Fe deficiency is the most common nutritional deficiency among pregnant women in both the developed and the developing world( Reference Lopez, Cacoub and Macdougall 1 ). During pregnancy Fe requirements considerably increase because of the expansion of maternal red cell mass and the growth of fetal-placental unit( Reference Lopez, Cacoub and Macdougall 1 ). Evidence exists that prenatal Fe use improves birth weight( Reference Haider, Olofin and Wang 2 ), gestational duration( Reference Zeng, Dibley and Cheng 3 ) and child intellectual development( Reference Christian, Murray-Kolb and Khatry 4 ), though some studies have not confirmed the associations( Reference Peña-Rosas, De-Regil and Garcia-Casal 5 , Reference Li, Zeng and Wang 6 ). Animal studies suggested that maternal Fe deficiency in pregnancy affected blood pressure and cardiovascular development in the offspring( Reference Andersen, Gambling and Holtrop 7 , Reference Gambling, Dunford and Wallace 8 ).
The main source of body Fe stores is from diets. Dietary Fe exists in two forms: haeme and non-haeme Fe. Haeme Fe comes mainly from animals and is better absorbed than non-haeme Fe that comes mainly from plants( Reference Hurrell and Egli 9 ). Studies suggested that haeme and non-haeme Fe may be differently associated with health outcomes( Reference Khambalia, Aimone and Nagubandi 10 , Reference Bao, Rong and Rong 11 ). Dietary Fe inadequacy during pregnancy is prevalent worldwide, especially in the developing countries( Reference Lee, Talegawkar and Merialdi 12 ). To meet the increased Fe requirements, Fe supplementation has been routinely recommended during pregnancy in the USA and Canada. However, because of the inconsistent effects of prenatal Fe use on birth outcomes and possible gastrointestinal side effects, the routine Fe supplements use during pregnancy is not recommended in most countries.
The relationship between dietary Fe intake and adverse birth outcomes such as low birth weight (LBW), preterm birth (PTB), small for gestational age (SGA) and birth defects has been explored in several studies( Reference Mathews, Yudkin and Neil 13 – Reference Shaw, Carmichael and Yang 18 ). However, the results were conflicting. Moreover, few studies considered the potential different effects of different sources of Fe (dietary or supplemental) and types of Fe (haeme or non-haeme) on birth outcomes( Reference Alwan, Greenwood and Simpson 16 ). The evidence for the effects of vitamin C, smoking, alcohol drinking and anaemia on the associations is also limited( Reference Baker, Wheeler and Sanders 15 , Reference Alwan, Greenwood and Simpson 16 ). To our knowledge, the associations between dietary and supplemental Fe intakes during pregnancy and birth outcomes have not been reported in China. China has been reported to undergo a nutrition transition during the past few decades, with animal-source foods intakes increasing markedly( Reference Zhai, Du and Wang 19 ). Pregnant women in Northwest China reported dietary Fe intake close to the recommended nutrient intake (RNI); however, they may have poor Fe absorption because of the high proportion of plant foods contributing to Fe intake and the low intake of vitamin C( Reference Yang, Dang and Cheng 20 ). In China, Fe supplements use during pregnancy is still not common. The present study conducted in Shaanxi Province of Northwest China aimed to examine the associations of maternal Fe intake (total Fe from diet and supplements, dietary total Fe, haeme Fe, non-haeme Fe and Fe supplements use) during pregnancy with birth outcomes (LBW, PTB, SGA, intra-uterine growth retardation (IUGR) and birth defects), and to investigate the potential effect modifications by vitamin C, passive smoking, alcohol drinking and anaemia.
Methods
Study design and participants
A population-based cross-sectional survey with the purpose of investigating the risk factors for birth outcomes and maternal nutritional status during pregnancy was conducted in Shaanxi, China between August and November 2013. This area can be divided into three regions: northern, southern and central Shaanxi, with natural resources, history, culture and lifestyle greatly differing among them. A total of 30 027 women who were pregnant during 2010–2013 were recruited in the survey using a stratified multistage random sampling method. According to the proportion of rural to urban residents, population size and fertility rate in Shaanxi, China, twenty counties and ten districts were randomly sampled. In each sampled county, six villages each from six townships were randomly selected; in each sampled district, six communities each from three streets were randomly selected. In all, thirty and sixty participants were randomly selected in each sampled village and community, respectively. Among the participants, 7750 women who were pregnant during 2012–2013 and had infants <12 months old were further interviewed to report their diets during pregnancy. Women who reported a multiple gestation or an implausible total energy intake (>20 920 or <2092 kJ/d) were excluded from the present study. The final sample included a total of 7375 eligible mothers, and the median month after delivery when mothers were interviewed was 3 (10th–90th percentiles 0–7). The flow diagram of sampling strategy with exclusion criteria is displayed in Fig. 1.

