Dietary PUFA are perceived to be healthier than SFA. Research has shown several health benefits of n-3 fatty acids (FA) to humans including a decrease in the incidence of cancer, CVD, hypertension and arthritis and an improvement in visual acuity(Reference Simopoulos1). As a result, there has been a great deal of interest in manipulating the FA profile of ruminant milk fat to respond to consumer interest. However, feeding PUFA may contribute to increasing plasma lipoperoxidation as reported in steers(Reference Scislowski, Bauchart and Gruffat2) and rats(Reference Gladine, Rock and Morand3), which may increase oxidative stress in animals. It is known that in dairy cows the peripartum period, particularly postpartum, is characterised by a depleted antioxidant status(Reference Bernabucci, Ronchi and Lacetera4). Nevertheless, supplying n-3 PUFA in association with antioxidants such as vitamin E and plant polyphenols reduced lipoperoxidation in lactating cows, thereby contributing to protecting them against the deleterious consequences of lipoperoxidation(Reference Gobert, Martin and Ferlay5).
Recent research on new antioxidants has focused on natural molecules to satisfy consumer concerns about safety and toxicity(Reference Gladine, Rock and Morand3). Plants lignans are naturally strong antioxidants and flax (Linum usitatissimum) is known as the richest dietary source of lignans, with glycosides of secoisolariciresinol and matairesinol as the major compounds(Reference Mazur, Fotsis and Wähälä6). Flax lignans are concentrated in the outer fibre-containing layers(Reference Adlercreutz and Mazur7), thus resulting in higher concentration of lignans in hulls than in seeds(Reference Côrtes, Gagnon and Benchaar8). In non-ruminant animals, the colonic microbiota converts plant into mammalian lignans, enterodiol and enterolactone (EL)(Reference Saarinen, Huovinen and Wärri9). In dairy cows, the rumen is the main site for the conversion of plant lignan into EL, the mammalian lignan excreted in urine, blood and milk(Reference Gagnon, Côrtes and Da Silva10). In humans, greater consumption of flax lignans may be potentially beneficial to health as shown by the inverse relationship between blood concentrations of EL and the incidence of CVD(Reference Vanharanta, Voutilainen and Lakka11). However, there is no information on the effects of lignans on health of animals.
Previous results have shown that the antioxidant activity of plant and mammalian lignans is greater than that of vitamin E(Reference Prasad12). Rajesha et al. (Reference Rajesha, Murthy and Kumar13) demonstrated in a rat model that flax lignans up-regulate the expression of hepatic genes encoding for enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX), which are involved in defence mechanisms against oxidative stress. Moreover, several studies have demonstrated that n-3 PUFA possess anti-inflammatory and antioxidative properties(Reference Fernandes, Rahman and Bhattacharya14). Nevertheless, as previous results(Reference Gobert, Martin and Ferlay5) have shown that n-3 PUFA also increase plasma lipoperoxidation in dairy cows, it is hypothesised that supplementing dairy cow diets with a source of antioxidants such as plant lignans may increase the activity of enzymes and expression of genes involved in the metabolism of antioxidants. The present study aimed at determining: (1) the effects of dietary flax hulls and flax oil on the activity of antioxidant enzymes (SOD, CAT and GPX) in blood and the mammary gland; (2) the mRNA abundance of antioxidant genes in the mammary gland of dairy cows. Concentrations of EL in milk and plasma were also determined.
Materials and methods
Animals and experimental treatments
A total of eight multiparous Holstein cows (including one square of four cows fitted with ruminal cannulas; 10 cm, Bar Diamond, Inc.) were assigned to a replicated 4 × 4 Latin square design with four 21 d periods balanced for residual effect and a 2 × 2 factorial arrangement of treatments. The four treatments were: (1) control with no flax hulls (CONT); (2) 9·88 % flax hulls in the DM (HULL); (3) control with 500 g flax oil/d infused in the abomasum (COFO); (4) 9·88 % flax hulls in the DM and 500 g flax oil/d infused in the abomasum (HUFO). Previous results have shown that infusing 509 g flax oil/d in the duodenum has no effect on milk yield although feed intake is reduced by 8 %(Reference Côrtes, Kazama and da Silva-Kazama15). Therefore, the amount of flax oil used in the present experiment was defined in order to optimise the effect of oil on the activity of antioxidant enzymes in the mammary gland without causing any negative effect on milk production.
