thus, enrichment of β-carotene after test-meal intake in the plasma-TRL fraction was relatively low, and is therefore not reported.
When comparing the percentage of cis-isomers of total lycopene in the sauce v. the plasma-TRL fraction, an average increase from approximately 5 to 42 % was observed for sauce A. For sauce B, this percentage increased from 45 to 52 % (Fig. 1). Similarly, trans:cis-lycopene abundance ratios were 18·2 in sauce A and 1·4 in the plasma-TRL AUC of the group consuming this sauce, while this ratio was 1·2 and 0·9 for sauce B, respectively.
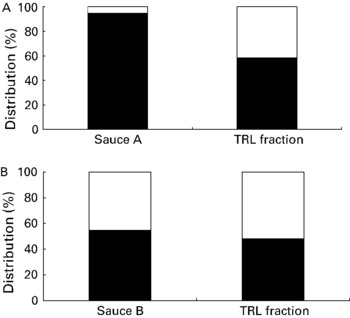
Fig. 1 Percentage distribution of lycopene isomers in the tomato sauces and the mean response in the plasma TAG-rich lipoprotein (TRL) fraction measured as baseline-corrected area under the curve after consumption of sauce A, rich in all-trans-lycopene (■) (A) and sauce B, rich in cis-lycopene (□) (B), respectively.
Baseline-corrected, dose-adjusted peak concentrations of 0·45 (sem 0·11) and 0·73 (sem 0·14) nmol/l plasma-TRL for total lycopene cis-isomers and 0·98 (sem 0·25) and 1·38 (sem 0·25) nmol/l plasma-TRL for total lycopene were reached in the plasma-TRL fraction at the 5th hour after the consumption of sauce A and sauce B, respectively (Fig. 2).
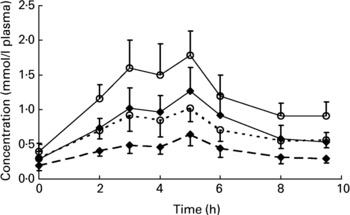
Fig. 2 Dose-adjusted plasma TAG-rich lipoprotein concentrations of total lycopene and lycopene cis-isomers following the consumption of sauce A (rich in all-trans-lycopene) and sauce B (rich in cis-lycopene) over 9.5 h. Values are means (n 12), with standard errors represented by vertical bars. (–♦–), Total lycopene in sauce A; (–○–), total lycopene in sauce B; (–♦–, dotted line), cis-lycopene in sauce A; (–○–, dotted line), cis-lycopene in sauce B.
A strong significant correlation (P < 0·0001) between the shortened approach to determine lycopene TRL AUC based on four blood samples (AUC4–6) v. AUC0–9 ·5 was found for total lycopene, lycopene cis-isomers and all-trans-lycopene (log-transformed (decadic) data, dose adjusted and baseline corrected). A linear model was developed to estimate the AUC0–9 ·5 by using AUC4–6 as:
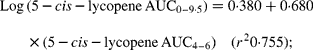


To validate the novel approach to determine lycopene TRL AUC and therefore to determine absorption, the AUC4–6 values were used to calculate the AUC0–9·5 values with the above equations and then compared with the observed values for total lycopene, all-trans-lycopene, total cis-lycopene and its isomers. No significant differences were found between the calculated and the measured results (Table 3).
Discussion
The results of the present study demonstrate a more than 55 % increase in fractional total lycopene absorption following the consumption of a cis-lycopene-rich tomato sauce v. a very similar all-trans-rich tomato sauce. In addition, the study shows an enhanced cis- compared with all-trans-lycopene plasma-TRL response when taking into account the amount of isomer consumed. These findings are in line with the hypothesis of preferential absorption of lycopene cis-isomers. To our knowledge, this is the first human study demonstrating enhanced cis-lycopene-isomer response compared with its all-trans-isomer from tomato products with a common food matrix, similar to those commercially available.