Fig. 1 Flow diagram of sampling strategy with exclusion criteria.
Ethics
This study was conducted in accordance with the Helsinki Declaration, and all procedures involving human subjects were approved by the Xi’an Jiaotong University Health Science Center. Written informed consent was obtained from all participants.
Assessment of nutrient intake
Dietary intakes of mothers during the whole pregnancy were measured by a 107-item semi-quantitative FFQ at 0–12 months (median 3; 10th–90th percentiles 0–7) after delivery. Maternal dietary patterns tended to change little from early to late pregnancy( Reference Crozier, Robinson and Godfrey 21 ). Diet assessment during the whole pregnancy at one time was reasonable, convenient and economical for large epidemiological studies( Reference Rifas-Shiman, Rich-Edwards and Willett 22 ), especially for our present large-scale survey with multiple dietary exposures and outcomes( Reference Yang, Dang and Cheng 20 ). The FFQ was established on the basis of the previously validated FFQ used for pregnant women in Shaanxi, China( Reference Cheng, Yan and Dibley 23 ). Pearson’s correlation coefficient for Fe between the FFQ and the average of six 24-h recalls was 0·65, with a range of 0·53–0·70 for other nutrients( Reference Cheng, Yan and Dibley 23 ). The frequency scales of five food items (animal oils, vegetable oils, salt, sugar and sauce) were open-ended, and were recorded as kilograms per month and the number of people regularly consuming them. Regarding the other 102 food items, women were asked to recall consumption frequency from the eight predefined categories ranging from never to two or more times per day and identify their portion sizes according to food portion images( Reference Yang, Dang and Cheng 20 ). Daily intakes of dietary total Fe, haeme Fe (mainly derived from animal meat sources), non-haeme Fe (derived from the difference between dietary total Fe and haeme Fe) and other nutrients were transformed using the China Food Composition Tables( 24 , 25 ). Participants were also asked to report the type/brand, lasting day and the amount of all dietary supplements they used during each trimester of pregnancy. Daily supplemental Fe intake was calculated by multiplying the Fe content of each reported supplement and the number of supplement tablets taken daily. Total Fe intake was derived from the sum of dietary Fe and supplemental Fe intakes. The RNI of Fe for Chinese pregnant women during the first, second and third trimesters were 20, 24 and 29 mg/d, respectively( 26 ).
Ascertainment of birth outcomes
Neonatal information including birth date, sex, birth weight and gestational age was obtained by reviewing birth certificates. Birth weight was measured with a baby scale with precision to the nearest 10 g. Gestational age at delivery was calculated according to the last menstrual period, and was confirmed by ultrasound scans. Birth defects information was collected with the pre-code structured questionnaire according to the International Classification of Diseases, 10th revision. Medical records including clinical diagnosis, physical examination, ultrasound imaging report and medical history were referred to ascertain birth outcomes. Birth outcomes were defined as follows: (1) LBW, birth weight <2500 g; (2) PTB, delivery <37 completed gestational week; (3) SGA, birth weight <10th percentile of the gestational age-sex specific Chinese reference for fetal growth( Reference Zhu, Zhang and Zhang 27 ); (4) IUGR, birth weight <3rd percentile of the gestational age-sex specific Chinese reference for fetal growth( Reference Zhu, Zhang and Zhang 27 ).
Assessment of socio-demographic and health-related characteristics
The general information of the participants during pregnancy was gathered face to face by well-trained interviewers via a standard questionnaire. The study information was classified as the following: (1) Socio-demographic characteristics: geographic area (northern, southern or central Shaanxi); residence (rural or urban); childbearing age (<25, 25–29 or ≥30 years); maternal education (primary school or below, junior high school or senior high school or above); maternal occupation (farmer or working outside); nulliparity (yes or no); (2) health-related characteristics: passive smoking (yes or no); alcohol drinking (yes or no); frequency of antenatal care visits (<6 or ≥6); folate supplements use (yes or no); anaemia (yes or no); medication use (yes or no). Passive smoking was defined as being exposed to another person’s tobacco smoke for ≥15 min/d. Alcohol drinking included a wide range of alcoholic beverages (liquor, wine and beer) consumed during pregnancy. Anaemia during pregnancy was diagnosed using the criteria of hb concentration <110 g/l.