The two total mixed diets (Table 1) were equal in protein. The diets were formulated to meet the requirements for cows that were 750 kg of body weight and producing 30 kg of milk with 3·5 % fat per d (National Research Council, 2001). Flax oil (Brenntag Canada, Inc.) contained, expressed as a percentage of total fatty acids, 5·0 % of C16 : 0, 4·2 % of C18 : 0, 17·4 % of C18 : 1cis-9, 14·9 % of C18 : 2cis-9, cis-12, 53·4 % of C18 : 3cis-9, cis-12, cis-15 and 5·1 % others. Flax hulls (Natunola Health, Inc.) contained, expressed as a percentage of DM, 23·5 % of crude protein, 19·4 % of neutral-detergent fibre, 14·3 % of acid-detergent fibre, 29·8 % of total lipids and 0·99 % of secoisolariciresinol diglucoside, and 6·86 kJ/g of DM. The cows averaged 163 (se 11) d in milk at the start of the experiment with an average body weight of 742 (se 11) kg. No antibiotics were given for at least 16 weeks before initiation of the experiment. The cows were kept in individual stalls and had free access to water. National guidelines for the care and use of animals were followed as recommended by the Canadian Council on Animal Care(16) and all experimental procedures were approved by the local Animal Care Committee Comité Institutionnel de Protection des Animaux.
Table 1 Ingredient and nutrient composition of experimental diets for lactating dairy cows
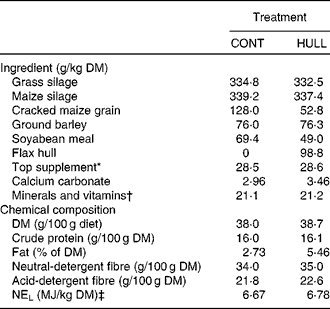
CONT, control with no flax hulls; HULL, diet with 9·88 % flax hulls; NEL, net energy for lactation.
* Contained 20 % of canola meal, 30 % of maize gluten meal, 20 % of soyabean meal and 30 % of brewer's maize.
† The premix contained (per kg of premix): 90·2 g Ca, 49·0 P, 48·9 g Mg, 17·6 g S, 140 g Na, 14·3 g K, 2068 mg Fe, 2718 mg Zn, 447 mg Cu, 1814 mg Mn, 69 mg I, 7 mg Co, 20 mg Se, 452 000 IU (473·6 μmol/l) of vitamin A, 58 000 IU (3619200 nmol/l) of vitamin D3 and 2692 IU (41951·8 μmol/l) of vitamin E.
‡ Calculated using published values of feed ingredients (National Research Council, 2001).
Sampling
Each experimental period consisted of 21 d with 7 d adaptation to the diets and 14 d of infusion. Cows were milked twice a day at 08.00 and 19.00 hours. All cows were fed for ad libitum intake (10 % refusals on an as-fed basis) twice a day (08.30 and 14.30 hours). Feed intake and milk yield were measured daily throughout the experiment. To perform abomasal infusions, an infusion line was inserted through the rumen cannula and the sulcus omasi into the abomasum as described by Gressley et al. (Reference Gressley, Reynal and Colmenero17). Placement of the infusion lines was monitored daily to ensure post-ruminal delivery. Oil was pumped into the abomasum by using peristaltic pumps (Masterflex L/S; Cole-Parmer Canada, Inc.). Samples of the diets were taken daily from day 15 to day 20 and pooled within period for each cow. All samples were frozen at − 20°C for subsequent drying at 55oC and analysed according to the procedures used by Côrtes et al. (Reference Côrtes, da Silva-Kazama and Kazama18). Milk samples were taken twice daily from day 15 (afternoon milking) to day 21 (morning milking) and pooled on a 6 d basis proportionally to the corresponding milk yield. Milk samples were kept frozen at − 20°C without preservative for chemical analysis.