In the present study, cis-lycopene formation was controlled in the all-trans-lycopene-rich tomato sauce by applying a mild processing temperature of 75°C, resulting in 5 % cis-isomer formation. Cis-lycopene formation was induced to 45 % of total lycopene in a sauce by adjusting the temperature to 127°C (40 min). The published literature regarding the effects of tomato processing on the formation of lycopene cis-isomers remains somewhat controversial. Nguyen & Schwartz (Reference Nguyen and Schwartz1998), Nguyen et al. (Reference Nguyen, Francis and Schwartz2001) and Re et al. (Reference Re, Bramley and Rice-Evans2002) have shown that modest thermal treatment did not cause a considerable shift in the distribution of trans:cis-lycopene isomers. However, the addition of fat, together with applying higher temperatures, such as may occur during the cooking of tomato products, has been suggested to cause an increase in lycopene cis-isomer formation during tomato processing. Agarwal et al. (Reference Agarwal, Shen, Agarwal and Rao2001) observed an increase from 6 to 29 % cis-isomers when heating tomato juice (1 h) with 10 % added maize oil. Similarly, van het Hof et al. (Reference van het Hof, Gartner, West and Tijburg1998) found elevated levels of cis-isomers (about 55 %) when tomato paste was heated to 100°C for 30 min in the presence of 10 % maize oil, whereas it was lower with 5 % oil addition (about 35 %) and low (about 5 %) in the absence of oil. Together, these studies suggest that most processed food products contain primarily all-trans-lycopene, but that additional processing or cooking methods could increase cis-lycopene content. In the present study, the addition of 10 % maize oil to both sauces before processing resulted in the formation of cis-lycopene isomers with concentrations comparable with the former studies. It can be speculated that a certain amount of fat needs to be present in the product to solubilise and release lycopene from the matrix to enhance isomerisation by thermal treatment.
Although estimated total lycopene and isomer content of the two tomato sauces differed, we understand that the HPLC method used to quantify isomers is limited by the fact that pure authentic standards of isomers are not readily available for calibration. Measurements for all detected lycopene isomers are based on response factors for the all-trans compound. When using UV-visible spectrophotometry methods of detection, this would result in an underestimation of lycopene isomer content because of the shift in absorbance spectra when isomerisation occurs. Electrochemical methods of detection were found to be similar in detector response to all-trans-lycopene but not for all lycopene isomeric forms (Ferruzzi et al. Reference Ferruzzi, Nguyen, Sander, Rock and Schwartz2001). As such, further refinements in methodology are necessary to quantify lycopene cis-isomers including other cis-carotenoid geometrical forms.
Previous studies (Stahl & Sies, Reference Stahl and Sies1992; Gartner et al. Reference Gartner, Stahl and Sies1997) have suggested that heat treatment alone might improve lycopene bioavailability without significantly altering the isomer composition within foods. In the present study, it is therefore possible that the higher lycopene absorption from the cis-isomer-rich product was due to the higher temperature treatment. The observation that not only the cis-lycopene plasma-TRL response, but also the all-trans-lycopene response increased following consumption of the cis-lycopene-rich product as compared with the all-trans-rich product, would support this hypothesis. However, in another study by our group (Unlu et al. Reference Unlu, Bohn, Francis, Clinton and Schwartz2007), we found a significantly higher plasma-TRL response from tomato sauce obtained from tangerine tomatoes rich in cis-lycopene (about 97 % cis-isomers) produced under similar mild processing conditions as compared with the all-trans-rich tomato sauce in the present study. Thus, both preferred absorption of lycopene cis-isomers per se, and improved absorption of total lycopene due to heat treatment could have contributed to the present results, as the effect of cis-lycopene isomerisation could not be separated from the heat processing. In addition to heat treatment, the lower dose of lycopene served within the cis-isomer-rich sauce could have added to an increased fractional lycopene absorption, as lycopene absorption has been reported to follow a curvilinear absorption pattern, as tested with doses ranging between 10 and 120 mg (Gustin et al. Reference Gustin, Rodvold, Sosman, Diwadkar-Navsariwala, Stacewicz-Sapuntzakis, Viana, Crowell, Murray, Tiller and Bowen2004). However, in the present study, even the non-dose-adjusted AUC suggested higher lycopene bioavailability from the cis-isomer-rich lycopene product.
