Excessive accumulation of lipids in the adipose tissue of modern broiler strains is a major concern for producers, because most of the fat depots are lost during evisceration of the carcass or processing of the meat, leading to lower meat yields. Studies in vitro and with intact chicks have demonstrated that liver is the major site of lipid biosynthesis in the chicken( Reference Leveille, Romsos and Yeh 1 , Reference Saadoun and Leclercq 2 ). Most fatty acids are synthesised in the liver and transported via LDL for storage in adipose tissues as TAG in chickens and other avian species( Reference Hermier 3 ). Fatty acid synthase (FAS) and malic enzyme (ME) are two enzymes necessary for de novo synthesis of long-chain fatty acids, and the activities of these enzymes are correlated with the rate of fatty acid synthesis in the livers of animals( Reference Morris, Winberry and Fisch 4 ). Hepatic FAS and ME activities and their mRNA expression levels were also correlated with the proportion of abdominal fat of broilers( Reference Whitehead, Hood and Heard 5 , Reference Yang 6 ). It was reported that the expression levels of hepatic FAS and ME mRNA correlated positively with the rate of synthesis of their respective enzyme proteins, indicating that the nutritional regulation of these enzyme levels is primarily at pre-translational steps( Reference Morris, Winberry and Fisch 4 , Reference Morris, Nilson and Jenik 7 , Reference Goodridge, Jenik and Mcdevitt 8 ).
Mn is an essential trace element for animals. It is involved in the metabolism of carbohydrates, amino acids, protein and lipid as a constituent or activator of numerous enzymes( Reference Erikson, Syversen and Aschner 9 ). The current National Research Council( 10 ) Mn requirement for broilers is 60 mg/kg, but this requirement is primarily based upon studies conducted more than 60 to 70 years ago with birds of markedly different productive potentials from modern birds today and semi-purified diets( Reference Wilgus, Norris and Heuser 11 – Reference Gallup and Norris 13 ). Results from our previous study( Reference Luo, Su, Huang and Liu 14 ) indicated that dietary Mn of 120 mg/kg was a satisfactory level for the growth performance, tissue Mn concentrations and Mn-containing superoxide dismutase (MnSOD) activity in heart of broilers fed a practical conventional diet from 1 to 28 d of age. More recent research findings indicate that the optimal Mn requirements for starter and grower broilers fed practical conventional diets are about 130 and 100 mg/kg, respectively( Reference Li, Lin and Lu 15 , Reference Lu, Chang and Liao 16 ), which are much higher than the value (60 mg/kg) recommended by National Research Council( 10 ). It is reported that supplemental Mn in the diet of pigs reduced the amount of fat deposited in the carcass( Reference Plumlee, Thrasher and Beeson 17 , Reference Atherton 18 ). Some previous studies from our laboratory have demonstrated that dietary Mn supplementation (100 mg/kg) decreased abdominal fat deposition of broilers, and Mn amino acid with a moderate chelation strength (Mn–AA) was as effective as manganese sulfate (MnSO4.H2O)( Reference Lu, Ji and Luo 19 , Reference Lu, Luo and Ji 20 ). These results suggest that feeding a normal level of Mn would decrease fat synthesis and body fat content. Our further study indicated that the addition of 100 mg/kg Mn as either MnSO4 or Mn–AA to the diets decreased the activities of FAS and ME, and mRNA expression of ME in the liver of broilers, and no differences were detected in these parameters between birds fed diets supplemented with MnSO4 and Mn–AA( Reference Wang, Chen and Lu 21 ). However, the above results are all obtained from in vivo studies, and might have been affected by other factors, such as feed intake, other dietary factors, environmental factors and so on. Whether supplemental Mn could directly regulate FAS and ME activities and their gene expressions in primary chicken hepatocytes, as well as the underlying relationship between the regulation of the two enzymes and supplemental Mn sources, remain unknown.
The aim of the present study was to investigate the effects of Mn source and Mn level on FAS and ME activities and their mRNA expressions in in vitro-cultured primary chicken hepatocytes in order to explore whether Mn could directly regulate the activities and mRNA expressions of FAS and ME.