On day 20, a sample of urine was taken 2 h after the morning meal by hand stimulation of the perineal region and kept frozen at − 20oC for analysis of the mammalian lignan EL. Blood sample from each cow was also collected on day 20 of each period from the jugular vein into 10 ml K3EDTA-vacuum tubes (Becton Dickinson and Cie) and centrifuged at 5000 g for 20 min at 4°C. Plasma and erythrocytes were removed and stored at − 20°C for further analysis of antioxidant enzyme activities and EL. On day 21, biopsies were taken from the upper portion of the mammary gland on the last day of each period using the method of Farr et al. (Reference Farr, Stelwagen and Cate19) and alternating between the left and right hindquarters. Although inflammation was restricted to a very small area and disappeared within 2 d, a site at least 10 cm apart from the first one was chosen when a quarter was used for the second time. Tissue obtained from the biopsies was rinsed in sterile saline solution to remove all traces of blood, cut into two parts: one part was immediately frozen in liquid N2 and stored at − 80°C for further analysis of relative mRNA abundance of antioxidant enzymes. The second half of the sample was ground immediately and used to determine antioxidant enzyme activity according to the manufacturer's instructions of each commercial assay kit.
Chemical analyses
DM of the diets was determined in a forced-air oven according to AOAC procedure 934.01(20). The acid-detergent fibre content in diets was determined according to the AOAC(20) (method 973.18). The neutral-detergent fibre and acid-detergent fibre procedures were adapted for use in an ANKOM200 Fibre Analyzer (ANKOM Technology Corporation). Total N content of diets was determined by thermal conductivity (LECO model FP-428 Nitrogen Determinator, LECO). Diethyl ether extraction of diets was conducted according to method no. 7.060(20). The activity of GPX, SOD and CAT in plasma, erythrocytes and mammary gland was determined enzymatically. Activity of GPX was determined at 340 nm using a coupled-enzymatic assay (kit 703102; Cayman Chemical) in which oxidation of NADPH was monitored spectrophotometrically, whereas reductase activity was determined using H2O2 as a substrate. CAT activity (kit 707002, Cayman Chemical) was determined by measuring the rate of disappearance of H2O2 at 540 nm. Activity of GPX and CAT was expressed as nmol/min per g (in erythrocytes and the mammary gland) or per mg (in plasma) of total protein. Activity of total protein. Activity of SOD (kit 706002, Cayman Chemical) was determined by measuring the production of superoxide radicals by xanthine oxidase and hypoxanthine at 440 nm. Here, one unit of activity was defined as the amount of enzyme needed to exhibit 50 % dismutation of the superoxide radical. Total protein concentration in plasma, erythrocytes and mammary gland was determined with the bicinchoninic acid protein assay (kits BCA1 and B 9643; Sigma-Aldrich) that relies on the formation of a Cu2+–protein complex under alkaline conditions, followed by reduction of the Cu2+ to Cu1+. Concentration of EL in milk and plasma was analysed as described previously by Gagnon et al. (Reference Gagnon, Côrtes and Da Silva10).
RNA extraction and complementary DNA synthesis
Extraction of total RNA from mammary gland tissue and complementary DNA synthesis was performed as previously described(Reference Labrecque, Beaudry and Mayhue21).