The main mechanism by which cis-lycopene accumulates in blood or tissue remains controversial. The higher plasma-TRL responses of cis-lycopene and its isomers compared with all-trans-lycopene (when taking into account the amount isomer consumed) from both, but especially from the all-trans-lycopene-rich product, could be explained either due to higher absorption of cis-lycopene, increased in vivo isomerisation from all-trans-lycopene, or both. The observation, however, that following consumption of the cis-rich product, only a slightly decreased ratio of trans:cis-lycopene was found in the TRL fraction indicated that an enhanced uptake of cis-lycopene alone cannot explain the observed results. This less pronounced difference in isomer composition between the plasma-TRL fraction and the tomato sauce consumed indicated that isomerisation continued toward an equilibrium between all-trans- and cis-lycopene isomers in the plasma-TRL fraction. An earlier in vitro study investigating trans:cis-isomer distribution in organic solutions suggested a ratio of 50 % all-trans- to 50 % cis-lycopene to be the most stable (Nguyen & Schwartz, Reference Nguyen and Schwartz1998), values which are close to the ones observed in the plasma-TRL fractions of the present study. The trans:cis-isomer ratio of the plasma-TRL fraction was 0·92 and 1·39 following the cis-lycopene and all-trans-lycopene-rich sauce consumption, respectively, suggesting that the higher the trans:cis-isomer ratio in the product consumed, the higher the trans:cis ratio in the TRL AUC response. Boileau et al. (Reference Boileau, Merchen, Wasson, Atkinson and Erdman1999) found significantly higher amounts of cis-lycopene isomers in mesenteric lymph (77 %) compared with dose, stomach and intestinal contents (6–18 %) in ferrets. It has thus been suggested that there is a continuous isomerisation of all-trans-lycopene to cis-lycopene isomers in the body, with higher proportions of cis-lycopene following the order tissue>serum>chylomicrons (Clinton et al. Reference Clinton, Emenhiser, Schwartz, Bostwick, Williams, Moore and Erdman1996). This is further supported by a previous human study by the observation that upon cessation of dietary (predominantly all-trans) lycopene intake, the percentage of cis-isomers of total lycopene increases in plasma (Frohlich et al. Reference Frohlich, Kaufmann, Bitsch and Bohm2006). Interestingly, the present study additionally indicates in vivo isomerisation of either all-trans- or cis-isomers of lycopene to 5-cis-lycopene, as this was not detected in the tomato sauce, while it was found in high concentrations in the plasma-TRL fractions following sauce consumption. As formation of 5-cis from other cis-isomers would require several isomerisation steps, and all-trans-lycopene was present in much higher concentration in the product and plasma-TRL, it appears more likely that all-trans-lycopene was isomerised to 5-cis-lycopene in vivo. 9-cis- and 13-cis-lycopene were present in both sauce and plasma-TRL, and their presence can thus be explained by absorption.
The carotenoid response measured in the TRL-fraction of plasma was suggested to be a valuable measure of carotenoid bioavailability from single meals, as this fraction represents newly absorbed carotenoids (O'Neill & Thurnham, Reference O'Neill and Thurnham1998). However, a large number of blood samples over a 10–12 h course are often employed. We found a significant correlation between TRL AUC4–6 and AUC0–9·5 following the consumption of test meals for total lycopene, lycopene cis-isomers and all-trans-lycopene, and a linear model was developed to estimate the AUC0–9·5 values based on the AUC4–6 . No significant differences in the AUC were found when comparing the estimated AUC0–9·5 values (based on the AUC4–6 ) with the measured AUC0–9·5. Thus, in future studies comparing lycopene bioavailability from different test meals, it seems feasible to collect only four blood samples at 0, 4, 5 and 6 h following test-meal consumption. The limited data obtained within the present study suggest a similar kinetic behaviour of the cis-isomers and all-trans-lycopene within the plasma-TRL fraction during the observed 9.5 h time period, as the TRL appearance pattern, Cmax, and the similar regression curves for estimating the AUC0–9.5 based on the AUC4–6 values indicate. As a smaller number of samples are beneficial both for the researcher and subject, future studies to extend this method to additional carotenoids are merited.
In conclusion, we observed a significant increase in lycopene absorption when tomato sauce was enriched in cis-isomers by heat treatment, being in agreement with the hypothesis that cis-lycopene isomers are preferentially absorbed in man. Additional heat treatment of the tomato matrix, enhancing availability of lycopene to digestive and absorptive processes, could also have contributed to this result. The present results further suggest that preferred absorption of cis-lycopene isomers alone cannot explain the dramatically increased proportion of lycopene cis-isomers in biological samples, an observation that probably reflects an additional in vivo isomerisation from all-trans- to cis-isomers. However, based upon these findings, tomato-based food products could be manipulated by temperature processing to favour the formation of specific isomer patterns to improve lycopene bioavailability and possibly health benefits.
Acknowledgements
Salaries and research support were provided by state and federal funds appropriated to: The OSU (Ohio Agricultural Research and Development Center (OARDC) Competitive Grants Program); The General Clinical Research Center at The OSU (grant M01-RR00034 from the National Center of Research Resources of the National Institutes of Health); National Cancer Institute grant RO1 CA 112632; the United States Department of Agriculture Initiative for Future Agriculture & Food Systems (IFAFS) grant no. 2001-52102-11333.