Statistical analysis
The study population characteristics and energy-adjusted nutrient intakes by residual method( Reference Willett 28 ) were described according to tertiles of dietary total Fe and haeme Fe intakes. Categorical variables were expressed as percentages and continuous variables as means. Univariable comparisons were made using χ 2 test for categorical variables and ANOVA for continuous variables. Household wealth index was established by principal component analysis according to the variables of family economic level (housing condition, vehicle type, income source, and type and number of household appliance), and this index was divided into thirds as an indicator for the poor, medium and rich households( Reference Filmer and Pritchett 29 ). To avoid multicollinearity of nutrients in regression analysis, principal component analysis was used according to the intakes of potential nutrients (protein, fat, carbohydrate, vitamin A, thiamin, riboflavin, folate, vitamin C, vitamin E, Ca, Zn and Se). The number of components extracted was based on eigenvalues >1, the scree plot and interpretability( Reference Schulze, Hoffmann and Kroke 30 ). The first component that explained 68·7 % of the total variance was finally extracted, and the factor loadings of protein, Zn, riboflavin, thiamin, Ca and Se were above 0·80.
In our study, the survey data collected from the stratified multistage random sampling design had a hierarchical structure in which lower levels were nested within higher levels. Considering this, multilevel models which were explicitly designed to analyse hierarchically structured data were applied. We first constructed four-level empty models representing county (district)–township (community)–village (street)–individual and observed non-significant within-group variations (all P>0·05) and low intra-class correlations (all lower than 0·001) in the village (street) level. Therefore, we adopted simplified multilevel models with a random intercept at the county (district) and the township (community) levels. Multilevel logistic regression models were used to estimate OR and 95 % CI for adverse birth outcomes (LBW, PTB, SGA, IUGR and birth defects) associated with maternal Fe intake and Fe supplements use, and multilevel linear regression models were used to evaluate the effect of every 1 mg/d increase in maternal haeme Fe intake on birth weight. The intake of total Fe, dietary Fe, haeme Fe and non-haeme Fe was stratified by tertile to avoid the possible influence of extreme values, whereas the Fe supplements use was categorised by trimester because of the low proportion of pregnant women taking Fe supplements in China and the possible different effects of Fe supplements use on birth outcomes during different trimesters reported before( Reference Alwan, Greenwood and Simpson 16 ). Four adjusted models were established: (1) model 1 adjusted for energy; (2) model 2 adjusted for energy and socio-demographic characteristics, including geographic area, residence, childbearing age, education, occupation, household wealth index and parity; (3) model 3 adjusted for all variables in model 2 plus health-related characteristics, including passive smoking, alcohol drinking, antenatal care visit frequency, folate supplements use, anaemia and medication use; (4) model 4 adjusted for all variables in model 3 plus principal component score based on the nutrient intakes. Models were additionally adjusted for Fe supplements use to examine the effect of dietary total Fe, adjusted for Fe supplements use and non-haeme Fe to examine the effect of haeme Fe, adjusted for Fe supplements use and haeme Fe to examine the effect of non-haeme Fe, and additionally adjusted for dietary total Fe to examine the effect of Fe supplements use. To test for a linear trend, we used the median for each tertile of iron intake as a continuous variable. We further conducted stratified analyses and evaluated interaction terms to explore the possible modifiable effects of vitamin C intake (the RNI of ≥ or <115 mg/d for Chinese pregnant women), passive smoking (yes or no), alcohol drinking (yes or no) and anaemia (yes or no) on the associations.
Statistical analyses were performed using STATA software (version 12.0; StataCorp LP). A two-tailed P<0·05 was regarded as statistically significant.