Methods
Isolation and cultivation of primary chick hepatocytes
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS–CAAS, Beijing, China), and performed in accordance with the guidelines. Ethical approval on animal survival was given by the animal ethics committee of IAS–CAAS. Hepatocytes were isolated from the liver tissue of 22-d-old male Arbor Acres broilers (Huadu Broiler Breeding Corp.) fed the Mn-deficient maize–soyabean meal diet (containing 19·69 mg Mn/kg by analysis) during a period of 1 to 21 d of age to deplete the body Mn storage. In a previous study from our laboratory( Reference Li, Luo and Liu 22 ), when broilers were fed an Mn-deficient diet containing 23 mg Mn/kg for 21 d, they showed a moderate Mn deficiency as reflected by the increased incidence of leg abnormality, and decreased bone Mn, heart Mn and MnSOD activity. In the present study, the Mn-deficient diet contained a lower Mn level as mentioned above; therefore, the chickens should be in Mn deficiency. The hepatocytes isolated from birds fed an Mn-deficient diet could be Mn-deficient, and more sensitive to Mn supplementation. Hepatocyte isolation was performed by using the collagenase perfusion method as described previously( Reference Fraslin, Touquette and Douaire 23 ), with some modifications. Isolated cells were suspended in 40 ml of L-15 medium (Invitrogen) supplemented with 10 % fetal bovine serum (FBS), 10 μg/ml of transferrin, 10 μg/ml of vitamin C, 10−6 m dexamethasone, 10−6 m insulin and 1 % antibiotics (100 IU/ml of penicillin and 100 mg/ml of streptomycin) at 4°C. Purification of hepatocytes was performed as described by Wu et al.( Reference Wu, Huang and Wei 24 ). The viability of the isolated hepatocytes was 95 (sd 1·3) % (n 6), which was analysed by the 0·4 % trypan blue dye exclusion method, and the cell yield from each liver preparation was 2·0 (SD 1·0)×109 hepatocytes (n 6). Evaluation of the cells by using light microscopy showed that about 95 % of the collected cells were hepatocytes.
The hepatocyte cultures were performed according to the method described by Fujii et al.( Reference Fujii, Yoshino and Suzuki 25 ), with some modifications. Hepatocytes were seeded into six-well plates (Corning Life Sciences) at a density of 1·5×106 viable cells per well in 1·5 ml of the above-mentioned L-15 medium. Cells were cultured at 37°C in a humidified incubator (Model 3110 series; Thermo Electron Corporation) with 5 % CO2 and 95 % air. After a plating period of 6 h, cell monolayers were washed twice with ice-cold PBS, and 3 ml of fresh L-15 medium (supplemented with 10 % FBS and 1 % antibiotics (100 IU/ml of penicillin and 100 mg/ml of streptomycin)) was added to each well. After about 20 h of incubation in addition to the above plating period of 6 h, these cells were used for the following experiments. In each experiment, hepatocytes were isolated individually from six replicate broilers per treatment and the cells from each replicate broiler were plated into six wells.
A preliminary experiment to evaluate the viability of chick hepatocytes
This experiment was conducted to measure the efflux of lactic dehydrogenase (LDH) from the hepatocytes treated with various amounts of Mn in order to find the optimal Mn supplementation range.
At the end of the above incubation, the culture medium of the cells was removed, and hepatocyte monolayers were washed three times with ice-cold PBS. Next, 3 ml of serum-free L-15 medium (supplemented with 1 % antibiotics (100 IU/ml of penicillin and 100 mg/ml of streptomycin)) was added to each well, and the hepatocytes were treated with 0, 0·02, 0·20, 0·50 and 1·00 mmol/l of Mn as inorganic manganese chloride (MnCl2.4H2O) (reagent grade; Beijing Biochemical Reagent Company) for 24- and 48-h incubation. After 24- or 48-h incubation, the culture medium was collected into 2-ml Eppendorf tubes, and then stored at –20°C. The activities of LDH were analysed according to the instructions in the commercial kits (Nanjing Jiancheng Bioengineering Institute). One unit of enzyme activity was defined as 1 mmol of reduced nicotinamide adenine dinucleotide oxidised per min. The LDH activity in the culture medium was expressed as units per litre (U/l). All of the samples were measured in duplicate.