Real-time PCR amplifications of studied genes
The relative mRNA abundance of studied genes was determined using real-time PCR amplifications. Primers were designed with the Primer Express software 3.0 (PE Applied BioSystems). The PCR amplifications were performed in a 10 μl reaction volume consisting of corresponding concentrations of forward and reverse primers (Table 2), 5 μl of 2 × Power SYBRGreen Master Mix (PE Applied BioSystems), 0·2 μl of complementary DNA and 0·05 μl of AmpErase (PE Applied BioSystems). Cycling conditions were 2 min at 50°C, followed by 10 min at 95°C and forty cycles of 3 s at 95°C and 30 s at 60°C. Amplification, detection and data analysis were performed with an ABI 7500 Fast Real-Time PCR System (PE Applied BioSystems). Specificity of amplified fragments was verified with the melting curve analysis. Following this, three reference genes were also amplified to identify the one that was the least affected by treatments. These reference genes are glyceraldehyde-3-phosphate dehydrogenase, peptidylprolyl isomerase A and actin-β. Amplifications were performed in triplicate. Data were analysed using the comparative C t method, by which the amount of target (studied gene) normalised to a reference gene (actin-β for the present study) and relative to a calibrator (known individual value) is calculated as 2− ΔΔC t, as described in the User Bulletin #2 (Applied Biosystems 1997; ABI PRISM 7700 Sequence Detection System).
Table 2 Primer sequences used for real-time PCR amplifications of selected genes in mammary gland biopsies

CAT, catalase; F, forward; R, reverse; SOD, superoxide dismutase; GPX, glutathione peroxidase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PPIA, peptidylprolyl isomerase A; ACTB, actin-β.
Determination of amplification efficiency
Amplification efficiency for each gene was determined using a standard curve. A pool of mammary gland complementary DNA from all biopsies collected (n 32) was used to prepare the different dilutions (1, 5, 10, 20, 50, 100, 150 and 200 ng) needed for construction of the standard curve. PCR were performed in duplicate and repeated twice (on different days) as described previously and amplification efficiency for each studied gene was determined according to Cikos et al. (Reference Cikos, Bukovska and Koppel22).
Statistical analysis
All results were analysed using the MIXED procedure of SAS (SAS 2000; SAS Institute) within a 2 × 2 factorial arrangement of treatments with flax oil (0 v. 500 g flax oil/d) and flax hulls (0 v. 9·88 % flax hulls) as fixed effects. Data on feed intake were averaged over the last 6 d of each period, and were subjected to ANOVA. Data on enzyme activity and feed intake were analysed using a replicated 4 × 4 Latin square design with the following general model:
where Y ijkl, the dependent variable; μ, overall mean; Si, fixed effect of square (i = 1–2); Cj(i), random effect of cow within square (j = 1–4); Pk, fixed effect of period (k = 1–4); Hl, fixed effect of Hull treatment, O m = fixed effect of oil treatment; HOlm, interaction; eijklm, residual error. Data on mRNA abundance were analysed using the normalised quantities (as shown previously) and are presented as relative quantification of mRNA abundance using the comparative C t method comparing all treatment combinations to the CONT diet, used here as reference. Data on EL concentrations were transformed (log) as previously performed by Hausner et al. (Reference Hausner, Johnsen and Hallund23) and Nesbitt et al. (Reference Nesbitt, Lam and Thompson24), but results in Table 3 are reported as adjusted mean values (with CI) on the original scale of measurements. The compound symmetry was used as the covariance structure. The two-sided level of significance was set at P ≤ 0·05, although probability values up to P < 0·10 are shown in the text if the data suggest a trend. Unless otherwise noted, results are reported as least square means with their standard errors.
Table 3 Concentration of enterolactone (EL) in plasma and milk of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g flax oil/d in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g flax oil/d in the abomasum (HUFO)(Adjusted mean values with their 95 % confidence intervals on the original scale of measurements)
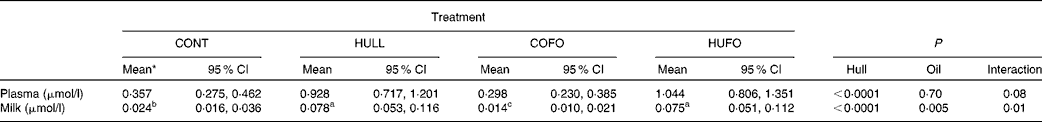
a,b,c Adjusted mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Data on EL concentrations were log-transformed for statistical analyses to represent a normal distribution.