Results
Status of iron intake
The status of Fe intake among pregnant women in Shaanxi, China is present in Table 1. Mean total Fe intake from diet and supplements was 29·8 (sd 12·7) mg/d, and the proportions of participants reporting total Fe intake below the RNI of 20, 24 and 29 mg/d were 27·9, 38·1 and 51·1 %, respectively. Mean dietary total Fe intake was 28·2 (sd 11·3) mg/d, and the proportions of women who had dietary Fe intake below the RNI of 20, 24 and 29 mg/d were 30·2, 40·9 and 53·8 %, respectively. Mean haeme Fe intake was 2·6 (sd 2·3) mg/d, and the main contributor to haeme Fe intake was red meat (76·9 %). Mean non-haeme Fe intake was 25·6 (sd 11·3) mg/d, with cereals and tubers contributing the most to non-haeme Fe intake (62·1 %). 19·8 % of participants reported taking Fe supplements during pregnancy, and women with Fe supplements use during the first, second and third trimesters accounted for 6·9, 12·1 and 8·2 % of the sample, respectively. Only 5·4 % of women reported taking Fe-only supplements during pregnancy, with those taking Fe-only supplements during the three gestational periods accounting for 2·1, 2·9 and 2·5 % of the sample, respectively.
Table 1 Daily iron intake from different sources among pregnant women in Shaanxi Province, Northwest China (Mean values and standard deviations)

* Red meat included pork, beef, mutton and their products.
† White meat included poultry, fish, shrimp, shellfish and their products.
Characteristics of the participants
The general characteristics, energy-adjusted nutrient intakes and pregnancy outcomes among the participants according to tertiles of dietary total Fe and haeme Fe intakes are displayed in Table 2. Pregnant women with higher dietary total Fe and haeme Fe intakes tended to be in central Shaanxi and southern Shaanxi, respectively. Participants with higher dietary total Fe and haeme Fe intakes were more likely to live in urban areas, be aged 25–29 years at delivery, be better educated, work outside, be wealthier, have more antenatal care visits and take supplements of folate and Fe. Women with higher dietary total Fe intake were less likely to drink during pregnancy, and women with higher haeme Fe intake were less likely to be exposed to secondhand smoke. Pregnant women consuming more haeme Fe also tended to be at their first delivery.
Table 2 Baseline characteristics of participants by tertiles (T) of dietary total iron and haeme iron intakes among pregnant women in Shaanxi Province, Northwest China (Mean values and percentages)

* P values for the differences among groups were derived from χ 2 tests for categorical variables and ANOVA for continuous variables.
Significant differences existed in all selected energy-adjusted nutrient intakes among tertiles of dietary total Fe and haeme Fe intakes. Dietary total Fe intake was positively associated with most nutrients intake, and negatively associated with fat, vitamin E and Se intake. Haeme Fe intake was positively associated with most nutrients intake, and negatively associated with dietary total Fe, non-haeme Fe, carbohydrate and vitamin E intake.
Pregnancy outcomes of the participants were as follows: (1) birth weight, 3264 (sd 440) g; (2) gestational age, 39·6 (sd 1·3) weeks; (3) LBW, 3·3 %; (4) PTB, 3·1 %; (5) SGA, 12·8 %; (6) IUGR, 5·8 %; (7) birth defects, 1·9 %. The results of univariable comparisons suggested significant differences in birth weight, LBW, SGA and IUGR among tertiles of haeme Fe intake.
Associations between total iron intake from diet and supplements and birth outcomes
Total Fe intake from diet and supplements was not associated with LBW, PTB, SGA, IUGR or birth defects and the tests for trend were not statistically significant in all models (online Supplementary Table S1).
Associations between dietary iron intakes and birth outcomes
There was no relationship between dietary total Fe intake and adverse birth outcomes (online Supplementary Table S1). The associations of dietary haeme Fe and non-haeme Fe intakes with birth outcomes are shown in Table 3. No associations of non-haeme Fe intake with LBW, SGA, IUGR or birth defects were observed. The highest tertiles of non-haeme Fe intakes compared with the lowest tertiles were significantly and negatively associated with PTB risk in model 1 (OR 0·65; 95 % CI 0·43, 0·97) and in model 2 (OR 0·66; 95 % CI 0·44, 0·98), and the tests for trend were significant in model 1 (P trend=0·039) and in model 2 (P trend=0·047). However, the associations attenuated and were not significant after further adjustment for health-related factors in model 3 and further adjustment for health-related plus dietary factors in model 4, and the tests for trend were not statistically significant in these two models (P trend>0·05).