Cell treatments
Expt 1 was performed to investigate the effect of supplemental Mn concentration on the activities and mRNA expression of FAS and ME in the hepatocytes at a different incubation time. Our preliminary experiment indicated that cells treated with about 0·50 mmol/l of Mn as long as 24 or 48 h had the best viability. Therefore, in Expt 1, the hepatocytes were incubated in fresh serum-free L-15 medium, and treated with 0 (control), 0·25, 0·50 or 0·75 mmol/l of Mn as manganese chloride for 24 or 48 h. The FAS and ME mRNA levels and enzymatic activities were determined after Mn treatments for 24 or 48 h.
Expt 2 was performed to evaluate the effect of supplemental Mn source and concentration on the activities and mRNA expression of FAS and ME in the primary hepatocytes. The results in Expt 1 indicated that the Mn treatment affected FAS and ME mRNA expression only at 48 h, and the dose of 0·25 or 0·50 mmol/l of Mn was effective in decreasing the mRNA expression of the two enzymes. Therefore, in Expt 2, the cells were incubated in the fresh serum-free L-15 medium, and treated with 0 (control), 0·25 or 0·50 mmol/l of Mn as manganese chloride or Mn–AA with a moderate chelation strength (quotient of formation (Q f)=16·85 between 10 and 100, containing Mn 9·06 % and total amino acids 29·09 %; Sanbao Additive Company) for 48 h. The Q f is a quantitative measurement of chelation strength between Mn and amino acids according to the shift in half-wave potential (E 1 / 2) in polarography, as described by Holwerda et al.( Reference Holwerda, Albin and Madsen 26 ) and Li et al.( Reference Li, Luo and Liu 22 ). Lysine or Met concentration in each treatment was balanced by supplementation of additional l-lysine monohydrochloride or dl-methionine. The FAS and ME mRNA levels and enzymatic activities in hepatocytes, as well as the LDH activities in the medium, were determined after Mn treatments for 48 h.
Sample collections and preparations
At the end of Expt 1, the medium was removed and the cell monolayer was washed three times with ice-cold PBS. The cells in the first two wells for each replicate were scraped in ice-cold 0·1 mol/l of potassium phosphate (pH=7·0 (sd 0·1)), and then sonicated at 4°C for 4 min (1 s with 10-s intervals). Lysates were centrifuged at 8000 g for 15 min at 4°C to harvest the supernatant to a new centrifuge tube, and then the supernatant was centrifuged at 20 000 g for 50 min at 4°C. The supernatant was collected for FAS activity analysis. The cells in the next two wells for each replicate were scraped in ice-cold saline, and then sonicated at 4°C for 4 min (1 s with 10-s intervals). Lysates were centrifuged at 2000 g for 10 min at 4°C to harvest the supernatants for ME activity analysis. The cells in the other two wells for each replicate were scraped in TRIzol reagent (Life Technologies) and frozen (–80°C) for FAS and ME mRNA expression analysis.
At the end of Expt 2, the culture medium in the first well for each replicate was collected and stored at –20°C for the subsequent analysis of LDH activities. In addition, the cells in the second and third wells and the other three wells for each replicate were collected and prepared according to the procedures mentioned in Expt 1 for the subsequent analyses of FAS and ME activities and mRNA expression levels, respectively.
Analysis of enzymatic activities
The measurement of LDH activity in the incubation medium was performed as described in the above preliminary experiment. The protein concentrations in supernatants were determined by BCA protein assay kit (catalogue no. 233225; Thermo). The FAS activity was measured as described by Tian et al.( Reference Tian, Hsu and Wang 27 ). The ME activity was determined by the modified method of Hsu and Lardy( Reference Hsu and Lardy 28 ). The enzymatic activities were expressed as units (U) per mg of protein (U/mg prot.). One U of FAS activity is noted as µmol of oxidised NADPH per min per mg of protein at 37°C. One U of ME activity is defined as µmol of reduced NADP per min per mg of protein at 30°C. All of the samples were measured in duplicate.