Results
DM intake
There was no interaction between flax hull supplementation and oil infusion for DM intake. Intake of DM, expressed in kg/d, averaged 18·9 and was similar among treatments (data not shown). However, DM intake, expressed as a percentage of body weight, was lower (P = 0·05) for cows infused with flax oil than for those not infused (2·64 and 2·82, respectively).
Concentration of enterolactone in plasma and milk
Concentration of EL in plasma was significantly higher for cows fed HULL and HUFO than for those fed CONT and COFO (Table 3). There was an interaction (P = 0·01) between flax hull supplementation and flax oil infusion for milk EL concentration; the highest concentrations were observed for cows fed HULL and HUFO and the lowest were obtained for cows fed COFO, with cows fed CONT having intermediate values.
Enzyme activity in plasma, mammary gland and erythrocytes
Plasma activity of CAT and SOD was similar among diets and GPX activity decreased with flax oil supplementation (Table 4). The activity of CAT in the mammary gland tended (P = 0·07) to increase with oil infusion, although the effect tended (P = 0·10) to be more pronounced in the absence than in the presence of hulls in the diet as suggested by the interaction. There was no effect of treatment on GPX activity in the mammary gland, but SOD activity tended (P = 0·09) to be higher with flax hull supplementation. Enzyme activity in erythrocytes was similar among treatments.
Table 4 Activity of antioxidant enzymes in plasma, mammary gland and erythrocytes of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g flax oil/d in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g flax oil/d in the abomasum (HUFO) (Mean values with their standard errors)

CAT, catalase; GPX, glutathione peroxidase; SOD, superoxide dismutase.
Relative quantification of antioxidant enzyme mRNA in the mammary gland
Cows fed the HULL diet had higher levels of CAT, GPX1 and SOD1 mRNA in the mammary gland and lower mRNA abundance of GPX3, SOD2 and SOD3 compared with those fed the CONT treatment (Table 5). Abundance of CAT, GPX1, GPX3, SOD2 and SOD3 mRNA was down-regulated in the mammary gland of cows fed HUFO compared to those fed CONT. Infusion of 500 g flax oil/d in the abomasum of cows fed the control diet (COFO) decreased the mRNA abundance of CAT, GPX1, GPX3 and SOD3 in the mammary gland when compared with the CONT treatment.
Table 5 Relative quantification (RQ) of antioxidant enzyme mRNA in the mammary gland of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g flax oil/d in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g flax oil/d in the abomasum (HUFO)
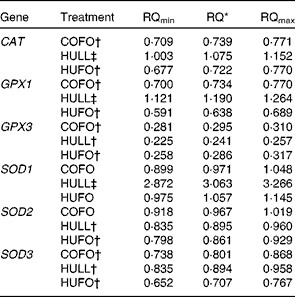
CAT, catalase; GPX, glutathione peroxidase; SOD, superoxide dismutase.
* RQ of mRNA abundance using the comparative cycle threshold method and the control (CONT) treatment as reference. Values represent ‘fold’ values relative to CONT with their corresponding RQmin and RQmax.
† Down-regulated genes (P < 0·05).
‡ Up-regulated genes (P < 0·05).
Discussion
Many studies have reported that feeding PUFA enhances fertility(Reference Petit and Twagiramungu25, Reference Ambrose, Kastelic and Corbett26) and modulates the immune status(Reference Lessard, Gagnon and Petit27, Reference Sordillo, Contreras and Aitken28) of dairy cows. However, PUFA may also increase the susceptibility of plasma and tissues to peroxidation as shown in rats(Reference Frémont, Gozzélino and Franchi29) and steers(Reference Scislowski, Bauchart and Gruffat2), which may result in impaired health of animals. The present experiment was specifically designed to investigate the ability of flax lignans to increase the activity of enzymes and expression of genes involved in the metabolism of antioxidants in cows subjected to oxidative stress. To this effect, abomasal infusion of flax oil was performed to increase the susceptibility of tissues to lipoperoxidation, as previously carried out by Gladine et al. (Reference Gladine, Rock and Morand3). However, infusing flax oil decreased intake of DM, expressed as a percentage of body weight. Previous studies(Reference Choi and Palmquist30, Reference Litherland, Thire and Beaulieu31) have reported that increased concentrations of TAG and free FA in the small intestine are correlated with increased concentrations of gut hormones such as cholecystokinin and glucagon-like peptide-1 in the plasma, which may result in satiety and reduction of gut motility and decreased DM intake.