Table 3 Birth outcomes associated with tertiles (T) of dietary haeme iron and non-haeme iron intakes during pregnancy in Shaanxi Province, Northwest ChinaFootnote * (Odds ratios and 95 % confidence intervals)

* Multilevel logistic regression models were used to estimate OR and 95 % CI. Models were additionally adjusted for Fe supplements use and non-haeme Fe to examine the effect of haeme Fe, and additionally adjusted for Fe supplements use and haeme Fe to examine the effect of non-haeme Fe.
† P for trend across tertiles was calculated using the median intake of each tertile as a continuous variable.
‡ Number of participants who had the corresponding adverse birth outcomes in the corresponding groups/the total number of participants in the corresponding groups.
§ Model 1 was adjusted for energy.
|| Model 2 was adjusted for energy and socio-demographic characteristics, including geographic area, residence, childbearing age, education, occupation, household wealth index and parity.
¶ Model 3 was adjusted for all variables in model 2 plus health-related characteristics, including passive smoking, alcohol drinking, antenatal check visit frequency, folate supplements use, anaemia and medication use.
** Model 4 was adjusted for all variables in model 3 plus principal component score based on the nutrient intakes.
Participants in the highest tertile of >2·65 mg/d haeme Fe intake were less likely to have a LBW baby (OR 0·68; 95 % CI 0·49, 0·94) and a SGA baby (OR 0·76; 95 % CI 0·62, 0·94) compared with those in the lowest tertile. Pregnant women in the medium and highest tertiles of haeme Fe intake were less likely to deliver an IUGR baby relative to those in the lowest tertile (medium tertile v. lowest tertile: OR 0·78; 95 % CI 0·61, 0·95; highest tertile v. lowest tertile: OR 0·76; 95 % CI 0·59, 0·93; P trend=0·045). We found a 45 % lower risk of birth defects for the highest compared with the lowest tertile of haeme Fe intake (OR 0·55; 95 % CI 0·32, 0·89). Moreover, the risks for LBW, SGA, IUGR and birth defects were reduced with increasing tertiles of haeme Fe intake in all models (P trend<0·05). However, we observed no relationship between haeme Fe intake and PTB risk.
We further explored the magnitude of change in birth weight in association with maternal haeme Fe intake during pregnancy (Table 4). In the energy-adjusted model, per 1 mg/d increase of haeme Fe intake resulted in an increase of 24 g of birth weight (95 % CI 10, 38; P=0·001). The significant association persisted when additional adjustments were performed. After adjustment for all possible confounders (socio-demographical, health-related and dietary factors), the birth weight increase was 16 g (95 % CI 3, 31; P=0·035) for per 1 mg/d increase in maternal haeme Fe intake during pregnancy.
Table 4 Birth weight changes associated with per 1 mg/d increase in maternal haeme iron intake during pregnancyFootnote * (Changes and 95 % confidence intervals)
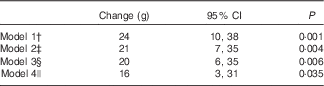
* Multilevel linear regression models were used to estimate changes and 95 % CI. Models were additionally adjusted for Fe supplements use and non-haeme Fe to examine the effect of haeme Fe.
† Model 1 was adjusted for energy.
‡ Model 2 was adjusted for energy and socio-demographic characteristics, including geographic area, residence, childbearing age, education, occupation, household wealth index and parity.
§ Model 3 was adjusted for all variables in model 2 plus health-related characteristics, including passive smoking, alcohol drinking, antenatal check visit frequency, folate supplements use, anaemia and medication use.
|| Model 4 was adjusted for all variables in model 3 plus principal component score based on the nutrient intakes.
Associations between iron supplements use and birth outcomes
The association between Fe supplements use and LBW risk is shown in Fig. 2. Women taking Fe supplements during pregnancy had a lower risk of delivering a LBW baby (OR 0·72; 95 % CI 0·50, 0·95). Fe supplements use during the second and third trimesters was associated with a significantly reduced risk of LBW (during the second trimester: OR 0·67; 95 % CI 0·42, 0·98; during the third trimester: OR 0·47; 95 % CI 0·24, 0·93), whereas no association was observed between Fe supplements use during the first trimester and LBW risk. The associations of Fe supplements use during pregnancy or during three different gestational periods with PTB, SGA, IUGR or birth defects were not statistically significant (data not shown).