Determinations of fatty acid synthase and malic enzyme mRNA expression levels
The concentrations of total RNA were estimated by measuring ultraviolet light absorbance at 260 nm with a spectrophotometer (NanoDrop 2000; Gene Company Ltd). In brief, complementary DNA was synthesised using the SuperScript_III First-Strand Synthesis for RT-PCR kit (Invitrogen) with DNA Engine PCR instrument (Bio-Rad). The FAS and ME mRNA expression levels were determined by relative quantitative real-time PCR using the power SYBR® Green PCR mater mix kit (catalogue no. 4367659; Applied Biosystems) with ABI Prism 7500 (Applied Biosystems) as described by Li et al.(
Reference Li, Lin and Liao
29
). The information of primers was listed in Table 1. The β-actin was used as the internal reference to normalise the FAS and ME mRNA expression levels using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
30
,
Reference Zhu, Lu and Li
31
). All of the samples were measured in duplicate.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
30
,
Reference Zhu, Lu and Li
31
). All of the samples were measured in duplicate.
Table 1 Primer sequences for real-time PCR amplification
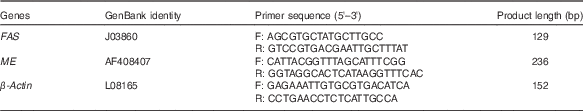
FAS, fatty acid synthase; ME, malic enzyme; F, forward; R, reverse.
Statistical analyses
The data in the preliminary experiment and Expt 1 were analysed by one-way ANOVA using the general linear model (GLM) procedure of the SAS 9.2 (SAS Institute Inc.). Orthogonal polynomials were used to assess linear and quadratic responses to added Mn levels. In addition, regression analysis was used to fit the data in the preliminary experiment to determine the optimal added Mn concentration in the medium. Data in Expt 2 were analysed using single degree of freedom contrast to compare all supplemental Mn treatments with the control( Reference Suo, Lu and Zhang 32 , Reference Zhang, Lu and Luo 33 ). Data excluding the control were analysed by two-way ANOVA using the GLM procedure (SAS Institute Inc.). The model included the effects of Mn source, supplemental Mn concentration and their interaction. The replicate served as the experimental unit. If the variances were significant, differences among means were tested by the LSD method, and the statistical significance was set at P≤0·05.
Results
Effect of supplemental manganese concentration on the lactic dehydrogenase release (preliminary experiment)
The addition of Mn significantly affected (P<0·0001) LDH activities (Table 2) in the medium after incubation for 24 and 48 h. The LDH activities decreased linearly (P<0·003) and quadratically (P<0·0001) as Mn concentrations increased at either 24 or 48 h, and the lowest LDH activity was at a supplemental Mn concentration of 0·50 mmol/l. On the basis of the fitted quadratic models of LDH activities with added Mn concentrations (24 h: Y=610·5−1271·6X+1110·3X 2, R 2=0·8194, P<0·0001; 48 h: Y=1268·7−1623·1X+1441·7X 2, R 2=0·6475, P<0·0001), the optimal added Mn concentrations were 0·57 mmol/l at 24 h and 0·56 mmol/l at 48 h, respectively.
Table 2 Effect of supplemental manganese concentration on the activities of lactic dehydrogenase (LDH) in the culture medium of chicken hepatocytes at a different incubation time (preliminary experiment)
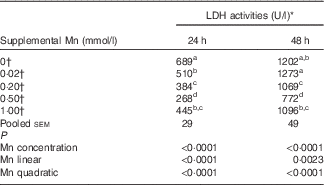
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P<0·0001).
* One unit of LDH activity was defined as 1 mmol of reduced nicotinamide adenine dinucleotide oxidised per min.
† Data represent the means of six replicates (n 6).
Effect of supplemental manganese concentration on the activities and mRNA expression levels of fatty acid synthase and malic enzyme (Expt 1)
Mn supplementation did not influence (P>0·17) the FAS and ME activities in chicken hepatocytes at 24 and 48 h, and their mRNA expression levels at 24 h, but did affect (P<0·001) their mRNA expression levels at 48 h (Table 3). As supplemental Mn concentrations increased, linear (P<0·0007) and quadratic (P<0·02) responses were observed in FAS and ME mRNA expression.
Table 3 Effect of supplemental manganese concentration on the activities and mRNA expression levels of fatty acid synthase (FAS) and malic enzyme (ME) in chicken hepatocytes at a different incubation time (Expt 1)
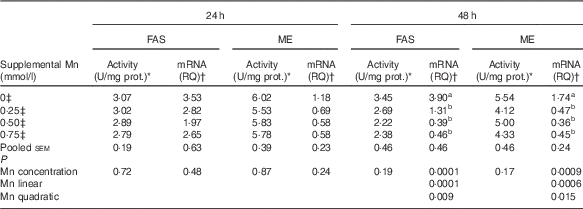
FAS, fatty acid synthase; ME, malic enzyme; U, unit; RQ, relative quantities.