Antioxidant supplementation has increased GPX activity in the liver of rats(Reference Alía, Horcajo and Bravo32) and depression in GPX activity has been observed in tissues during sickness such as diabetes as a result of important adaptive response to conditions of increased peroxidative stress(Reference Ananthan, Latha and Ramkumar33). In this context, plants rich in antioxidant compounds could be employed as a prophylactic strategy against metabolic diseases (e.g. ketosis) in dairy herds. Indeed, ketotic dairy cows present clinical signs similar to those observed in diabetic people, such as high concentrations of plasma NEFA and β-hydroxybutyrate(Reference Bernabucci, Ronchi and Lacetera4). Moreover, previous studies have shown that the antioxidant properties of plant extracts can be achieved by the activation of antioxidant enzymes(Reference Ananthan, Latha and Ramkumar33) as observed for mammary gland SOD activity in the present experiment. Similarly, higher SOD activity in the rumen of cows fed antioxidants has been reported 2 h after feeding(Reference Aoki, Furukawa and Sato34).
SOD is the first enzyme involved in the conversion of oxygen radicals to peroxides(Reference Yu35). SOD activity in plasma of dairy cows has been shown to increase with antioxidant supplementation, but the effect was dependent on the type of fat fed, with the highest activity being observed when oxidised fat was added to the diet(Reference Vázquez-Añón, Nocek and Bowman36). In the present experiment, SOD activity and SOD1 mRNA abundance in the mammary gland increased with the addition of flax hulls in the diet, whereas there was no effect of abomasal infusion of flax oil. Flax hull is one of the richest sources of the plant lignan secoisolariciresinol diglucoside, which is metabolised by the ruminal microbiota to produce the mammalian lignan EL(Reference Gagnon, Côrtes and Da Silva10). Using different in vitro systems, it has been demonstrated that both lignan metabolites, EL and enterodiol, have hydroxyl and peroxyl radical-scavenging activities that can make an impact on antioxidant status(Reference Kitts, Yuan and Wijewickreme37, Reference Hu, Yuan and Kitts38). Mammalian lignans may also act directly on endogenous antioxidant enzyme systems. In fact, flax lignans are composed of polyphenolic compounds present in plants as glycoside conjugates(Reference Hu, Yuan and Kitts38) and many plant-derived phenolic and polyphenolic compounds have demonstrated antioxidant properties(Reference Morton, Abu-Amsha Caccetta and Puddey39). It has also been reported that different phenolic acids can induce mRNA abundance of SOD1, GPX and CAT genes in the liver of rats and that the nuclear factor (erythroid-derived 2)-like 2 may play a key role in this induction(Reference Yeh and Yen40). The transcription factor nuclear factor (erythroid-derived 2)-like 2 recognises antioxidant response elements in the promoter regions of target genes, including antioxidant encoding genes(Reference Kaspar, Niture and Jaiswal41). Of interest, SOD1 has an antioxidant response elements consensus sequence in its promoter region, whereas SOD2 and SOD3 do not(Reference Miao and St Clair42), which may explain that SOD1 transcript abundance was higher with HULL. This suggests that the different response to the HULL treatment between transcript abundance of SOD1 and that of SOD2 and SOD3 may result from the absence of the antioxidant response elements consensus sequence in the promoter region of SOD2 and SOD3. Feeding HULL also increased the abundance of GPX1 and CAT mRNA, which are two genes known to be nuclear factor (erythroid-derived 2)-like 2 downstream targets(Reference Yeh and Yen40, Reference Dreger, Westphal and Weller43).