Fig. 2 OR of low birth weight associated with iron supplements use during pregnancy in Shaanxi Province, Northwest China. Multilevel logistic regression models were used to estimate OR and 95 % CI. Model 1 was adjusted for energy. Model 2 was adjusted for energy and socio-demographic characteristics, including geographic area, residence, childbearing age, education, occupation, household wealth index and parity. Model 3 was adjusted for all variables in model 2 plus health-related characteristics, including passive smoking, alcohol drinking, antenatal check visit frequency, folate supplements use, anaemia and medication use. Model 4 was adjusted for all variables in model 3 plus principal component score based on the nutrient intakes. Models were additionally adjusted for dietary total iron intake to examine the effect of iron supplements use.
Roles of modifiable factors
The associations between maternal Fe intake (total Fe intake from diet and supplements, dietary total Fe, haeme Fe, non-haeme Fe and Fe supplements use) and birth outcomes did not vary by vitamin C intake, passive smoking, alcohol drinking or anaemia (data not shown), and the tests for interaction were not significant (P>0·05).
Discussion
In this population-based cross-sectional study, we observed significant and negative associations between maternal haeme Fe intake during pregnancy and birth outcomes, including LBW, SGA, IUGR and birth defects. Fe supplements use during pregnancy and during the second and third trimesters was associated with a reduced risk of LBW. We found no significant associations of total Fe from diet and supplements, dietary total Fe or non-haeme Fe intake with birth outcomes.
Previous studies on maternal Fe intake and birth outcomes have generally reported on dietary total Fe intake and Fe supplements use only. Similar to the current study, several studies reported no association of dietary total Fe intake during pregnancy with birth weight( Reference Mathews, Yudkin and Neil 13 , Reference Lagiou, Mucci and Tamimi 14 ). A recent comprehensive meta-analysis showed a significantly negative association of prenatal Fe use with LBW and no associations with PTB or SGA( Reference Haider, Olofin and Wang 2 ), which is also consistent with our present study. To our knowledge, only one study conducted in the UK investigated the associations of different sources and types of Fe intakes with birth weight and gestational age. The result of this previous study showed a positive association between both total Fe intake and non-haeme Fe intake, derived by 24 h dietary recall in the first trimester, and birth weight, which is different from the result of our study. The discrepancy may be partially due to different study designs, nutrient assessment methods and population characteristics such as genetic backgrounds and dietary habits. However, the result of no association between maternal Fe intake and PTB in this previous study is in agreement with our study result. Studies on the association between maternal Fe intake and birth defects are sparse. A previous study reported a negative association between dietary total Fe intake and spinal bifida in the offspring( Reference Groenen, van Rooij and Peer 17 ). In the current study, we observed a negative association between haeme Fe intake and birth defects. However, we cannot further evaluate the associations of maternal Fe intake with birth defects subtypes because of the limited cases. Further large studies with better designs are needed to explore the effect of maternal Fe intake on birth defects, especially for the subtypes with a high prevalence such as congenital heart defects( Reference Yang, Qiu and Qu 31 ).
Fe is an essential constituent of hb and myoglobin, which account for 60 % of total body Fe. Fe is necessary for DNA synthesis, various enzymatic processes and mitochondrial energy generation( Reference Lopez, Cacoub and Macdougall 1 ), which may exert effects on pregnant women and their fetus. Dietary Fe is the main determinant of body Fe store, and exists as haeme and non-haeme Fe. Consistent with the current study, previous studies reported that the two forms of Fe may have different relations with health outcomes( Reference Khambalia, Aimone and Nagubandi 10 , Reference Bao, Rong and Rong 11 ). Although precise mechanisms are not clear, the different bioavailability of haeme and non-haeme Fe could partially explain the discrepancy in their relations with birth outcomes. Haeme Fe absorption is more efficient and affected little by other dietary factors, whereas non-haeme Fe absorption is well regulated and depends on accompanying dietary components such as vitamin C, the enhancer of Fe absorption, and Ca and polyphenols, the inhibitors of Fe absorption( Reference Hurrell and Egli 9 , Reference Cook 32 ). It has been reported that haeme Fe absorption was 10–15 times higher than non-haeme Fe absorption( Reference Khambalia, Aimone and Nagubandi 10 ). Therefore, it is plausible that higher haeme Fe intake is more likely to increase body Fe stores and thus is more likely to be