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·01).
* One U of FAS activity is noted as µmol of oxidised NADPH per min per mg of protein at 37°C; one U of ME activity is defined as µmol of reduced NADP per min per mg of protein at 30°C.
† The FAS or ME mRNA abundances were calculated as the RQ of the FAS or ME mRNA to the internal reference gene β-actin mRNA; RQ=
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
(C
t
=threshold cycle).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
(C
t
=threshold cycle).
‡ Data represent the means of six replicates (n 6).
Effects of supplemental manganese source and concentration on the lactic dehydrogenase release (Expt 2)
Compared with the control, the addition of either 0·25 or 0·50 mmol/l of Mn as either manganese chloride or Mn–AA significantly decreased (P<0·01) LDH activities in the culture medium (Table 4). However, Mn source, Mn concentration and their interaction did not affect (P>0·13) LDH activities in the culture medium.
Table 4 Effects of supplemental manganese source and concentration on the activity of lactic dehydrogenase (LDH) in the culture medium of chicken hepatocytes (Expt 2)

Manganese chloride, MnCl2.4H2O; Mn–AA, Mn–amino acid chelate with a moderate chelation strength (Q f=16·85).
* One unit of LDH activity was defined as 1 mmol of reduced nicotinamide adenine dinucleotide oxidised per min.
† Data represent the means of six replicates (n 6).
‡ Different (P<0·01) from all Mn supplemental groups.
§ Data represent the means of twelve replicates (n 12).
Effects of supplemental manganese source and concentration on the activities and mRNA expression levels of fatty acid synthase and malic enzyme (Expt 2)
Compared with the control, supplemental Mn as either Mn chloride or Mn–AA at either 0·25 or 0·50 mmol/l significantly decreased (P<0·004) FAS activities and the mRNA expression levels of FAS and ME in chicken hepatocytes, but had no effect (P>0·76) on the ME activities (Table 5). The Mn source, Mn concentration and their interaction did not affect (P>0·19) FAS and ME activities, and FAS mRNA expression levels. The Mn source or an interaction between Mn source and Mn concentration had no effect (P>0·22) on the ME mRNA expression levels. However, the ME mRNA levels were influenced (P<0·03) by Mn concentration, and were lower (P<0·03) at 0·50 mmol/l than at 0·25 mmol/l.
Table 5 Effects of supplemental manganese source and concentration on the activities and mRNA expression levels of fatty acid synthase (FAS) and malic enzyme (ME) in chicken hepatocytes (Expt 2)
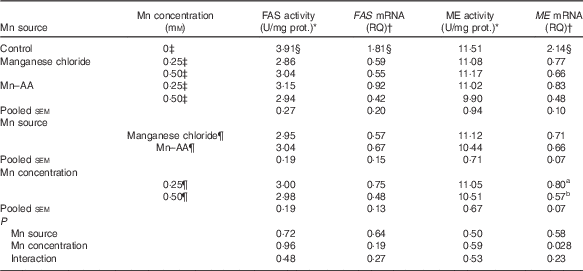
FAS, fatty acid synthase; RQ, relative quantities; ME, malic enzyme; U, unit; manganese chloride, MnCl2.4H2O; Mn–AA, Mn–amino acid chelate with a moderate chelation strength (Q f=16·85).
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·03).
* One U of FAS activity is noted as µmol of oxidised NADPH per min per mg of protein at 37°C. One U of ME activity is defined as µmol of reduced NADP per min per mg of protein at 30°C.
† The FAS or ME mRNA abundances were calculated as the RQ of the FAS or ME mRNA to the internal reference gene β-actin mRNA; RQ=
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
(C
t
=threshold cycle).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
(C
t
=threshold cycle).
‡ Data represent the means of six replicates (n 6).
§ Different (P<0·004) from all Mn supplemental groups.
¶ Data represent the means of twelve replicates (n 12).