In the present study, the mRNA abundance of SOD2, SOD3 and GPX3 was lower when cows were fed HULL and HUFO and mRNA levels of CAT, GPX1 and SOD1 were lower or not affected by feeding HUFO compared to when cows were fed CONT. This down-regulation or absence of effect on antioxidant enzymes when flax hulls were added to the diet may be related to the hydroxyl and peroxyl radical-scavenging activities of the polyphenolic compounds present in mammalian lignans(Reference Kitts, Yuan and Wijewickreme37, Reference Hu, Yuan and Kitts38). Indeed, these polyphenols may contribute to the reduction of the cellular hydroxyl and peroxyl radical load, thus decreasing the need for antioxidant enzymes. A similar observation was reported in the liver of rats that were fed polyphenol-rich apple juice, where the expression of many antioxidant genes was down-regulated or not affected by treatments(Reference Soyalan, Minn and Schmitz44).
The reason for the lower mRNA abundance of CAT, GPX1, GPX3 and SOD3 genes of cows fed the COFO diet is unknown. An earlier study has reported that n-3 PUFA enhances serum and spleen homogenate peroxides and activities of SOD, GPX and CAT in the spleen of mice(Reference Reddy Avula and Fernandes45). Similarly, rats fed a diet rich in fish oil had higher SOD1, CAT and GPX hepatic activities(Reference Ruiz-Gutiérrez, Pérez-Espinosa and Vázquez46). However, the opposite also has been reported, with reduced antioxidant activities in animals fed diets enriched with n-3 PUFA. For example, rats fed linseed oil or fish oil supplements had higher lipid peroxidation and CAT activity in the liver, whereas GPX activity was decreased(Reference Ramaprasad, Baskaran and Krishnakantha47). Similarly, rats fed a 10 % fish oil supplement had higher CAT activity and lower SOD and GPX activities in the liver(Reference Venkatraman, Angkeow and Satsangi48). Myocardial enrichment in n-3 PUFA was also associated with decreased SOD and GPX activities in the heart of rats(Reference Schimke, Haberland and Wirth49). Moreover, treating DHL-4 cells, a human cancer cell line, with the n-3 FA DHA increased lipid peroxidation although this was associated with a decrease in SOD1 transcript abundance(Reference Ding, Vaught and Yamauchi50). Altogether, these results may demonstrate that n-3 PUFA can either increase or decrease antioxidant activities in different tissues and cell lines.
In the present study, there was a tendency for increased CAT activity when flax oil was infused in the abomasum, whereas the inverse was observed for CAT mRNA abundance in the mammary gland. Moreover, there was no effect of flax oil on GPX and SOD activities, while the mRNA abundance of GPX1, GPX3 and SOD3 genes was lower in the mammary gland of cows fed the COFO diet. Differences between activity and mRNA abundance in response to dietary treatments may be explained in part by the fact that activity of all GPX and SOD isoforms was considered, whereas the different isoforms (e.g. GPX1, GPX3, SOD1, SOD2 and SOD3) were discriminated at the transcript levels.
In the present experiment, differences were observed in the activity of antioxidant enzymes between the different tissues. Indeed, there was no effect of treatment on the activity of enzymes in erythrocytes, whereas an effect of flax hulls on SOD and flax oil on CAT activity was observed in the mammary gland. Flax oil also had an effect on GPX activity in plasma, but not in the mammary gland and erythrocytes. Discrepancies between the effects of treatment on enzyme activities might be explained by differences in bioavailability and absorption of the polyphenolic metabolites and flax oil among tissues. Similar differences in tissue absorption and elimination of tea polyphenols were observed in rats(Reference Chen, Lee and Li51) and mice(Reference Kim, Lee and Hong52). Moreover, the protective effect of antioxidants has been highlighted only in the most ‘sensibilised’ tissues(Reference Gladine, Morand and Rock53) and lipoperoxidation has been shown to differ among tissues (e.g. more important in the liver than in the Longissimus thoracis).