associated with reduced risks for adverse birth outcomes. It is also plausible that pregnant women with a higher intake of haeme Fe may have a much better baseline Fe status because of their more concern about nutrition, such that their subsequent increase in Fe stores resulting from haeme Fe intake may have raised Fe levels enough to be beneficial. In fact, the participants in our study with a higher intake of haeme Fe tended to have better overall nutrition status, which was supported by the higher intakes of most energy-adjusted nutrients in the higher tertile of haeme Fe intake shown in Table 2. Previous studies supported significant associations between maternal Fe status (hb, ferritin and transferrin receptor) and birth outcomes (LBW, PTB and SGA)( Reference Haider, Olofin and Wang 2 , Reference Rahman, Abe and Rahman 33 , Reference Alwan, Cade and McArdle 34 ). Maternal hepcidin, the master regulator of systemic Fe bioavailability, was reported to influence placental uptake of dietary haeme and non-haeme Fe( Reference Young, Griffin and Pressman 35 ) and relate with adverse pregnancy outcomes( Reference Simavli, Derbent and Keskin 36 ). Moreover, a previous study demonstrated gene–Fe interaction affected birth weight( Reference Hur, Kim and Ha 37 ). People with distinct genetic backgrounds may respond variously to Fe intake and Fe status. Future studies integrating different sources and types of Fe intake, measures of body Fe status with genetic backgrounds are warranted.
The present study was conducted in Shaanxi Province of Northwest China using a stratified multistage random sampling method, with the large sample size accounting for 3 % of neonates in Shaanxi, China. The Shaanxi Province was similar to the Northwest China in the diversity of culture and lifestyle. Thus, our results can be generalised to the overall Shaanxi Province and Northwest China, and may also partly reflect the status among pregnant women in China. Another strength of this study included the relatively accurate birth outcomes collected by reviewing birth certificates and medical records. However, some limitations should be acknowledged. First, the information on nutrient intakes and non-dietary characteristics during pregnancy was retrospectively self-reported by the mothers at 0–12 months (median: 3; 10th–90th percentiles: 0–7) after delivery. Although previous studies suggested that nutrient intakes and events during pregnancy could be recalled rather well even after years( Reference Bunin, Gyllstrom and Brown 38 – Reference Kvalvik, Nilsen and Skjaerven 40 ), we cannot rule out the possible exposure misclassification due to recall bias. To minimise bias, we made efforts to help participants recall accurately in the study. For one thing, we used standard questionnaires and detailed supporting materials such as food portion images and calendars to gather information. For another thing, we conducted a pilot study to test the survey instruments and trained interviewers rigorously according to the detailed guides before the formal survey. Second, we cannot reveal a real causal association because of the cross-sectional design. Third, we cannot fully rule out all other unobserved and unknown confounders even after controlling for some potential confounders, including socio-demographic, health-related and dietary factors. For example, we did not collect data on maternal pre-pregnancy weight and height, which were reported to be associated with birth outcomes( Reference Alwan, Greenwood and Simpson 16 ). Fourth, we lacked data on biomarkers of body Fe status such as hb, ferritin and transferrin receptor during pregnancy, which limited us to further explore the associations between maternal Fe status and birth outcomes.
In conclusion, findings from the present study suggest that higher haeme Fe intake is associated with a reduced risk for LBW, SGA, IUGR and birth defects. The result also suggests that Fe supplements use during pregnancy is negatively associated with LBW risk. The increase in maternal haeme Fe intake and Fe supplements use during pregnancy may reduce adverse birth outcomes risk. Future studies with data on different sources and types of Fe, measures of body Fe status and genetic backgrounds are warranted to further explore their associations and to decipher underlying mechanisms.
Acknowledgements
The authors are grateful to all mothers who participated in this study, all staff who coordinated field work and all investigators who contributed to data collection.
This study was supported by the National Natural Science Foundation of China (grant no.: 81230016) and the Shaanxi Health and Family Planning Commission (Sxwsjswzfcght2016-013).
The authors’ contributions are as follows: J. Y., S. D. and H. Y. conceived and designed the study; J. Y., S. D., Y. C. and H. Y. drafted and revised the manuscript; J. Y., L. P., Y. J. and F. L. analysed and interpreted the data; J. Y., L. P., L. Z., Q. W., Q. L., Y. K. and Y. S. collected and cleared the data. All authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000691