Discussion
LDH is a stable cytosolic enzyme, which becomes extracellular when the cell membrane is damaged. The activity of LDH in the culture medium is an indicator of cellular integrity and vitality, and are commonly used to evaluate the cytotoxicity( Reference Mitchell and Acosta 34 , Reference Mcqueen and Williams 35 ). The effect of Mn on the viabilities of primary cultured chicken hepatocytes has not been reported. The results from the present study showed that the addition of Mn as either manganese chloride or Mn–AA decreased the leakage of LDH from hepatocytes, and thereby improved the integrity and vitality of hepatocytes. On the basis of the data of LDH activities from the preliminary experiment and Expt 2, the appropriate supplemental Mn concentration was about 0·50 mg/kg. Similar results were observed in an experiment( Reference Gao, Wang and Li 36 ) with primary broiler myocardial cells, suggesting that Mn supplementation with 0·50 mmol/l could have the best cell viability.
FAS, a key enzyme in lipogenesis of animals, is involved in the conversion of acetyl CoA and malonyl CoA to palmitate. The content of FAS in liver changes drastically when animals are subjected to different nutritional states. Refeeding starved rats and chickens caused a substantial increase in the rate of FAS synthesis in liver( Reference Morris, Winberry and Fisch 4 , Reference Lakshmanan, Nepokroeff and Porter 37 ). It is confirmed that the nutritional regulation of the FAS synthesis was primarily due to regulation of the amount of FAS mRNA in liver of chicks( Reference Morris, Winberry and Fisch 4 , Reference Morris, Nilson and Jenik 7 , Reference Goodridge, Jenik and Mcdevitt 8 ). However, the information is still limited regarding the effect of Mn on the activity and gene expression of FAS in the liver of animals. In the present study, the FAS activity was not altered in Expt 1, but decreased in Expt 2. The disparity in these results might be majorly because of the greater variations within the groups in Expt 1 than in Expt 2, which led to the non-significant results in Expt 1. Wang et al. ( Reference Wang, Chen and Lu 21 ) demonstrated that the inclusion of 100 mg/kg of Mn as either MnSO4 or Mn–AA in the diets reduced FAS activity, but did not affect its mRNA expression in the liver of broilers. However, the results from the current study showed that the addition of Mn as either manganese chloride or Mn–AA decreased both the activity and mRNA expression of FAS in the chicken hepatocytes. The disparities in the results of FAS mRNA among the two studies might be due to the differences in experimental type (present in vitro v. previous in vivo ( Reference Luo, Su, Huang and Liu 14 ) studies), supplemental Mn levels and so on. The results from the present study also suggest that the regulation of hepatic FAS activity by Mn might be at a pre-translational level.
Our previous studies have demonstrated that when Mn sources were supplied through either diets, or i.v. injections, the organic Mn with a moderate chelation strength was more effective than MnSO4 or organic Mn sources with weak or strong chelation strength in activating MnSOD gene expression in the heart of broilers at both mRNA and protein levels( Reference Li, Luo and Liu 22 , Reference Li, Luo and Lu 38 – Reference Li, Lu and Hao 41 ). This might result from greater intestinal absorption and/or tissue utilisation of the organic Mn source with moderate chelation strength in broilers( Reference Li, Lu and Hao 41 – Reference Ji, Luo and Lu 43 ). A further in vitro study from our laboratory also indicated that Mn–AA was more effective than manganese chloride in enhancing MnSOD mRNA expression in the primary broiler myocardial cells( Reference Li, Lin and Liao 29 ). However, Wang et al.( Reference Wang, Chen and Lu 21 ) found that both Mn–AA and MnSO4 exhibited similar efficacies in regard to enhancing the FAS activity in the liver of broilers. Similarly, in the present study, no differences were detected in FAS activity and its mRNA expression of broiler hepatocytes between Mn–AA and manganese chloride. Differences in parameters chosen in different studies (heart MnSOD mRNA( Reference Li, Luo and Liu 22 , Reference Li, Luo and Lu 38 – Reference Li, Luo and Lu 40 ) or protein expression( Reference Li, Lu and Hao 41 ) v. liver FAS activity( Reference Wang, Chen and Lu 21 ); MnSOD mRNA expression in myocardial cells( Reference Li, Lin and Liao 29 ) v. present FAS activity and its mRNA expression in hepatocytes) may offer explanations to why the efficacies of Mn–AA M differed among the experiments.