GPX is the enzyme involved in the second step of removing the peroxides produced by SOD enzyme and converting them into water(Reference Yu35). Plasma GPX activity was increased by antioxidant supplementation when dairy cows were fed diets containing 2 % of fresh non-stabilised or stabilised soyabean oil(Reference Vázquez-Añón, Nocek and Bowman36). It might be required for both enzymes (SOD and GPX) to be active for the adequate removal of end products of oxidation and to result in enhancement of the antioxidant status of animals(Reference Collins54). The mechanism by which flax hull increases the activity of antioxidant enzymes in plasma and the mammary gland is not clear. It is possible that flax lignans, by removing reactive oxygen molecules and end products of oxidation from the diet and digesta, could reduce the load of peroxides coming into the animal. As a result, lower input of peroxides would have a saving effect on the endogenous antioxidant defence system. Flax products are rich in plant lignans, which are strong antioxidants(Reference Martin, Haourigui and Pelissero55), and feeding flax hulls increased plasma and milk concentrations of EL in the present experiment. Similar increases in EL concentrations have been reported following the conversion of plant lignans into the mammalian lignan EL by ruminal microbiota(Reference Gagnon, Côrtes and Da Silva10). However, contrary to the results of Gagnon et al. (Reference Gagnon, Côrtes and Da Silva10), abomasal infusion of flax oil decreased milk EL concentration when cows were fed flax hulls in the present experiment, which was observed in parallel with the lower activity of CAT in the mammary gland. The mammalian lignan EL is known for its antioxidant properties(Reference Kitts, Yuan and Wijewickreme37). Therefore, EL could modulate the immune status of dairy cows through the activation of enzymes involved in the antioxidant defence system(Reference Kitts, Yuan and Wijewickreme37). Moreover, increases in milk EL concentration may contribute to enhanced milk composition for better human health. Indeed, a Finnish study has shown a decrease in the incidence of CVD with greater blood concentration of EL in human subjects(Reference Vanharanta, Voutilainen and Lakka11). However, it is unknown if the health effects of mammalian lignans are similar in dairy cows. Further investigations are required to better understand the impact of increasing mammalian lignans in biological fluids on reproduction, lactation and health of dairy cows.
In conclusion, the present study shows that supplementing dairy cow diets with flax products high in n-3 PUFA and lignans has no effect on the activity of antioxidant enzymes in erythrocytes. However, n-3 PUFA supplementation tends to decrease GPX activity in plasma. In the mammary gland, the activities of CAT and SOD, which are responsible for the removal of free radicals leading to oxidative stress, were modulated by n-3 PUFA and lignans, respectively. Moreover, SOD activity and SOD1 mRNA abundance in the mammary gland increased with the addition of flax hulls in the diet, which was related to the scavenging activities of the polyphenolic compounds present in mammalian lignans. Therefore, flax hulls may contribute to increasing the mammalian lignan EL in milk and the abundance of some antioxidant genes in the mammary gland, which can contribute to protect against oxidative stress damage occurring in the mammary gland and other tissues of dairy cows.
Acknowledgements
C. C. was a recipient of a fellowship from the National Science and Engineering Research Council of Canada. The present study was funded by Agriculture and Agri-Food Canada. The authors would like to express their gratitude to the staff of the Dairy & Swine Research & Development Centre for their contribution to the present study. The authors especially thank Véronique Roy, Liette Veilleux, Danielle Beaudry and Sylvie Dallaire for technical assistance and Steve Méthot for his help in the statistical analyses. None of the authors had a personal or professional conflict of interest. The authors' contributions to the present study were as follows: C. C., M.-F. P. and H. V. P. drafted the manuscript. H. V. P. and M.-F. P. conceived and directed the study. C. C. coordinated the study and was in charge of the infusions and of collecting data from animals. N. G. performed the laboratory work on enzyme and antioxidant activities. C. B. contributed to the conception and design of the experiment and to the interpretation and discussion of the results. P. L. performed the mammary biopsies. All authors were involved in revising the paper critically and approved the final version of the paper.