ME is one of the key lipogenic enzymes in mammals and chickens. This enzyme carries out the irreversible decarboxylation of malate to pyruvate with the formation of NADPH from NADP+, and then this NADPH is vital for fatty acid biosynthesis( Reference Yoshimoto, Nakamura and Ichihara 44 , Reference Ma, Salati and Ash 45 ). Nutritional regulation of the ME gene in animals has been investigated. Switching rats from a regular chow diet to a fat-free high-carbohydrate diet induced hepatic ME activity and its mRNA level( Reference Katsurada, Iritani and Fukuda 46 ). In poultry, liver is the primary site of ME expression( Reference Goodridge, Crish and Hillgartner 47 ). It is reported that hepatic ME activity and the abundance of its mRNA in chickens were tightly controlled by nutritional conditions( Reference Katsurada, Iritani and Fukuda 46 ), and nutritional regulation of the synthesis of the enzyme is primarily at a pre-translational level( Reference Morris, Winberry and Fisch 4 ). A previous study( Reference Wang, Chen and Lu 21 ) from our laboratory demonstrated that dietary Mn supplementation with 100 mg/kg as either Mn–AA or MnSO4 decreased hepatic ME activity and its mRNA expression in broilers, indicating that Mn could regulate the chicken ME gene expression at a transcriptional level. In the current study, the ME mRNA was not significantly decreased at 24 h but only at 48 h, indicating that Mn could decrease ME mRNA expression in the primary broiler hepatocytes in a time-dependent manner. In addition, our present study showed that the addition of either 0·25 or 0·50 mmol/l of Mn as either Mn–AA or manganese chloride inhibited the ME mRNA expression, but had no effect on the ME activity in chicken hepatocytes, suggesting that this enzyme expression might be also modulated by Mn at both transcriptional and post-transcriptional levels. The discrepancy in results of ME activity between the present study and our previous study( Reference Wang, Chen and Lu 21 ) might be partially due to the differences in Mn levels and experimental types (present in vitro v. previous in vivo ( Reference Wang, Chen and Lu 21 ) studies) used in the two studies. Further studies are needed to address how Mn affects gene expressions of FAS and ME in the future.
Until now, the influence of Mn source on ME activity and expression has only been reported by researchers from our laboratory. An earlier study( Reference Lu, Luo and Ji 20 ) from our laboratory demonstrated that the addition of either 100 or 200 mg/kg Mn as Mn–AA or MnSO4 decreased ME activities in abdominal fat of broilers, but no differences were detected between Mn–AA and MnSO4 treatments. Subsequently, we observed that Mn–AA and MnSO4 had similar efficacies in regulating the hepatic ME mRNA and activity of broilers( Reference Wang, Chen and Lu 21 ). The results from the current study are consistent with the above results, indicating that Mn–AA was as effective as manganese chloride in reducing ME mRNA expression in primary cultured chicken hepatocytes. These results also implied that hepatic ME activity and its mRNA expression were also not sensitive indicators in distinguishing the effectiveness of Mn sources.
In conclusion, the results from the present study indicate that Mn supplementation as either Mn–AA or manganese chloride decreased the activity of FAS and the mRNA expression of FAS and ME in cultured primary chicken hepatocytes. Mn supplementation also reduced the hepatocyte damage. Compared with manganese chloride, Mn–AA had a similar effect on the measured cellular parameters. These results suggest that Mn might directly modulate the FAS and ME expressions at a transcriptional level. These findings might provide a further insight into the specific regulatory mechanism of the two enzymes by Mn.
Acknowledgements
The authors are grateful to the staff in this laboratory for their valuable input to this study.
The present study was supported by the Agricultural Science and Technology Innovation Program (ASTIPIAS08), the China Agriculture Research System (grant no. CARS-41) and the National Natural Science Foundation of China (grant no. 30771575).
The authors’ contributions are as follows: X. L. and L. L. designed the experiment; L. L. drafted the manuscript; X. L. and X. L. participated in writing and editing of the manuscript; M. W. conducted most of the experiments and analysed the data; L. Z. performed the analysis of some parameters; and X. L. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.








